Drought-Tolerance Gene Identification Using Genome Comparison and Co-Expression Network Analysis of Chromosome Substitution Lines in Rice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Evaluation of Physiological Responses at Vegetative Stage under Drought-Stress Conditions
2.2.1. Rice Growth Condition
2.2.2. Net Photosynthesis Rate and Leaf Water Status Detection
2.3. Identification of Drought-Tolerance Gene
2.3.1. Whole Genome Sequencing
2.3.2. Gene Co-Expression Network Analysis
2.4. Identification of Drought-Tolerance Gene Function in Arabidopsis
2.4.1. Arabidopsis Homologous Gene
2.4.2. Arabidopsis Growth Condition
2.5. Statistical Analysis
3. Results
3.1. Evaluation of Physiological Responses of CSSLs of KDML105 under Drought-Stress Conditions
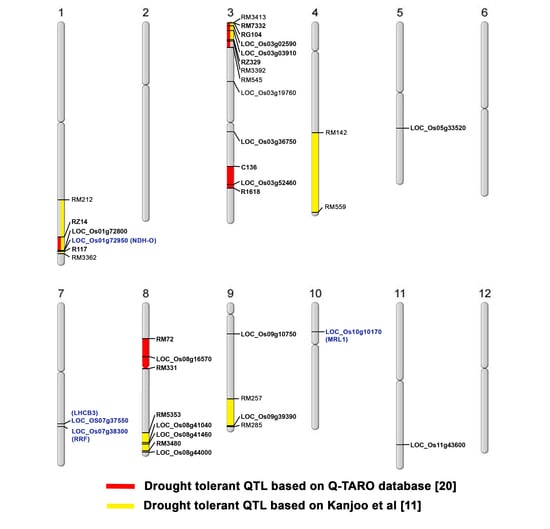
3.2. Whole Genome Sequence Comparison between CSSL104 and KDML10’ and Co-Expression Network Analysis Revealed that Major Hub Genes Have a Role in Photosynthesis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Saikumar, S.; Gouda, P.K.; Saiharini, A.; Varma, C.M.K.; Vineesha, G.; Padmavathi, G.; Shenoy, V.V. Major QTL for enhancing rice grain yield under lowland reproductive drought stress identify using O. sativa/O. glaberrima introgression line. Field Crops Res. 2014, 163, 119–131. [Google Scholar] [CrossRef]
- Tulyathan, V.; Leeharatanaluk, B. Change in quality of rice (Oryza sativa L.) cv. Khao Dawk Mali 105 during storage. J. Food Biochem. 2007, 31, 415–425. [Google Scholar] [CrossRef]
- Siangliw, J.; Jongdee, B.; Pantuwan, G.; Toojinda, T. Developing KDML105 backcross introgression lines using marker-assisted selection for QTLs associated with drought tolerance in rice. Sci. Asia 2007, 33, 207–214. [Google Scholar] [CrossRef]
- Siddique, M.R.B.; Hamid, A.; Islam, M.S. Drought stress effects on water relations of wheat. Bot. Bull. Acad. Sin. 2001, 41, 35–39. [Google Scholar]
- Hussain, M.; Malik, M.A.; Farooq, M.; Ashraf, M.Y.; Cheema, M.A. Improving drought tolerance by exogenous application of glycine-betaine and salicylic acid in sunflower. J. Agron. Crop Sci. 2008, 194, 193. [Google Scholar] [CrossRef]
- Cornic, G.; Massacci, A. Leaf photosynthesis under drought. In Photosynthesis and the Environment; Baker, N.R., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1996; pp. 347–366. [Google Scholar]
- Anjum, F.; Yaseen, M.; Rasul, E.; Wahid, A.; Anjum, S. Water stress in barley (Hordeum valgare L.). I. effect on chemical composition and chlorophyll content. Pak. J. Agron. Sci. 2003, 40, 45–49. [Google Scholar]
- Du, Y.C.; Kawamitsu, Y.; Nose, A.; Hiyane, S.; Murayama, S.; Wasano, K.; Uchida, Y. Effect on water stress on carbon exchange rate and activities of photosynthetic enzymes in leaves of sugarcane (Saccharum sp.). Aust. J. Plant Physiol. 1996, 23, 719–726. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef] [Green Version]
- Zargar, S.M.; Gupta, N.; Nazir, M.; Mahajan, R.; Malik, F.A.; Sofi, N.R.; Shikari, A.B.; Salgotra, R.K. Impact of drought on photosynthesis: Molecular perspective. Plant Gene 2017, 11, 154–159. [Google Scholar] [CrossRef]
- Kanjoo, V.; Punyawaew, K.; Siangliw, J.L.; Jearakongman, S.; Vanavichit, A.; Toojinda, T. Evaluation of agronomic traits in chromosome segment substitution lines of KDML105 containing drought tolerance QTL under drought stress. Rice Sci. 2012, 19, 117–124. [Google Scholar] [CrossRef]
- Punchkhon, C.; Kasetranunt, W.; Kositsup, B.; Siengliw, J.; Chadchawan, S. Evaluation of drought tolerance ability at seedling stage of CSSL rice populations with drought tolerant genes on chromosome 8. In Proceedings of the 9th Botanical Conference of Thailand, Bangkok, Thailand, 3–5 June 2015; pp. 61–71. [Google Scholar]
- Vajrabhaya, M.; Vajrabhaya, T. Somaclonal variation of salt tolerance in rice. In Biotechnology Agriculture Forestry; Bajaj, Y.P.S., Ed.; Springer: Berlin/Heidelberg, Germany, 1991; pp. 368–382. [Google Scholar]
- Pongprayoon, W.; Roytrakul, S.; Pichayangkura, R.; Chadchawan, S. The role of hydrogen peroxide in chitosan-induced resistance to osmotic stress in rice (Oryza sativa L.). Plant Growth Regul. 2013, 70, 159–173. [Google Scholar] [CrossRef]
- Chintakovid, N.; Maipoka, M.; Phaonakrop, N.; Mickelbart, M.V.; Roytrakul, S.; Chadchawan, S. Proteomic analysis of drought-responsive proteins in rice reveals photosynthesis-related adaptations to drought stress. Acta Physiol. Plant. 2017, 39, 240. [Google Scholar] [CrossRef]
- Missirian, V.; Comai, L.; Filkov, V. Statistical mutation calling from sequenced overlapping DNA pools in TILLING experiments. BMC Bioinf. 2011, 12, 287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouyang, S.; Zhu, L.; Hamilton, J.; Lin, H.; Campbell, M.; Childs, K.; Thibaud-Nissen, F.; Malek, R.L.; Lee, Y.; Zheng, L.; et al. The TIGR rice genome annotation resource: Improvements and new features. Nucleic Acids Res. 2007, 35, 883–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawahara, Y.; de la Bastide, M.; Hamilton, J.P.; Kanamori, H.; McCombie, W.R.; Ouyang, S.; Schwartz, D.C.; Tanaka, T.; Zhou, S.; Childs, K.L.; et al. Improvement of the Oryza sativa Nipponbare reference genome using next generation sequence and optical map data. Rice 2013, 6, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, P.; Jung, K.; Choi, D.; Hwang, D.; Zhu, J.; Ronald, P. The rice oligonucleotide array database: An atlas of rice gene expression. Rice 2012, 5, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, L.; Zou, D.; Sang, J.; Xu, X.J.; Yin, H.Y.; Li, M.W.; Wu, S.Y.; Hu, S.N.; Hao, L.L.; Zhang, Z. Rice Expression Database (RED): An integrated RNA-Seq-derived gene expression database for rice. J. Genet. Genom. 2017, 44, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Lamesch, P.; Berardini, T.Z.; Li, D.; Swarbreck, D.; Wilks, C.; Sasidharan, R.; Muller, R.; Dreher, K.; Alexander, D.L.; Garcia-Hernandez, M.; et al. The arabidopsis information resource (TAIR): Improved gene annotation and new tools. Nucleic Acids Res. 2012, 40, D1202–D1210. [Google Scholar] [CrossRef] [PubMed]
- De Diego, N.; Fürst, T.; Humplik, J.; Ugena, L.; Podlešáková, K.; Spíchal, L. An automated method for high-throughput screening of Arabidopsis rosette growth in multi-well plates and its validation stress condition. Front. Plan Sci. 2017, 8, 1702. [Google Scholar] [CrossRef] [Green Version]
- Yonemaru, J.; Yamamoto, T.; Fukuoka, S.; Uga, Y.; Hori, K.; Yano, M. Q-TARO:QTL Annotation rice online database. Rice 2010, 3, 194. [Google Scholar] [CrossRef] [Green Version]
- Tu, C.J.; Schuenemann, D.; Hoffman, N.E. Chloroplast FtsY, chloroplast signal recognition particle, and GTP are required to reconstitute the soluble phase of light-harvesting chlorophyll protein transport into thylakoid membranes. J. Biochem. Chem. 1999, 274, 27219–27224. [Google Scholar] [CrossRef] [Green Version]
- Rumeau, D.; Bécuwe-Linka, N.; Beyly, A.; Louwagie, M.; Garin, J.; Peltier, G. New subunit NDH-M, -N, -O, encoded by nuclear genes, are essential for plastid Ndh complex functioning in higher plants. Plant Cell 2005, 17, 219–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooks, M.D.; Sylak-Glassman, E.J.; Fleming, G.R.; Niyogi, K.K. A thioredoxin-like/β-propeller protein maintains the efficiency of light harvesting in Arabidopsis. Proc. Natl. Acad. Sci. USA 2013, 110, E2733–E2740. [Google Scholar] [CrossRef] [Green Version]
- Adamiec, M.; Gibasiewicz, K.; Luciński, R.; Giera, W.; Chełminiak, P.; Szewczyk, S.; Sipińska, W.; Grondelle, R.; Jackowski, G. Excitation energy transfer and charge separation are affected in Arabidopsis thaliana mutants lacking light-harvesting chlorophyll a/b binding protein Lhcb3. J Photochem Photobiol. B Biol. 2015, 153, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Wang, W.; Yu, X.; Lin, W.; Maio, Y. Comparative proteomic analysis of coregulation of CIPK14 and WHIRLY1/3 mediated pale yellowing of leaves in Arabidopsis. Int. J. Mol. Sci. 2018, 19, 2331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hertle, A.P.; Blunder, T.; Wunder, T.; Pesaresi, P.; Pribil, M.; Armbruster, U.; Leister, D. PGRL1 is the elusive ferredoxin-plastoquinone reductase in photosynthetic cyclic electron flow. Mol. Cell 2013, 49, 511–523. [Google Scholar] [CrossRef] [Green Version]
- Hey, D.; Grimm, B. ONE-HELIX PROTEIN2 (OHP2) is required for the stability of OHP1 and assembly factor HCF244 and is functionally linked to PSII biogenesis. Plant Physiol. 2018, 177, 1453–1472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Liu, B.; Zhang, J.; Kong, F.; Zhang, L.; Meng, H.; Li, W.; Rochaix, J.D.; Li, D.; Peng, L. OHP1, OHP2, and HCF244 form a transient functional complex with the photosystem II reaction center. Plant Physiol. 2019, 179, 195–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chotewutmontri, P.; Williams-Carrier, R.; Barkan, A. Exploring the link between photosystem II assembly and translation of the chloroplast psbA mRNA. Plants 2020, 9, 152. [Google Scholar] [CrossRef] [Green Version]
- Motohashi, R.; Yamazaki, T.; Myouga, F.; Ito, T.; Ito, K.; Satou, M.; Kobayashi, M.; Nagata, N.; Yoshida, S.; Nagashima, A.; et al. Chloroplast ribosome release factor 1 (AtcpRF1) is essential for chloroplast development. Plant Mol. Biol. 2007, 646, 481–497. [Google Scholar] [CrossRef]
- Tezara, W.; Mitchell, V.J.; Driscoll, S.D.; Lawlor, D.W. Water stress inhibits plant photosynthesis by decreaseing coupling factor and ATP. Nature 1999, 401, 914–917. [Google Scholar] [CrossRef]
- Teulat, B.; Monneveux, P.; Wery, J.; Borries, C.; Souyris, I.; Charrier, A.; This, D. Relationships between relative water content and growth parameters under water stress in barley: A QTL study. New Phytol. 1997, 137, 99–107. [Google Scholar] [CrossRef]
- González, L.; González-Vilar, M. Determination of relative water content. In Handbook of Plant Ecophysiology Techniques; Springer: Berlin/Heidelberg, Germany, 2001; pp. 207–212. [Google Scholar]
- Nikkanen, L.; Toivola, J.; Trotta, A.; Diaz, M.G.; Tikkanen, M.; Aro, E.; Rintamäki, E. Regulation of cyclic electron flow by chloroplast NADPH-dependent thioredoxin system. Plant Direct 2018, 2, e00093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damkjaer, J.T.; Kereïche, S.; Johnson, M.; Kovacs, L.; Kiss, A.Z.; Boekema, E.J.; Ruban, A.V.; Horton, P.; Jansson, S. The photosystem II light-harvesting protein Lhcb3 affects the macrostructure of photosystem II and the rate of state transitions in Arabidopsis. Plant Cell 2009, 21, 3245–3256. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Ouyang, M.; Li, Q.; Zou, M.; Gou, J.; Ma, J.; Lu, C.; Zhang, L. The Arabidopsis chloroplast ribosome recycling factor is essential for embryogenesis and chloroplast biogenesis. Plant Mol. Biol. 2010, 74, 47–59. [Google Scholar] [CrossRef]
- DalCorso, G.; Pesaresi, P.; Masiero, S.; Aseeva, E.; Schünemann, D.; Finazzi, G.; Joliot, P.; Barbato, R.; Leister, D. A complex containing PGRL1 and PGRL5 is involved in the switch between linear and cyclic electron flow in Arabidopsis. Cell 2008, 132, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Suorsa, M.; Rossi, F.; Tadini, L.; Labs, M.; Colombo, M.; Jahns, P.; Kater, M.M.; Leister, D.; Finazzi, G.; Aro, E.; et al. PGRL5-PGRL1-dependent cyclic electron transport modulates linear electron transport rate in Arabidopsis thaliana. Mol. Plant 2016, 9, 271–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Yan, J.; Ahammed, G.J.; Wang, X.; Bu, X.; Xiang, H.; Li, Y.; Lu, J.; Liu, Y.; Qi, H.; et al. PGR5/PGRL1 and NDH mediate far-red light-induced photoprotection in response to chilling stress in tomato. Front. Plant Sci. 2020, 11, 669. [Google Scholar] [CrossRef] [PubMed]
- Johnson, X.; Wostrikoff, K.; Finazzi, G.; Kuras, R.; Schwarz, C.; Bujaldon, S.; Nickelsen, J.; Stern, D.B.; Wollman, F.; Vallon, O. MRL1, a conserved pentatricopeptide repeat protein, is required for stabilization of rbcL mRNA in Chlamydomas and Arabidopsis. Plant Cell 2010, 22, 234–248. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |





| Conditions | Normal (0% PEG) | Drought Stress (15% PEG) | ||||||
|---|---|---|---|---|---|---|---|---|
| Timing (Days after Stress) | 0 | 3 | 6 | 9 | 0 | 3 | 6 | 9 |
| Net photosynthesis rate (μmol·m−2·s−1) | ||||||||
| KDML105 | 19.44 ± 4.46 | 14.50 ± 0.81 | 17.85 ± 1.85 | 20.17 ± 0.88 | 19.44 ± 4.46 | 10.30 ± 1.74 | 5.51 ± 0.42 b | 8.64 ± 1.11 |
| DH103 | 14.47 ± 2.31 | 17.45 ± 1.21 | 18.92 ± 1.53 | 21.35 ± 2.02 | 14.47 ± 2.31 | 10.67 ± 0.38 | 10.20 ± 1.23 a | 12.35 ± 0.59 |
| CSSL104 | 15.12 ± 1.28 | 16.74 ± 1.10 | 18.47 ± 1.61 | 15.76 ± 0.87 | 15.12 ± 1.28 | 11.31 ± 1.88 | 10.31 ± 1.33 a | 9.38 ± 1.79 |
| Transpiration rate (mmol·m−2·s−1) | ||||||||
| KDML105 | 5.26 ± 0.56 | 4.86 ± 0.44 | 0.23 ± 0.01 | 5.10 ± 0.39 b | 5.26 ± 0.56 | 2.42 ± 0.14 | 0.18 ± 0.02 | 1.87 ± 0.26 b |
| DH103 | 4.99 ±0.48 | 5.87 ± 0.12 | 0.26 ± 0.02 | 6.77 ± 0.71 a | 4.99 ±0.48 | 2.65 ± 0.24 | 0.17 ± 0.02 | 3.33 ± 0.10 a |
| CSSL104 | 4.54 ± 0.28 | 4.99 ± 0.34 | 0.25 ± 0.01 | 4.48 ± 0.27 b | 4.54 ± 0.28 | 2.57 ± 0.32 | 0.17 ± 0.02 | 2.16 ± 0.34 b |
| Stomatal conductance (mmol·m−2·s−1) | ||||||||
| KDML105 | 0.35 ± 0.09 | 0.32 ± 0.05 | 0.32 ± 0.06 b | 0.33 ± 0.04 b | 0.35 ± 0.09 | 0.12 ± 0.01 | 0.08 ± 0.01 | 0.09 ± 0.01 b |
| DH103 | 0.43 ± 0.05 | 0.44 ± 0.02 | 0.44 ± 0.03 a | 0.53 ± 0.07 a | 0.43 ± 0.05 | 0.12 ± 0.01 | 0.19 ± 0.04 | 0.17 ± 0.01 a |
| CSSL104 | 0.36 ± 0.03 | 0.33 ± 0.06 | 0.33 ± 0.06 b | 0.28 ± 0.02 b | 0.36 ± 0.03 | 0.15 ± 0.02 | 0.11 ± 0.02 | 0.10 ± 0.02 b |
| ΦPSII | ||||||||
| KDML105 | 0.25 ± 0.00 | 0.23 ± 0.01 | 0.23 ± 0.01 | 0.23 ± 0.01 b | 0.25 ± 0.00 | 0.20 ± 0.01 | 0.18 ± 0.02 | 0.15 ± 0.01 |
| DH103 | 0.22 ± 0.02 | 0.25 ± 0.02 | 0.26 ± 0.02 | 0.29 ± 0.01 a | 0.22 ± 0.02 | 0.21 ± 0.03 | 0.17 ± 0.02 | 0.18 ± 0.02 |
| CSSL104 | 0.24 ± 0.01 | 0.21 ± 0.01 | 0.25 ± 0.01 | 0.22 ± 0.00 b | 0.24 ± 0.01 | 0.21 ± 0.01 | 0.17 ± 0.02 | 0.17 ± 0.01 |
| Electron transport rate | ||||||||
| KDML105 | 163.02 ± 2.37 | 149.08 ± 9.83 | 153.51 ± 4.96 | 152.38 ± 3.91 b | 163.02 ± 2.37 | 130.73 ± 4.36 | 117.25 ± 10.71 | 100.65 ± 8.13 |
| DH103 | 143.72 ± 10.16 | 163.29 ± 11.08 | 168.02 ± 11.52 | 188.68 ± 4.99 a | 143.72 ± 10.16 | 139.94 ± 16.65 | 110.58 ± 11.43 | 118.43 ± 10.95 |
| CSSL104 | 155.86 ± 6.09 | 141.86 ± 7.08 | 161.10 ± 6.45 | 144.28 ± 2.79 b | 155.86 ± 6.09 | 133.31 ± 11.19 | 113.12 ± 12.52 | 112.18 ± 8.52 |
| Intercellular CO2 concentration (μmol·mol−1) | ||||||||
| KDML105 | 327.27 ± 5.37 | 305.04 ± 11.48 | 282.47 ± 14.96 b | 282.98 ± 10.67 | 327.27 ± 5.37 | 255.39 ± 18.30 | 269.74 ± 18.42 | 215.42 ± 21.55 a |
| DH103 | 330.97 ± 5.82 | 318.58 ± 3.45 | 312.79 ± 8.84 a | 313.36 ± 8.12 | 330.97 ± 5.82 | 260.64 ± 19.39 | 286.68 ± 18.62 | 265.87 ± 8.18 b |
| CSSL104 | 317.29 ± 2.12 | 294.48 ± 11.18 | 281.92 ± 13.68 b | 289.50 ± 9.46 | 317.29 ± 2.12 | 241.03 ± 14.35 | 227.89 ± 18.71 | 237.02 ± 13.81 ab |
| Fv’/Fm’ | ||||||||
| KDML105 | 0.54 ± 0.01 b | 0.53 ± 0.01 | 0.50 ± 0.01 | 0.50 ± 0.02 | 0.54 ± 0.01 b | 0.50 ± 0.01 | 0.48 ± 0.01 | 0.45 ± 0.02 |
| DH103 | 0.63 ± 0.02 a | 0.60 ± 0.03 | 0.55 ± 0.02 | 0.52 ± 0.01 | 0.63 ± 0.02 a | 0.53 ± 0.04 | 0.53 ± 0.08 | 0.47 ± 0.03 |
| CSSL104 | 0.53 ± 0.01 b | 0.54 ± 0.01 | 0.53 ± 0.03 | 0.52 ± 0.01 | 0.53 ± 0.01 b | 0.52 ± 0.03 | 0.48 ± 0.01 | 0.43 ± 0.02 |
| Relative chlorophyll content | ||||||||
| KDML105 | 35.93 ± 0.41 | 36.63 ± 0.95 | 36.40 ± 0.81 | 38.30 ± 1.05 | 35.93 ± 0.41 | 37.58 ± 0.40 | 37.10 ± 0.57 | 34.20 ± 1.02 |
| DH103 | 34.43 ± 0.43 | 35.78 ± 0.21 | 36.55 ± 0.34 | 38.15 ± 0.95 | 34.43 ± 0.43 | 33.25 ± 1.12 | 32.58 ± 2.18 | 34.38 ± 0.97 |
| CSSL104 | 34.90 ± 0.65 | 35.70 ± 0.39 | 38.65 ± 1.70 | 39.00 ± 0.38 | 34.90 ± 0.65 | 35.93 ± 1.06 | 36.25 ± 0.71 | 34.48 ± 1.21 |
| Leaf water potential (MPa) | ||||||||
| KDML105 | −1.20 ± 0.14 | −2.70 ± 0.77 | −5.15 ± 1.08 | −2.90 ± 0.26 | −1.60 ± 0.08 | −4.05 ± 0.48 | −9.00 ± 1.00 | −6.85 ± 0.43 |
| DH103 | −1.35 ± 0.10 | −2.60 ± 0.54 | −3.90 ± 0.33 | −3.25 ± 0.10 | −2.05 ± 0.32 | −4.60 ± 0.50 | −7.00 ± 0.38 | −6.95 ± 1.03 |
| CSSL104 | −1.20 ± 0.14 | −1.95 ± 0.15 | −4.20 ± 1.29 | −2.70 ± 0.13 | −1.65 ± 0.05 | −4.08 ± 0.31 | −7.10 ± 1.04 | −7.75 ± 1.50 |
| Rice Locus ID | Arabidopsis Locus ID | Gene Description | Mutant Stock | Homozygous | Involved in Photosynthesis |
|---|---|---|---|---|---|
| LOC_Os01g72800 | AT2G45770 | Chloroplast signal recognition particle (SRP) receptor homolog, alpha subunit CPFTSY. Required for light-harvesting chlorophyll a/b-binding protein (LHCP) integration into isolated thylakoids. | SALK_070410C | ✓ | ✓ |
| LOC_Os01g72950 | AT1G74880 | NDH-O, encoding subunit NDH-O of NAD(P)H: plastoquinone dehydrogenase complex (Ndh complex) present in the thylakoid membrane of chloroplasts. This subunit is thought to be required for Ndh complex assembly. | SALK_097351C | ✓ | ✓ |
| LOC_Os03g02590 | AT1G01820 | PEROXIN11C, member of the peroxin11 (PEX11) gene family, integral to peroxisome membrane, controls peroxisome proliferation | SALK_057358C | ✓ | |
| LOC_Os03g03910 | AT4G35090 | CAT2 | SALK_076998 | ✓ | |
| LOC_Os03g19760 | AT1G56500 | SOQ1 (Suppressor of quenching 1) prevents the formation of a slowly reversible form of antenna quenching, thereby maintaining the efficiency of light harvesting. | SALK_097577 | ✓ | |
| LOC_Os03g36750 | AT3G48420 | Haloacid dehalogenase-like hydrolase (HAD) superfamily protein | SALK_025204 | ✓ | |
| LOC_Os03g52460 | AT5G19220 | APL1, the large subunit of ADP-glucose pyrophosphorylase, which catalyzes the first and rate-limiting step in starch biosynthesis. | CS478981 | ✓ | |
| LOC_Os05g33520 | AT2G48070 | RPH1 is a chloroplast protein RPH1 (resistance to Phytophthora 1) involved in immune response to Phytophthora brassicae | SALK_102558C | ✓ | |
| LOC_Os07g37550 | AT5G54270 | LHCB3 is a component of the main light-harvesting chlorophyll a/b-protein complex of Photosystem II (LHC II) | SALK_020314C | ✓ | ✓ |
| LOC_Os07g38300 | AT3G63190 | RRF, encoding a chloroplast ribosome recycling factor homolog. | SALK_015954C | ✓ | ✓ |
| LOC_Os08g16570 | AT1G16080 | Nuclear protein | SALK_007790C | ✓ | |
| LOC_Os08g41040 | AT4G31115 | DUF1997 family protein | SALK_010690C | ✓ | |
| LOC_Os08g41460 | AT4G11960 | PGRL1B—a transmembrane protein present in thylakoids. Plants lacking PGRL1 show perturbation of cyclic electron flow. | SALK_059238C | ✓ | ✓ |
| AT4G22890 | SALK_133856C | ✓ | ✓ | ||
| LOC_Os09g10750 | AT2G42220 | Rhodanese/cell cycle control phosphatase superfamily protein | SALK_045769 | ✓ | |
| LOC_Os09g39390 | AT2G27680 | NAD(P)-linked oxidoreductase superfamily protein | SALK_073120C | ✓ | ✓ |
| LOC_Os10g10170 | AT4G34830 | MRL1 (a conserved pentatricopeptide repeat protein) required for stabilization of rbcL mRNA | SALK_060806C | ✓ | ✓ |
| LOC_Os11g43600 | AT3G62910 | Chloroplast ribosome release factor 1, CPRF1, encoding a plastid-localized ribosome release factor 1 that is essential in chloroplast development | SALK_117765C |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Punchkhon, C.; Plaimas, K.; Buaboocha, T.; Siangliw, J.L.; Toojinda, T.; Comai, L.; De Diego, N.; Spíchal, L.; Chadchawan, S. Drought-Tolerance Gene Identification Using Genome Comparison and Co-Expression Network Analysis of Chromosome Substitution Lines in Rice. Genes 2020, 11, 1197. https://doi.org/10.3390/genes11101197
Punchkhon C, Plaimas K, Buaboocha T, Siangliw JL, Toojinda T, Comai L, De Diego N, Spíchal L, Chadchawan S. Drought-Tolerance Gene Identification Using Genome Comparison and Co-Expression Network Analysis of Chromosome Substitution Lines in Rice. Genes. 2020; 11(10):1197. https://doi.org/10.3390/genes11101197
Chicago/Turabian StylePunchkhon, Chutarat, Kitiporn Plaimas, Teerapong Buaboocha, Jonaliza L. Siangliw, Theerayut Toojinda, Luca Comai, Nuria De Diego, Lukáš Spíchal, and Supachitra Chadchawan. 2020. "Drought-Tolerance Gene Identification Using Genome Comparison and Co-Expression Network Analysis of Chromosome Substitution Lines in Rice" Genes 11, no. 10: 1197. https://doi.org/10.3390/genes11101197