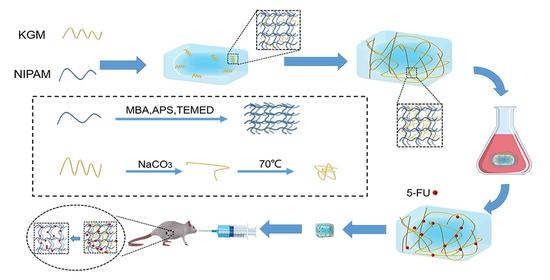
Preparation and Application of Double Network Interpenetrating Colon Targeting Hydrogel Based on Konjac Glucomannan and N-Isopropylacrylamide
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of IPN (KGM/NIPAM) Hydrogel
| Sample | m(KGM):m(NIPAM) | m(MBA):m(NIPAM) | m(APS):m(NIPAM) | v(TEMED):m(NIPAM) |
|---|---|---|---|---|
| A | 0.5:1 | 3:100 | 1:100 | 1:1000 |
| B | 1:1 | 3:100 | 1:100 | 1:1000 |
| C | 2:1 | 3:100 | 1:100 | 1:1000 |
2.2. Scanning Electron Microscope (SEM)
2.3. Structure Characterization
2.4. Thermosensitivity and Uptake of IPN Hydrogen
2.5. Drug Loading of IPN Hydrogel
2.6. In Vitro Release of IPN Hydrogel
2.7. In Vivo Administration of IPN Gel
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of KGM, NIPAM and IPN (KGM/NIPAM) Hydrogel
4.3. Preparation of IPN (KGM/NIPAM) Drug-Loaded Hydrogel
4.4. Structure Characterization
4.5. Thermosensitivity and Uptake of IPN Hydrogel
4.6. Study on Temperature Sensitivity of IPN Hydrogel
4.7. Drug Loading
4.8. Study of Drug Release from IPN Hydrogel In Vitro
4.9. In Vivo Administration of IPN Gel
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dai, S.H.; Jiang, F.T.; Shah, N.P.; Corke, H. Functional and pizza bake properties of Mozzarella cheese made with konjac glucomannan as a fat replacer. Food Hydrocoll. 2019, 92, 125–134. [Google Scholar] [CrossRef]
- Shahbuddin, M.; Bullock, A.J.; MacNeil, S.; Rimmer, S. Glucomannan-poly (N-vinyl pyrrolidinone) bicomponent hydrogels for wound healing. J. Mater. Chem. B 2014, 2, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, J.D.; Yang, F.Q. Konjac glucomannan, a promising polysaccharide for OCDDS. Carbohydr. Polym. 2014, 104, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Zheng, S.; Xie, W.; Cao, G.; Wang, L.; Pang, J. Konjac glucomannan: A review of structure, physicochemical properties, and wound dressing applications. J. Appl. Polym. Sci. 2022, 139, 51780. [Google Scholar] [CrossRef]
- Hayeeawaema, F.; Wichienchot, S.; Khuituan, P. Amelioration of gut dysbiosis and gastrointestinal motility by konjac oligo-glucomannan on loperamide-induced constipation in mice. Nutrition 2020, 73, 110715. [Google Scholar] [CrossRef]
- Liu, C.; Chen, Y.; Chen, J. Synthesis and characteristics of pH-sensitive semi-interpenetrating polymer network hydrogels based on konjac glucomannan and poly (aspartic acid) for in vitro drug delivery. Carbohydr. Polym. 2010, 79, 500–506. [Google Scholar] [CrossRef]
- Fan, J.; Wang, K.; Liu, M.; He, Z. In vitro evaluations of konjac glucomannan and xanthan gum mixture as the sustained release material of matrix tablet. Carbohydr. Polym. 2008, 73, 241–247. [Google Scholar] [CrossRef]
- Chen, L.-G.; Liu, Z.-L.; Zhuo, R.-X. Synthesis and properties of degradable hydrogels of konjac glucomannan grafted acrylic acid for colon-specific drug delivery. Polymer 2005, 46, 6274–6281. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, R.; Perkins, W.S.; Cheng, Y. Morphology evolution and gelation mechanism of alkali induced konjac glucomannan hydrogel. Food Chem. 2018, 269, 80–88. [Google Scholar] [CrossRef]
- Zhu, F. Modifications of konjac glucomannan for diverse applications. Food Chem. 2018, 256, 419–426. [Google Scholar] [CrossRef]
- Wen, X.; Cao, X.L.; Yin, Z.H.; Wang, T.; Zhao, C.S. Preparation and characterization of konjac glucomannan-poly(acrylic acid) IPN hydrogels for controlled release. Carbohydr. Polym. 2009, 78, 193–198. [Google Scholar] [CrossRef]
- Alves, A.; Miguel, S.P.; Araujo, A.R.T.S.; de Jesus Valle, M.J.; Navarro, A.S.; Correia, I.J.; Ribeiro, M.P.; Coutinho, P. Xanthan Gum-Konjac Glucomannan Blend Hydrogel for Wound Healing. Polymers 2020, 12, 99. [Google Scholar] [CrossRef] [Green Version]
- Cumpstey, I. Chemical modification of polysaccharides. Int. Sch. Res. Not. 2013, 2013, 417672. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Y.; Lin, L.; Mu, R.; Pang, J. Novel synthesis of mussel inspired and Fe3+ induced pH-sensitive hydrogels: Adhesion, injectable, shapeable, temperature properties, release behavior and rheological characterization. Carbohydr. Polym. 2020, 236, 116045. [Google Scholar] [CrossRef]
- Liu, M.; Fan, J.; Wang, K.; He, Z. Synthesis, characterization, and evaluation of phosphated cross-linked konjac glucomannan hydrogels for colon-targeted drug delivery. Drug Deliv. 2007, 14, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; Hu, H.; Zhuo, R.X. Konjac glucomannan-graft-acrylic acid hydrogels containing azo crosslinker for colon-specific delivery. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 4370–4378. [Google Scholar] [CrossRef]
- Zou, G.; Shen, J.; Duan, P.; Xia, X.; Chen, R.; Jin, B. Temperature-sensitive poly (N-isopropylacrylamide)/konjac glucomannan/graphene oxide composite membranes with improved mechanical property, swelling capability, and degradability. Int. J. Polym. Sci. 2018, 2018, 7906747. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.-F.; Sun, T.; Li, S.; Huang, Q.; Yue, L.; Zhu, L.; Wang, R. Oral colon-targeted konjac glucomannan hydrogel constructed through noncovalent cross-linking by cucurbit [8] uril for ulcerative colitis therapy. ACS Appl. Bio Mater. 2019, 3, 10–19. [Google Scholar] [CrossRef]
- Silverstein, M.S. Interpenetrating polymer networks: So happy together? Polymer 2020, 207, 122929. [Google Scholar] [CrossRef]
- Xu, Q.; Huang, W.J.; Jiang, L.B.; Lei, Z.J.; Li, X.Y.; Deng, H.B. KGM and PMAA based pH-sensitive interpenetrating polymer network hydrogel for controlled drug release. Carbohydr. Polym. 2013, 97, 565–570. [Google Scholar] [CrossRef]
- Yao, X.; Luo, X.G.; Han, B.C. Synthesis and Characteristics of Interpenetrating Polymer Network Hydrogels Based on Konjac Glucomannan with Different Molecular Weights and Poly (acrylic acid). In Advanced Materials Research; Trans Tech Publications Ltd.: Zurich, Switzerland, 2012; Volume 393, pp. 1004–1007. [Google Scholar] [CrossRef]
- Tang, L.; Wang, L.; Yang, X.; Feng, Y.; Li, Y.; Feng, W. Poly (N-isopropylacrylamide)-based smart hydrogels: Design, properties and applications. Prog. Mater. Sci. 2021, 115, 100702. [Google Scholar] [CrossRef]
- Halperin, A.; Kröger, M.; Winnik, F.M. Poly (N-isopropylacrylamide) phase diagrams: Fifty years of research. Angew. Chem. Int. Ed. 2015, 54, 15342–15367. [Google Scholar] [CrossRef] [PubMed]
- Sharker, K.K.; Takeshima, S.; Toyama, Y.; Ida, S.; Kanaoka, S.; Yusa, S.-I. pH-and thermo-responsive behavior of PNIPAM star containing terminal carboxy groups in aqueous solutions. Polymer 2020, 203, 122735. [Google Scholar] [CrossRef]
- Nagase, K.; Yamato, M.; Kanazawa, H.; Okano, T. Poly (N-isopropylacrylamide)-based thermoresponsive surfaces provide new types of biomedical applications. Biomaterials 2018, 153, 27–48. [Google Scholar] [CrossRef]
- Rice, C.V. Phase-transition thermodynamics of N-isopropylacrylamide hydrogels. Biomacromolecules 2006, 7, 2923–2925. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Q.; Tu, K.H.; Li, Y.P.; Fu, J.; Yu, F.S. Micellization behavior of temperature-responsive poly (N-isopropylacrylamide) grafted dextran copolymers. J. Mater. Sci. Lett. 2002, 21, 1453–1455. [Google Scholar] [CrossRef]
- Zhang, J.T.; Huang, S.W.; Gao, F.Z.; Zhuo, R.X. Novel temperature-sensitive, beta-cyclodextrin-incorporated poly(N-isopropylacrylamide) hydrogels for slow release of drug. Colloid Polym. Sci. 2005, 283, 461–464. [Google Scholar] [CrossRef]
- Rigas, A.; Dervenis, C.; Giannakou, N.; Kozoni, V.; Shiff, S.J.; Rigas, B. Selective induction of colon cancer cell apoptosis by 5-fluorouracil in humans. Cancer Investig. 2002, 20, 657–665. [Google Scholar] [CrossRef]
- Samy, M.; Abd El-Alim, S.H.; Rabia, A.; Amin, A.; Ayoub, M.M.H. Formulation, characterization and in vitro release study of 5-fluorouracil loaded chitosan nanoparticles. Int. J. Biol. Macromol. 2020, 156, 783–791. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, Y.; Pan, W.; Tong, X.; Zeng, Q.; Su, T.; Qi, X.; Shen, J. Polydopamine-incorporated dextran hydrogel drug carrier with tailorable structure for wound healing. Carbohydr. Polym. 2021, 253, 117213. [Google Scholar] [CrossRef]
- Qi, X.; Su, T.; Zhang, M.; Tong, X.; Pan, W.; Zeng, Q.; Zhou, Z.; Shen, L.; He, X.; Shen, J. Macroporous hydrogel scaffolds with tunable physicochemical properties for tissue engineering constructed using renewable polysaccharides. ACS Appl. Mater. Interfaces 2020, 12, 13256–13264. [Google Scholar] [CrossRef] [PubMed]
- Zdravkovic, A.S.; Nikolic, L.B.; Ilic-Stojanovic, S.S.; Nikolic, V.D.; Savic, S.R.; Kapor, A.J. The evaluation of temperature and pH influences on equilibrium swelling of poly(N-isopropylacrylamide-co-acrylic acid) hydrogels. Hem. Ind. 2017, 71, 395–405. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Shin, Y.; Park, S.; Jeong, J.P.; Kim, Y.; Jung, S. Multifunctional Oxidized Succinoglycan/Poly(N-isopropylacrylamide-co-acrylamide) Hydrogels for Drug Delivery. Polymers 2023, 15, 122. [Google Scholar] [CrossRef]
- Liang, L.; Li, Q.H.; Jin, K.; Luo, T.; Yang, Z.A.; Xu, X.D.; Cheng, H. In vitro and in vivo Evaluation of Novel Ocular Delivery System of 5-Fluorouracil Peptide Hydrogel. Asian J. Chem. 2014, 26, 2977–2981. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, R.; Yu, X.; Deng, R.; Zou, H.; He, Q.; Huang, W.; Li, C.; Zou, K. Preparation and Application of Double Network Interpenetrating Colon Targeting Hydrogel Based on Konjac Glucomannan and N-Isopropylacrylamide. Gels 2023, 9, 221. https://doi.org/10.3390/gels9030221
Yao R, Yu X, Deng R, Zou H, He Q, Huang W, Li C, Zou K. Preparation and Application of Double Network Interpenetrating Colon Targeting Hydrogel Based on Konjac Glucomannan and N-Isopropylacrylamide. Gels. 2023; 9(3):221. https://doi.org/10.3390/gels9030221
Chicago/Turabian StyleYao, Renhua, Xiaoqin Yu, Rui Deng, Huarong Zou, Qingwen He, Wenfeng Huang, Chunxiao Li, and Kun Zou. 2023. "Preparation and Application of Double Network Interpenetrating Colon Targeting Hydrogel Based on Konjac Glucomannan and N-Isopropylacrylamide" Gels 9, no. 3: 221. https://doi.org/10.3390/gels9030221