Ultrasonic-Assisted Glycosylation with Glucose on the Functional and Structural Properties of Fish Gelatin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Glycosylation Degree (DG) Analysis
2.2. Functional Properties Analysis
2.2.1. Emulsifying Properties
2.2.2. Foaming Properties
2.2.3. Gel Strength
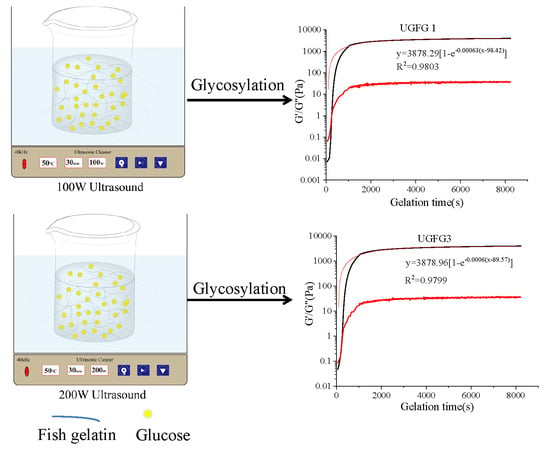
2.3. Rheological Properties
2.3.1. Gelatin Kinetics Analysis
2.3.2. Flow Behaviors
2.4. Structural Analysis
2.4.1. Fluorescence Analysis
2.4.2. FTIR Analysis
2.4.3. LF-NMR Analysis
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Modification of FG
4.3. Degree of Grafting (DG) Analysis
4.4. Functional Properties Analysis
4.4.1. Emulsifying Properties
4.4.2. Foaming Properties
4.4.3. Surface Hydrophobicity(H0) Analysis
4.4.4. Gel Strength Analysis
4.5. Rheological Properties Analysis
4.5.1. Gelation Kinetics
4.5.2. Flow Behavior
4.6. Structural Analysis
4.6.1. Fluorescence Analysis
4.6.2. Fourier Transform Infrared (FTIR) Analysis
4.6.3. Low-Field Nuclear Magnetic Resonance (LF-NMR)
4.7. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Yang, H.J.; Wang, H.F.; Huang, M.; Cao, G.T.; Tao, F.; Zhou, G.H.; Shen, Q.; Yang, H.S. Repurposing fish waste into gelatin as a potential alternative for mammalian sources: A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 942–963. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Tu, Z.C.; Shangguan, X.C.; Sha, X.M.; Wang, H.; Zhang, L.; Bansal, N. Fish gelatin modifications: A comprehensive review. Trends Food Sci. Technol. 2019, 86, 260–269. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Liu, G.X.; Tu, Z.C. Effect of urea on glycosylation of BSA based on spectral techniques. Spectrosc. Spectr. Anal. 2021, 41, 478–483. [Google Scholar] [CrossRef]
- Zhao, H.Z.; Kang, X.Z.; Zhou, X.L.; Tong, L.; Yu, W.W.; Zhang, J.J.; Yang, W.G.; Lou, Q.M.; Huang, T. Glycosylation fish gelatin with gum Arabic: Functional and structural properties. LWT 2021, 139, 7. [Google Scholar] [CrossRef]
- Huang, T.; Zhao, H.Z.; Fang, Y.Y.; Lu, J.P.; Yang, W.G.; Qiao, Z.H.; Lou, Q.M.; Xu, D.L.; Zhang, J.J. Comparison of gelling properties and flow behaviors of microbial transglutaminase (MTGase) and pectin modified fish gelatin. J. Texture Stud. 2019, 50, 400–409. [Google Scholar] [CrossRef]
- Kolodzlejska, I.; Kaczorowski, K.; Piotrowska, B.; Sadowska, M. Modification of the properties of gelatin from skins of Baltic cod (Gadus morhua) with transglutaminase. Food Chem. 2004, 86, 203–209. [Google Scholar] [CrossRef]
- Kaewruang, P.; Benjakul, S.; Prodpran, T. Characteristics and gelling property of phosphorylated gelatin from the skin of unicorn leatherjacket. Food Chem. 2014, 146, 591–596. [Google Scholar] [CrossRef]
- Bhat, R.; Karim, A.A. Ultraviolet irradiation improves gel strength of fish gelatin. Food Chem. 2009, 113, 1160–1164. [Google Scholar] [CrossRef]
- Tu, Z.C.; Huang, T.; Wang, H.; Sha, X.M.; Shi, Y.; Huang, X.Q.; Man, Z.Z.; Li, D.J. Physico-chemical properties of gelatin from bighead carp (Hypophthalmichthys nobilis) scales by ultrasound-assisted extraction. J. Food Sci. Technol. 2015, 52, 2166–2174. [Google Scholar] [CrossRef]
- Da Silva, R.S.G.; Pinto, L.A.A. Physical cross-linkers: Alternatives to improve the mechanical properties of fish gelatin. Food Eng. Rev. 2012, 4, 165–170. [Google Scholar] [CrossRef]
- Lin, D.R.; Zhang, Q.T.; Xiao, L.J.; Huang, Y.C.; Yang, Z.F.; Wu, Z.J.; Tu, Z.C.; Qin, W.; Chen, H.; Wu, D.T.; et al. Effects of ultrasound on functional properties, structure and glycation properties of proteins: A review. Crit. Rev. Food Sci. Nutr. 2021, 61, 2471–2481. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Q.; Tu, Z.C.; Wang, H.; Zhang, Q.T.; Hu, Y.M.; Zhang, L.; Niu, P.P.; Shi, Y.; Xiao, H. Glycation promoted by dynamic high pressure microfluidisation pretreatment revealed by high resolution mass spectrometry. Food Chem. 2013, 141, 3250–3259. [Google Scholar] [CrossRef]
- Liu, G.X.; Tu, Z.C.; Yang, W.H.; Wang, H.; Zhang, L.; Ma, D.; Huang, T.; Liu, J.; Li, X. Investigation into allergenicity reduction and glycation sites of glycated β-lactoglobulin with ultrasound pretreatment by high-resolution mass spectrometry. Food Chem. 2018, 252, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Liu, Q.; Dong, W.Y.; Cai, Z.X. Effect of high intensity ultrasound assisted glycosylation on the gel properties of ovalbumin: Texture, rheology, water state and microstructure. Food Chem. 2022, 372, 9. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.W.; Ge, W.; Li, X.D.; Du, L.L.; Zhao, G.X.; Wang, H.X.; Qu, X.W. Effects of ultrasonic pretreatment and glycosylation on functional properties of casein grafted with glucose. J. Food Process. Preserv. 2017, 41, 8. [Google Scholar] [CrossRef]
- Sha, X.M.; Hu, Z.Z.; Tu, Z.C.; Zhang, L.Z.; Duan, D.L.; Huang, T.; Wang, H.; Zhang, L.; Li, X.; Xiao, H. Influence of dynamic high pressure microfluidization on functional properties and structure of gelatin from bighead carp (Hypophthalmichthys nobilis) scale. J. Food Process. Preserv. 2018, 42, 11. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, R.; Ding, M.Z.; Li, L.; Tao, N.P.; Wang, X.C.; Zhong, J. Commercial cold-water fish skin gelatin and bovine bone gelatin: Structural, functional, and emulsion stability differences. LWT 2020, 125, 8. [Google Scholar] [CrossRef]
- Li, S.G.; Zhang, S.; Liu, Y.; Fu, X.; Xiang, X.L.; Gao, S.H. Effects of ultrasound-assisted glycosylation on the interface and foaming characteristics of ovotransferrin. Ultrason. Sonochem. 2022, 84, 10. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Q.; Wu, Y.Y.; Wang, G.Z.; Geng, F.; Song, H.B.; Luo, P.; Huang, Q. Effect of ball milling-assisted glycosylation modification on the structure and foaming property of egg white protein. J. Food Sci. 2022, 87, 3117–3128. [Google Scholar] [CrossRef]
- Lu, J.P.; Fang, Q.; Ma, N.; Yang, W.G.; Zhang, L.Y.; Huang, T. Gelation behaviour of fish skin gelatin in the presence of methanol-water and ethanol-water solvent system. Int. J. Food Sci. Technol. 2022, 57, 1598–1608. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Zhang, T.; Tao, L.; Nie, Y.; Wang, X.; Zhong, J. Effect of carbon numbers and structures of monosaccharides on the glycosylation and emulsion stabilization ability of gelatin. Food Chem. 2022, 389, 133128. [Google Scholar] [CrossRef] [PubMed]
- Cen, S.J.; Zhang, L.Y.; Liu, L.W.; Lou, Q.M.; Wang, C.C.; Huang, T. Phosphorylation modification on functional and structural properties of fish gelatin: The effects of phosphate contents. Food Chem. 2022, 380, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, Z.; Zhang, J.T.; Zhang, L.; Wang, S.; Wang, L.; Chen, J.; Zou, C.H.; Hu, J.D. Identification of edible gelatin origins by data fusion of NIRS, fluorescence spectroscopy, and LIBS. Food Anal. Meth. 2021, 14, 525–536. [Google Scholar] [CrossRef]
- Hansen, M.R.; Blennow, A.; Farhat, I.; Norgaard, L.; Pedersen, S.; Engelsen, S.B. Comparative NMR relaxometry of gels of amylomaltase-modified starch and gelatin. Food Hydrocolloid. 2009, 23, 2038–2048. [Google Scholar] [CrossRef]
- Kang, X.Z.; Guo, W.W.; Ding, K.Y.; Zhan, S.N.; Lou, Q.M.; Huang, T. Microwave processing technology influences the functional and structural properties of fish gelatin. J. Texture Stud. 2022. ahead of print. [Google Scholar] [CrossRef]
- Fang, Q.; Ma, N.; Ding, K.Y.; Zhan, S.N.; Lou, Q.M.; Huang, T. Interaction between Negatively Charged Fish Gelatin and Cyclodextrin in Aqueous Solution: Characteristics and Formation Mechanism. Gels 2021, 7, 260. [Google Scholar] [CrossRef]




| Samples | FAA (mg/mL) | DG (%) | EAI (m2/g) | ESI (%) | FC (%) | FS (%) | H0 | Gel Strength (g) |
|---|---|---|---|---|---|---|---|---|
| FG | 0.152 ± 0.004 f | - | 73.62 ± 1.30 a | 92.42 ± 8.92 de | 60.83 ± 4.25 a | 240.97 ± 21.42 bc | 303.23 ± 10.70 cd | 300.14 ± 32.96 de |
| FG-GLU | 0.147 ± 0.001 e | - | 79.07 ± 0.27 c | 51.41 ± 9.01 a | 65.00 ± 7.36 ab | 230.17 ± 18.99 b | 289.13 ± 9.74 c | 252.05 ± 7.30 a |
| UGFG1 | 0.097 ± 0.001 d | 33.14 ± 0.85 a | 76.84 ± 0.87 b | 83.21 ± 8.79 c | 65.83 ± 9.65 ab | 203.65 ± 24.92 ab | 263.17 ± 8.63 ab | 280.74 ± 6.66 d |
| UGFG2 | 0.082 ± 0.005 c | 45.37 ± 0.24 b | 86.78 ± 0.76 d | 72.76 ± 1.74 b | 79.83 ± 5.22 bc | 200.79 ± 12.49 ab | 258.50 ± 9.32 a | 270.34 ± 2.22 c |
| UGFG3 | 0.075 ± 0.01 b | 47.42 ± 1.06 c | 97.42 ± 0.86 f | 98.84 ± 2.50 e | 70.17 ± 5.89 b | 195.24 ± 14.97 a | 270.30 ± 5.88 b | 309.43 ± 0.41 e |
| UGFG4 | 0.06 ± 0.001 a | 57.39 ± 0.49 d | 94.77 ± 1.53 e | 91.17 ± 9.10 de | 81.67 ± 4.71 c | 199.88 ± 16.46 a | 252.03 ± 6.94 a | 258.71 ± 0.18 ab |
| Gelling Systems | G′∞ (Pa) | K (1/s) | tg (s) | R2 |
|---|---|---|---|---|
| FG | 3899.62 | 0.0006 | 59.72 | 0.977 |
| FG-GLU | 3414.39 | 0.0006 | 83.42 | 0.979 |
| UGFG1 | 3878.29 | 0.0006 | 98.42 | 0.980 |
| UGFG3 | 3878.96 | 0.0006 | 89.57 | 0.980 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, W.; Ding, K.; Su, K.; Sun, W.; Zhan, S.; Lou, Q.; Huang, T. Ultrasonic-Assisted Glycosylation with Glucose on the Functional and Structural Properties of Fish Gelatin. Gels 2023, 9, 119. https://doi.org/10.3390/gels9020119
Guo W, Ding K, Su K, Sun W, Zhan S, Lou Q, Huang T. Ultrasonic-Assisted Glycosylation with Glucose on the Functional and Structural Properties of Fish Gelatin. Gels. 2023; 9(2):119. https://doi.org/10.3390/gels9020119
Chicago/Turabian StyleGuo, Wenwen, Keying Ding, Kaiyuan Su, Wanyi Sun, Shengnan Zhan, Qiaoming Lou, and Tao Huang. 2023. "Ultrasonic-Assisted Glycosylation with Glucose on the Functional and Structural Properties of Fish Gelatin" Gels 9, no. 2: 119. https://doi.org/10.3390/gels9020119