A Modified Sol–Gel Synthesis of Anatase {001}-TiO2/Au Hybrid Nanocomposites for Enhanced Photodegradation of Organic Contaminants
Abstract
:1. Introduction
2. Results and Discussion
2.1. X-ray Diffraction (XRD)
2.2. X-ray Photoemission Spectroscopy (XPS)
2.3. Transmission Electron Microscopy-Energy Dispersive X-ray (TEM-EDS)
2.4. Ultraviolet–Visible Diffuse Reflectance Spectroscopy (UV–Vis DRS)
2.5. Photocatalytic Activity
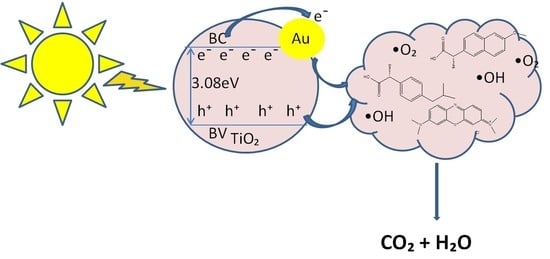
2.6. Proposed Mechanism
3. Conclusions
4. Materials and Methods
4.1. Materials and Reagents
4.2. Synthesis of Au/TiO2 Photocatalyst
4.3. X-ray Diffraction (XRD) Analysis
4.4. X-ray Photolectron Spectroscopy (XPS)
4.5. Transmission Electron Microscopy (TEM)
4.6. UV–Vis Diffuse Reflectance Spectroscopy (DRS) Analysis
4.7. Photocatalytic Experiment
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Göbel, A.; Thomsen, A.; McArdell, C.S.; Joss, A.; Giger, W. Occurrence and sorption behavior of sulfonamides, macrolides, and trimethoprim in activated sludge treatment. Environ. Sci. Technol. 2005, 39, 3981–3989. [Google Scholar] [CrossRef]
- Radjenovic, J.; Sedlak, D.L. Challenges and Opportunities for Electrochemical Processes as Next-Generation Technologies for the Treatment of Contaminated Water. Environ. Sci. Technol. 2015, 49, 11292–11302. [Google Scholar] [CrossRef]
- Shah, A.; Shah, M. Characterisation and bioremediation of wastewater: A review exploring bioremediation as a sustainable technique for pharmaceutical wastewater. Groundw. Sustain. Dev. 2020, 11, 100383. [Google Scholar] [CrossRef]
- Lee, Y.; von Gunten, U. Advances in predicting organic contaminant abatement during ozonation of municipal wastewater effluent: Reaction kinetics, transformation products, and changes of biological effects. Environ. Sci. Water Res. Technol. 2016, 2, 421–442. [Google Scholar] [CrossRef]
- Ikehata, K.; Gamal El-Din, M.; Snyder, S.A. Ozonation and Advanced Oxidation Treatment of Emerging Organic Pollutants in Water and Wastewater. Ozone Sci. Eng. 2008, 30, 21–26. [Google Scholar] [CrossRef]
- Jin, X.; Ma, J. Synthesis and comparison of photocatalytic activity under UV-visible or visible light irradiation for Bi3TaO7 and Bi2YTaO7 photocatalysts. J. Mater. Sci. Mater. Electron. 2018, 29, 18751–18759. [Google Scholar] [CrossRef]
- Trellu, C.; Oturan, N.; Pechaud, Y.; Hullebush, E.D.V.; Esposito, G.; Outran, M.A. Anodic oxidation of surfactants and organic compounds entrapped in micelles-selective degradation mechanisms and soil washing solution reuse. Water Res. 2017, 118, 1–11. [Google Scholar] [CrossRef]
- Wang, T.; Costan, J.; Centeno, A.; Pang, J.S.; Darvill, D.; Ryan, M.P.; Xie, F. Broad and enhanced fluorescence using zinc-oxide nanoflower arrays. J. Mater. Chem. 2015, 3, 2656–2663. [Google Scholar]
- Kiran, S.V.; Rajesh, J.T.; Kinjal, J.S.; Pradyuman, A.J.; Atindra, D.S.; Vimal, G.G. Photocatalytic degradation of pharmaceutical and pesticide compounds (PPCs) using doped TiO2 nanomaterials: A review. Water-Energy Nexus 2020, 3, 46–61. [Google Scholar]
- Maolin, Z.; Tiedan, C.B.; Yunjian, W. Insights into TiO2 polymorphs: Highly selective synthesis, phase transition, and their polymorph-dependent properties. R. Soc. Chem. 2017, 7, 52755–52761. [Google Scholar]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why is anatase a better photocatalyst than rutile?—Model studies on epitaxial TiO2 films. Sci. Rep. 2014, 4, 4043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piler, K.; Bahrim, C.; Twagirayezu, S.; Benson, J.T. Chapter Two—Lattice disorders of TiO2 and their significance in the photocatalytic conversion of CO2. Adv. Catal. 2020, 66, 109–233. [Google Scholar]
- Panayotov, A.D.; Frenkel, A.; Morris, J.R. Catalysis and Photocatalysis by Nanoscale Au/TiO2: Perspectives for Renewable Energy. ACS Energy Lett. 2017, 2, 1223–1231. [Google Scholar] [CrossRef]
- Conte, F.; Rossetti, I.; Ramis, G.; Vaulot, C.; Hajjar-Garreau, S.; Bennici, S. Low Metal Loading (Au, Ag, Pt, Pd) Photo-Catalysts Supported on TiO2 for Renewable Processes. Materials 2022, 15, 2915. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhou, D.; Han, S.; Li, Y.; Liu, J.; Zhou, Y.; Shen, W. Metal–Support Interaction in Pt/TiO2: Formation of Surface Pt–Ti Alloy. J. Phys. Chem. C 2021, 125, 10386–10396. [Google Scholar] [CrossRef]
- Bellardita, M.; Yurdakal, S.; Palmisano, L. 4—Synthesis and Characterization of Titanium Dioxide and Titanium Dioxide–Based Materials, Metal Oxides, Titanium Dioxide (TiO2) and Its Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 87–165. ISBN 9780128199602. [Google Scholar] [CrossRef]
- Zikalala, S.A.; Kuvarega, A.T.; Mamba, B.B.; Mhlanga, S.D.; Nxumalo, E.N. The effect of synthetic routes on the physicochemical properties and optical response of N-doped titania–oxidized carbon nanotube nanohybrids. Mater. Today Chem. 2018, 10, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Chebil, W.; Fouzri, A.; Fargi, A.; Azeza, B.; Zaaboub, Z.; Sallet, V. Characterization of ZnO thin films grown on different p-Si substrate elaborated by solgel spin-coating method. Mater. Res. Bull. 2015, 70, 719–727. [Google Scholar] [CrossRef]
- Yasir, M.; Azizan, M.T.; Ramli, A.; Ameen, M. Solvothermal Synthesis of Anatase TiO2 Nanosheets with Exposed {001} Facets. SainsMalaysiana 2017, 46, 2515–2521. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, S.Q.; Fu, X.Z.; Xu, Y.J. Synthesis of M@TiO2 (M = Au, Pd, Pt) core–shell with tunable photoreactivity. J. Phys. Chem. C 2011, 115, 9136–9145. [Google Scholar] [CrossRef]
- Gu, L.; Wang, J.; Cheng, H.; Du, Y.; Han, X. Synthesis of nano-sized anatase TiO2 with reactive {001} facets using lamellar protonated titanate as precursor. Chem. Commun. 2012, 48, 6978–6980. [Google Scholar] [CrossRef]
- Pang, Y.; Xu, G.; Feng, Q.; Lv, J.; Qin, Y.; Zhang, Y.; Zheng, Z.; Wu, Y. Crystalline orientation preference for TiO2 nanotube arrays with efficient photoelectrochemical properties. Phys. Lett. A 2018, 382, 2759–2762. [Google Scholar] [CrossRef]
- Machín, A.; Cotto, M.; Ducongé, J.; Arango, J.C.; Morant, C.; Márquez, F. Synthesis and Characterization of Au@TiO2 NWs and their Catalytic Activity by Water Splitting: A Comparative Study with Degussa P25. Am. J. Eng. Appl. Sci. 2017, 10, 298–311. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Wen, W.; Qian, X.Y.; Liu, J.B.; Wu, J.M. UV and visible light photocatalytic activity of Au/TiO2 nanoforests with Anatase/Rutile phase junctions and controlled Au locations. Sci. Rep. 2017, 7, 41253. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, B.; Amoli, V.; Maurya, A. Green Synthesis of Pt-Doped TiO2 Nanocrystals with Exposed (001) Facets and Mesoscopic Void Space for Photo-Splitting of Water under Solar Irradiation. Nanoscale 2015, 7, 10504–10512. [Google Scholar] [CrossRef] [PubMed]
- Ida, S.; Justin, S.J.; Wilson, P.; Neppolian, B. Facile synthesis of black N-TiO2/N-RGO nanocomposite for hydrogen generation and electrochemical applications: New insights into the structure-performance relationship. Appl. Surf. Sci. Adv. 2022, 9, 100249. [Google Scholar] [CrossRef]
- Nyholm, R.; Berndtsson, A.; Martensson, N. Core level binding energies for the elements Hf to Bi (Z = 72−83). J. Phys. C Solid State Phys. 1980, 13, L1091. [Google Scholar] [CrossRef]
- Sahoo, S.R.; Ke, S.C. Spin-Orbit Coupling Effects in Au 4f Core-Level Electronic Structures in Supported Low-Dimensional Gold Nanoparticles. Nanomaterials 2021, 11, 554. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.D.; Liu, H.Y. Photocatalytic reduction activity of {001} TiO2 co-doped with F and Fe under visible light for bromate removal. J. Nanomater. 2016, 2016, 5646175. [Google Scholar] [CrossRef] [Green Version]
- Balsamo, S.A.; Sciré, S.; Condorelli, M.; Fiorenza, R. Photocatalytic H2 Production on Au/TiO2: Effect of Au Photo deposition on Different TiO2 Crystalline Phases. J 2022, 5, 92–104. [Google Scholar] [CrossRef]
- Wang, B.-Y.; Hsiao, Y.-S.; Wei, P.-C.; Liu, Y.-T.; Chu, C.-C.; Hsiao, V.K.S. Visible Light-Induced Photocatalyst with Au/TiO2 Nanocomposites Fabricated through Pulsed Laser-Induced Photolysis. Catalysts 2022, 12, 564. [Google Scholar] [CrossRef]
- Mihai, S. Synthesis of Gold Nanoparticles Using Schiff Base. Acta Phys. Pol. A 2013, 123, 254–255. [Google Scholar] [CrossRef]
- Zhang, L.Y.; You, J.; Li, Q.W.; Dong, Z.H.; Zhong, Y.J.; Han, Y.L.; You, Y.H. Preparation and Photocatalytic Property of Ag Modified Titanium Dioxide Exposed High Energy Crystal Plane (001). Coatings 2020, 10, 27. [Google Scholar] [CrossRef]











| Cycle 1 Photodegradation Efficiency (%) | ||||
| Time | 60 min | 120 min | 180 min | 240 min |
| MB (4.10 × 10−4 M) | 50.00 | 65.00 | 77.75 | 91.75 |
| Ibuprofen (200 mg/L) | 39.00 | 59.00 | 79.00 | 91.20 |
| Naproxen (4.4 mg/L) | 34.10 | 51.10 | 67.00 | 82.95 |
| Cycle 2 Photodegradation Efficiency (%) | ||||
| Time | 15 min | 30 min | 45 min | 60 min |
| MB (4.10 × 10−4 M) | 30.00 | 50.00 | 75.00 | 79.00 |
| Ibuprofen (200 mg/L) | 15.00 | 22.00 | 30.00 | 37.00 |
| Naproxen (4.4 mg/L) | 18.18 | 42.05 | 55.00 | 64.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usman, A.K.; Cursaru, D.-L.; Brănoiu, G.; Şomoghi, R.; Manta, A.-M.; Matei, D.; Mihai, S. A Modified Sol–Gel Synthesis of Anatase {001}-TiO2/Au Hybrid Nanocomposites for Enhanced Photodegradation of Organic Contaminants. Gels 2022, 8, 728. https://doi.org/10.3390/gels8110728
Usman AK, Cursaru D-L, Brănoiu G, Şomoghi R, Manta A-M, Matei D, Mihai S. A Modified Sol–Gel Synthesis of Anatase {001}-TiO2/Au Hybrid Nanocomposites for Enhanced Photodegradation of Organic Contaminants. Gels. 2022; 8(11):728. https://doi.org/10.3390/gels8110728
Chicago/Turabian StyleUsman, Abubakar Katsina, Diana-Luciana Cursaru, Gheorghe Brănoiu, Raluca Şomoghi, Ana-Maria Manta, Dănuţa Matei, and Sonia Mihai. 2022. "A Modified Sol–Gel Synthesis of Anatase {001}-TiO2/Au Hybrid Nanocomposites for Enhanced Photodegradation of Organic Contaminants" Gels 8, no. 11: 728. https://doi.org/10.3390/gels8110728