Alginate-Based Composites for Corneal Regeneration: The Optimization of a Biomaterial to Overcome Its Limits
Abstract
:1. Introduction
Corneal Diseases: Current Strategies and Their Limitations
2. Material Properties to Match the Corneal Microenvironment
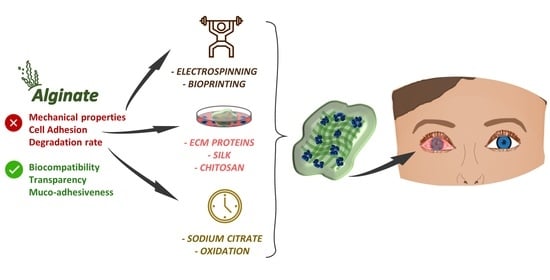
3. Advantages and Limitations of Using Alginate for Corneal TERM
Structure and Factors That Influence the Gelation of Alginates
4. Alginate Composite
4.1. Combinations to Reinforce Alginate Hydrogel-Based-Scaffolds
4.2. Strategies to Improve Cell Incorporation and Survival into Alginate Hydrogels
4.3. Oxidized Alginate to Control Alginate Degradation Rate and More
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Griffith, M.; Jackson, W.B.; Lagali, N.; Merrett, K.; Li, F.; Fagerholm, P. Artificial corneas: A regenerative medicine approach. Eye 2009, 23, 1985–1989. [Google Scholar] [CrossRef]
- Sridhar, M.S. Anatomy of cornea and ocular surface. Indian J. Ophthalmol. 2018, 66, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Oie, Y.; Nishida, K. Corneal regenerative medicine. Regen. Ther. 2016, 5, 40–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guérin, L.-P.; Le-Bel, G.; Desjardins, P.; Couture, C.; Gillard, E.; Boisselier, E.; Bazin, R.; Germain, L.; Guérin, S.L. The Human Tissue-Engineered Cornea (hTEC): Recent Progress. Int. J. Mol. Sci. 2021, 22, 1291. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, E.H.M.; Poulsen, E.T.; Thogersen, I.B.; Yamamoto, K.; Bruel, A.; Enghild, J.J. The low-density lipoprotein receptor-related protein 1 (LRP1) interactome in the human cornea. Exp. Eye Res. 2022, 219, 109081. [Google Scholar] [CrossRef]
- Dua, H.S.; Azuara-Blanco, A. Limbal Stem Cells of the Corneal Epithelium. Surv. Ophthalmol. 2000, 44, 415–425. [Google Scholar] [CrossRef]
- Osei-Bempong, C.; Figueiredo, F.C.; Lako, M. The limbal epithelium of the eye—A review of limbal stem cell biology, disease and treatment. BioEssays 2012, 35, 211–219. [Google Scholar] [CrossRef]
- Park, M.; Richardson, A.; Pandzic, E.; Lobo, E.P.; Whan, R.; Watson, S.L.; Lyons, J.G.; Wakefield, D.; Di Girolamo, N. Visualizing the Contribution of Keratin-14+ Limbal Epithelial Precursors in Corneal Wound Healing. Stem Cell Rep. 2019, 12, 14–28. [Google Scholar] [CrossRef] [Green Version]
- Eghrari, A.O.; Riazuddin, A.; Gottsch, J.D. Overview of the Cornea: Structure, Function, and Development. Prog. Mol. Biol. Transl. Sci. 2015, 134, 7–23. [Google Scholar] [CrossRef]
- Espana, E.M.; Birk, D.E. Composition, structure and function of the corneal stroma. Exp. Eye Res. 2020, 198, 108137. [Google Scholar] [CrossRef]
- Fini, M.E.; Stramer, B.M. How the cornea heals: Cornea-specific repair mechanisms affecting surgical outcomes. Cornea 2005, 24, S2–S11. [Google Scholar] [CrossRef] [PubMed]
- Di Mundo, R.; Recchia, G.; Parekh, M.; Ruzza, A.; Ferrari, S.; Carbone, G. Sensing inhomogeneous mechanical properties of human corneal Descemet’s membrane with AFM nano-indentation. J. Mech. Behav. Biomed. Mater. 2017, 74, 21–27. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, R.C.; Wilson, S.E. Descemet’s membrane development, structure, function and regeneration. Exp. Eye Res. 2020, 197, 108090. [Google Scholar] [CrossRef] [PubMed]
- Dikstein, S.; Maurice, D.M. The metabolic basis to the fluid pump in the cornea. J. Physiol. 1972, 221, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Gutermuth, A.; Maassen, J.; Harnisch, E.; Kuhlen, D.; Sauer-Budge, A.; Skazik-Voogt, C.; Engelmann, K. Descemet’s Membrane Biomimetic Microtopography Differentiates Human Mesenchymal Stem Cells Into Corneal Endothelial-Like Cells. Cornea 2019, 38, 110–119. [Google Scholar] [CrossRef]
- Doillon, C.J.; Watsky, M.A.; Hakim, M.; Wang, J.; Munger, R.; Laycock, N.; Osborne, R.; Griffith, M. A collagen-based scaffold for a tissue engineered human cornea: Physical and physiological properties. Int. J. Artif. Organs. 2003, 26, 764–773. [Google Scholar] [CrossRef]
- World Report on Vision. Available online: https://cdn.who.int/media/docs/default-source/infographics-pdf/world-vision-infographic-final.pdf?sfvrsn=85b7bcde_2 (accessed on 31 May 2022).
- Ting, D.S.J.; Gopal, B.P.; Deshmukt, R.; Seitzman, G.D.; Said, D.G.; Dua, H.S. Diagnostic armamentarium of infectious keratitis: A comprehensive review. Ocul. Surf. 2022, 23, 27–39. [Google Scholar] [CrossRef]
- Soh, Y.Q.; Kocaba, V.; Weiss, J.S.; Jurjunas, U.V.; Kinoshita, S.; Aldave, A.J.; Mehta, J.S. Corneal dystrophies. Nat. Rev. Dis. Primers 2020, 6, 46. [Google Scholar] [CrossRef]
- Wilson, S.E. Corneal wound healing. Exp. Eye Res. 2020, 197, 108089. [Google Scholar] [CrossRef]
- Nicholas, M.P.; Mysore, N. Corneal neovascularization. Exp. Eye Res. 2021, 202, 108363. [Google Scholar] [CrossRef]
- Mehdizadeh, A.; Hoseinzadeh, A.; Fazelzadeh, A. Central corneal thickness as a risk factor for glaucoma. Med. Hypotheses 2007, 69, 1205–1207. [Google Scholar] [CrossRef] [PubMed]
- Griffith, M.; Alarcon, E.I.; Brunette, I. Regenerative approaches for the cornea. J. Intern. Med. 2016, 280, 276–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palchesko, R.; Carrasquilla, S.; Feinberg, A. Natural biomaterials for corneal tissue engineering, repair, and regeneration. Adv. Healthc. Mater. 2018, 7, 1701434. [Google Scholar] [CrossRef]
- Pellegrini, G.; Traverso, C.E.; Franzi, A.T.; Zingirian, M.; Cancedda, R.; Luca, M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet 1997, 349, 990–993. [Google Scholar] [CrossRef]
- Tan, D.T.H.; Dart, J.K.G.; Holland, E.J.; Kinoshita, S. Corneal Transplantation. Lancet 2012, 379, 1749–1761. [Google Scholar] [CrossRef]
- Price, M.O.; Gorovoy, M.; Price, F.W.; Benetz, B.A.; Menegay, H.J.; Lass, J.H. Descemet’s stripping automated endothelial keratoplasty: Three-year graft and endothelial cell survival compared with penetrating keratoplasty. Ophthalmology 2013, 120, 246–251. [Google Scholar] [CrossRef] [Green Version]
- Engelmann, K.; Bednarz, J.; Valtink, M. Prospects for endothelial transplantation. Exp. Eye Res. 2004, 78, 573–578. [Google Scholar] [CrossRef]
- Heinzelmann, S.; Bohringer, D.; Eberwein, P.; Reinhard, T.; Maier, P. Outcomes of Descemet membrane endothelial keratoplasty, Descemet stripping automated endothelial keratoplasty and penetrating keratoplasty from a single centre study. Graefes Arch. Clin. Exp. Ophthalmol. 2016, 254, 515–522. [Google Scholar] [CrossRef]
- Eye BankAssociation of America. Eye Banking Statistical Report; EBAA, no. 202; Eye BankAssociation of America: Washington, DC, USA, 2015. [Google Scholar]
- Kenyon, K.R.; Tseng, S.C. Limbal autograft transplantation for ocular surface disorders. Ophthalmology 1989, 96, 709–722; discussion 722–723. [Google Scholar] [CrossRef]
- Sangwan, V.S.; Basu, S.; MacNeil, S.; Balasubramanian, D. Simple limbal epithelial transplantation (SLET): A novel surgical technique for the treatment of unilateral limbal stem cell deficiency. Br. J. Ophthalmol. 2012, 96, 931–934. [Google Scholar] [CrossRef] [Green Version]
- Sacchetti, M.; Rama, P.; Bruscolini, A.; Lambiase, A. Limbal Stem Cell Transplantation: Clinical Results, Limits, and Perspectives. Stem Cells Int. 2018, 2018, 8086269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dufrane, D. Impact of age on human adipose stem cells for bone tissue engineering. Cell Transplant. 2017, 26, 1496–1504. [Google Scholar] [CrossRef]
- Baptista, L.S.; Silva, K.R.; Borojevic, R. Obesity and weight loss could alter the properties of adipose stem cells? World J. Stem Cells 2015, 7, 165–173. [Google Scholar] [CrossRef]
- Mo, M.; Wang, S.; Zhou, Y.; Li, H.; Wu, Y. Mesenchymal stem cell subpopulations: Phenotype, property and therapeutic potential. Cell Mol. Life Sci. 2016, 73, 3311–3321. [Google Scholar] [CrossRef] [PubMed]
- Joswig, A.J.; Mitchell, A.; Cummings, K.J.; Levine, G.J.; Gregory, C.A.; Smith, R.; Watts, A.E. Repeated intra-articular injection of allogeneic mesenchymal stem cells causes an adverse response compared to autologous cells in the equine model. Stem Cell Res. Ther. 2017, 8, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lohan, P.; Treacy, O.; Griffin, M.D.; Ritter, T.; Ryan, A.E. Anti-donor immune responses elicited by allogeneic mesenchymal stem cells and their extracellular vesicles: Are we still learning? Front. Immunol. 2017, 8, 1626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghiasi, M.; Jadidi, K.; Hashemi, M.; Zare, H.; Salimi, A.; Aghamollaei, H. Application of mesenchymal stem cells in corneal regeneration. Tissue Cell 2021, 73, 101600. [Google Scholar] [CrossRef]
- Chang, M.C.; Kuo, Y.J.; Hung, K.H.; Peng, C.L.; Chen, K.Y.; Yeh, L.K. Liposomal dexamethasone–moxifloxacin nanoparticle combinations with collagen/gelatin/alginate hydrogel for corneal infection treatment and wound healing. Biomed. Mater. 2020, 15, 055022. [Google Scholar] [CrossRef]
- Al-Jaibaji, O.; Swioklo, S.; Gijbels, K.; Vaes, B.; Figueiredo, F.C.; Connon, C.J. Alginate encapsulated multipotent adult progenitor cells promote corneal stromal cell activation via release of soluble factors. PLoS ONE 2018, 13, e0202118. [Google Scholar] [CrossRef]
- Reakasame, S.; Boccaccini, A.R. Oxidized alginate-based hydrogels for tissue engineering applications: A review. Biomacromolecules 2018, 19, 3–21. [Google Scholar] [CrossRef]
- Holoclar. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/holoclar (accessed on 30 May 2022).
- Pellegrini, G.; De Luca, M. Eyes on the prize: Limbal stem cells and corneal restoration. Cell Stem Cell 2014, 15, 121–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, B.; Dudenhoefer, E.; Dohlman, C.H. Keratoprosthesis: An update. Curr. Opin. Ophthalmol. 2001, 12, 282–287. [Google Scholar] [CrossRef] [PubMed]
- von Nussbaum, A.; Nepomuk, J. Cornea Artificials; Schurich: Munich, Germany, 1853. [Google Scholar]
- Avadhanam, V.S.; Smith, H.E.; Liu, C. Keratoprostheses for corneal blindness: A review of contemporary devices. Clin. Ophthalmol. 2015, 9, 697–720. [Google Scholar] [CrossRef] [Green Version]
- Gomaa, A.; Comyn, O.; Liu, C. Keratoprostheses in clinical practice—A review. Clin. Exp. Ophthalmol. 2010, 38, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, W.; Zhang, Z.; Lee, B.P. Recent developments in tough hydrogels for biomedical applications. Gels 2018, 4, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madl, A.C.; Myung, D. Supramolecular Host–Guest Hydrogels for Corneal Regeneration. Gels 2021, 7, 163. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Marchant, R.E. Design properties of hydrogel tissue-engineering scaffolds. Expert. Rev. Med. Devices 2011, 8, 607–626. [Google Scholar] [CrossRef]
- Pita-López, M.L.; Fletes-Vargas, G.; Espinosa-Andrews, H.; Rodriguez-Rodriguez, R. Physically cross-linked chitosan-based hydrogels for tissue engineering applications: A state-of-the-art review. Eur. Polym. J. 2021, 145, 110176. [Google Scholar] [CrossRef]
- Nakagawa, M.; Teraoka, F.; Fujimoto, S.; Hamada, Y.; Kibayashi, H.; Takahashi, J. Improvement of cell adhesion on poly(L-lactide) by atmospheric plasma treatment. J. Biomed. Mater. Res.-Part A 2006, 77, 112–118. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, D.; Chen, J.; Zhang, X.; Li, X.; Zhao, W.; Xu, T. Biomaterials Based on Marine Resources for 3D Bioprinting Applications. Mar. Drugs 2019, 17, 555. [Google Scholar] [CrossRef] [Green Version]
- Kong, B.; Mi, S. Electrospun scaffolds for corneal tissue engineering: A review. Materials 2016, 9, 614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, D.; Liu, Z.; Qian, C.; Huang, J.; Zhou, Y.; Mao, X.; Qu, Q.; Liu, B.; Wang, J.; Hu, Z.; et al. 3D Bioprinting of a Gelatin-Alginate Hydrogel for Tissue-Engineered Hair Follicle Regeneration. Acta Biomater. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Williams, D. Revisiting the definition of biocompatibility. Med. Device Technol. 2003, 14, 10–13. [Google Scholar] [PubMed]
- Tathe, A.; Ghodke, M.; Nikalje, A.P. A brief review: Biomaterials and their apllication. Int. J. Pharm. Pharm. Sci. 2010, 2, 19–23. [Google Scholar]
- Mancuso, A.; Barone, A.; Cristiano, M.C.; Cianflone, E.; Fresta, M.; Paolino, D. Cardiac Stem Cell-Loaded Delivery Systems: A New Challenge for Myocardial Tissue Regeneration. Int. J. Mol. Sci. 2020, 21, 7701. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, A.; Cianflone, E.; Crsiatiano, M.C.; Salerno, N.; Tarsitano, M.; Marino, F.; Molinaro, C.; Fresta, M.; Torella, D.; Paolino, D. Lyotropic Liquid Crystals: A Biocompatible and Safe Material for Local Cardiac Application. Pharmaceutics 2022, 14, 452. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef]
- Wilson, S.L.; Sidney, L.E.; Dunphy, S.E.; Rose, J.B.; Hopkinson, A. Keeping an Eye on Decellularized Corneas: A Review of Methods, Characterization and Applications. J. Funct. Biomater. 2013, 4, 114–161. [Google Scholar] [CrossRef] [Green Version]
- Sani, E.S.; Kheirkhah, A.; Rana, D.; Sun, Z.; Foulsham, W.; Sheikhi, A.; Khademhosseini, A.; Dana, R.; Annabi, N. Sutureless repair of corneal injuries using naturally derived bioadhesive hydrogels. Sci. Adv. 2019, 5, eaav1281. [Google Scholar] [CrossRef] [Green Version]
- Tan, G.Z.; Zhou, Y. Tunable 3D Nanofiber Architecture of Polycaprolactone by Divergence Electrospinning for Potential Tissue Engineering Applications. Nano Micro Lett. 2018, 10, 73. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Favi, P.; Cheng, X.; Golshan, N.H.; Ziemer, K.S.; Keidar, M.; Webster, T.J. Cold atmospheric plasma (CAP) surface nanomodified 3D printed polylactic acid (PLA) scaffolds for bone regeneration. Acta Biomater. 2016, 46, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Kruse, M.; Walter, P.; Bauer, B.; Rütten, S.; Schaefer, K.; Plange, N.; Gries, T.; Jockenhoevel, S.; Fuest, M. Electro-Spun Membranes as Scaffolds for Human Corneal Endothelial Cells. Curr. Eye Res. 2018, 43, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.M.; Liu, X.H. Advancing biomaterials of human origin for tissue engineering. Prog. Polym. Sci. 2016, 53, 86–168. [Google Scholar] [CrossRef] [Green Version]
- Mogoşanu, G.D.; Grumezescu, A.M. Natural and synthetic polymers for wounds and burns dressing. Int. J. Pharm. 2014, 463, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Mano, J.F.; Silva, G.A.; Azevedo, H.S.; Malafaya, P.B.; Sousa, R.A.; Silva, S.S.; Boesel, L.F.; Oliveira, J.; Santos, T.C.; Marques, A.P.; et al. Natural origin biodegradable systems in tissue engineering and regenerative medicine: Present status and some moving trends. J. Royal Soc. Interface 2007, 4, 999–1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- del Barrio, A.J.L.; Chiesa, M.; Gallego Ferrer, G.; Garagorri, N.; Briz, N.; Fernandez-Delgado, J.; De Miguel, M.P. Biointegration of corneal macroporous membranes based on poly (ethyl acrylate) copolymers in an experimental animal model. J. Biomed. Mater. Res. A 2015, 103, 1106–1118. [Google Scholar] [CrossRef] [PubMed]
- De Miguel, M.P.; Casaroli-Marano, R.P.; Nieto-Nicolau, N.; Martínez-Conesa, E.M.; Alió del Barrio, J.L.; Alió, J.L.; Arnalich-Montiel, F. Frontiers in regenerative medicine for cornea and ocular surface. In Frontiers in Stem Cell and Regenerative Medicine Research; ur-Rahman, A., Anjum, S., Eds.; Bentham Books: Sharjah, United Arab Emirates, 2015; Volume 1, pp. 92–138. [Google Scholar] [CrossRef] [Green Version]
- Del Barrio, J.L.A.; Arnalich-Montiel, F.; De Miguel, M.P.; El Zarif, M.; Alió, J.L. Corneal stroma regeneration: Preclinical studies. Exp. Eye Res. 2021, 202, 108314. [Google Scholar] [CrossRef]
- Anumolu, S.S.; Singh, Y.; Gao, D.; Stein, S.; Sinko, P.J. Design and evaluation of novel fast forming pilocarpine-loaded ocular hydrogels for sustained pharmacological response. J. Control Release 2009, 137, 152–159. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.Y.; Zhang, Y.; Zhu, D.; Lu, Y.; Zhou, G.; Liu, W.; Cao, Y.; Zhang, W.J.; Ljubimov, A.V. Corneal stroma regeneration with acellular corneal stroma sheets and keratocytes in a rabbit model. PLoS ONE 2021, 10, e0132705. [Google Scholar] [CrossRef]
- Ghezzi, C.E.; Marelli, B.; Omenetto, F.G.; Funderburgh, J.L.; Kaplan, D.L. 3D functional corneal stromal tissue equivalent based on corneal stromal stem cells and multi-layered silk film architecture. PLoS ONE 2017, 12, e0169504. [Google Scholar] [CrossRef] [Green Version]
- Formisano, N.; van der Putten, C.; Grant, R.; Sahin, G.; Truckenmüller, R.K.; Bouten, C.V.; Giselbrecht, S. Mechanical Properties of Bioengineered Corneal Stroma. Adv. Healthc. Mater. 2021, 10, 2100972. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Porta, N.; Fernandes, P.; Queiros, A.; Salgado-Borges, J.; Parafita-Mato, M.; González-Méijome, J.M. Corneal Biomechanical Properties in Different Ocular Conditions and New Measurement Techniques. ISRN Ophthalmol. 2014, 2014, 724546. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Zhang, J.; Li, G.; Ma, G.; Huang, Y.; Sun, J.; Wang, Y.; Nener, B. Determination of Young’s modulus of jet grouted coalcretes using an intelligent model. Eng. Geol. 2019, 252, 43–53. [Google Scholar] [CrossRef]
- Parsajoo, M.; Armaghani, D.J.; Mohammed, A.S.; Khari, M.; Jahandari, S. Tensile strength prediction of rock material using non-destructive tests: A comparative intelligent study. Transp. Geotech. 2021, 31, 100652. [Google Scholar] [CrossRef]
- Dias, J.M.; Ziebarth, N.M. Anterior and posterior corneal stroma elasticity assessed using nanoindentation. Exp. Eye Res. 2013, 115, 41–46. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Sisley, A.M.G.; Anderson, A.J.; Taberner, A.J.; McGhee, C.N.J.; Patel, D.V. Characterization of a novel collagen scaffold for corneal tissue engineering. Tissue Eng.-Part C Methods 2016, 22, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Ahearne, M.; Fernández-Pérez, J.; Masterton, S.; Madden, P.W.; Bhattacharjee, P. Designing Scaffolds for Corneal Regeneration. Adv. Funct. Mater. 2020, 30, 10. [Google Scholar] [CrossRef] [Green Version]
- Borene, M.L.; Barocas, V.H.; Hubel, A. Mechanical and Cellular Changes During Compaction of a Collagen-Sponge-Based Corneal Stromal Equivalent. Ann. Biomed. Eng. 2004, 32, 274–283. [Google Scholar] [CrossRef]
- Madden, P.W.; Lai, J.N.; George, K.A.; Giovenco, T.; Harkin, D.G.; Chirila, T.V. Human corneal endothelial cell growth on a silk fibroin membrane. Biomaterials 2011, 32, 4076–4084. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, X.; Gunawidjaja, R.; Lin, Y.H.; Gupta, M.K.; Kaplan, D.L.; Naik, R.R.; Tsukruk, V.V. Mechanical Properties of Robust Ultrathin Silk Fibroin Films. Adv. Funct. Mater. 2007, 17, 2229–2237. [Google Scholar] [CrossRef]
- Chen, J.; Yan, C.; Zhu, M.; Yao, Q.; Shao, C.; Lu, W.; Wang, J.; Mo, X.; Gu, P.; Fu, Y.; et al. Electrospun nanofibrous SF/P(LLA-CL) membrane: A potential substratum for endothelial keratoplasty. Int. J. Nanomed. 2015, 10, 3337–3350. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, S.; Dawson, R.A.; Chirila, T.V.; Shadforth, A.; Hogerheyde, T.A.; Edwards, G.A.; Harkin, D.G. Treatment of silk fibroin with poly(ethylene glycol) for the enhancement of corneal epithelial cell growth. J. Funct. Biomater. 2015, 6, 345–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joo, C.; Hayan, J.; Jeong, S.; Joaquim, O.; Rui, R.; Gilson, K. Biofunctionalized lysophosphatidic acid/silk fibroin film for cornea endothelial cell regeneration. Nanomaterials 2018, 8, 290. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.K.; Sim, B.R.; Kim, J.I.; Khang, G. Functionalized silk fibroin film scaffold using β-Carotene for cornea endothelial cell regeneration. Colloids Surf. B 2018, 164, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Hazra, S.; Nandi, S.; Naskar, D.; Guha, R.; Chowdhury, S.; Pradhan, N.; Kundu, S.C.; Konar, A. Non-mulberry silk fibroin biomaterial for corneal regeneration. Sci. Rep. 2016, 6, 21840. [Google Scholar] [CrossRef] [Green Version]
- Aghaei-Ghareh-Bolagh, B.; Guan, J.; Wang, Y.; Martin, A.D.; Dawson, R.; Mithieux, S.M.; Weiss, A.S. Optically robust, highly permeable and elastic protein films that support dual cornea cell types. Biomaterials 2019, 188, 50–62. [Google Scholar] [CrossRef]
- Farasatkia, A.; Kharaziha, M.; Ashrafizadeh, F.; Salehi, S. Transparent silk/gelatin methacrylate (GelMA) fibrillar film for corneal regeneration. Mater. Sci. Eng. C 2021, 120, 111744. [Google Scholar] [CrossRef]
- Qi, X.; Wang, J.; Sun, D.; Zhou, Q.; Xie, L. Postoperative changes in amniotic membrane as a carrier for allogeneic cultured limbal epithelial transplantation. Am. J. Ophthalmol. 2014, 158, 1192–1198. [Google Scholar] [CrossRef]
- Khosravimelal, S.; Mobaraki, M.; Eftekhari, S.; Ahearne, M.; Seifalian, A.M.; Gholipourmalekabadi, M. Hydrogels as Emerging Materials for Cornea Wound Healing. Small 2021, 17, e2006335. [Google Scholar] [CrossRef]
- Trujillo-de Santiago, G.; Sharifi, R.; Yue, K.; Sani, E.S.; Kashaf, S.S.; Alvarez, M.M.; Leijten, J.; Khademhosseini, A.; Dana, R.; Annabi, N. Ocular adhesives: Design, chemistry, crosslinking mechanisms, and applications. Biomaterials 2019, 197, 345–367. [Google Scholar] [CrossRef] [Green Version]
- Shou, Y.; Zhang, J.; Yan, S.; Xia, P.; Xu, P.; Li, G.; Zhang, K.; Yin, J. Thermoresponsive Chitosan/DOPA-Based Hydrogel as an Injectable Therapy Approach for Tissue-Adhesion and Hemostasis. ACS Biomater. Sci. Eng. 2020, 6, 3619–3629. [Google Scholar] [CrossRef] [PubMed]
- Isobe, N.; Tsudome, M.; Kusumi, R.; Wada, M.; Uematsu, K.; Okada, S.; Deguchi, S. Moldable crystalline α-chitin hydrogel with toughness and transparency toward ocular applications. ACS Appl. Polym. Mater. 2020, 2, 1656–1663. [Google Scholar] [CrossRef]
- Liang, W.; Luo, Z.; Zhou, L. Preparation and characterization of the n-HA/PVA/CS porous composite hydrogel. Chin. J. Chem. Eng. 2020, 28, 598–602. [Google Scholar] [CrossRef]
- Irimia, T.; Dinu-Pîrvu, C.E.; Ghica, M.V.; Lupuleasa, D.; Muntean, D.L.; Udeanu, D.I.; Popa, L. Chitosan-based in situ gels for ocular delivery of therapeutics: A state-of-the-art review. Mar. Drugs 2018, 16, 373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, N.; Dmour, I.; Taha, M.O. Degradability of Chitosan Micro/Nanoparticles for Pulmonary Drug Delivery. Heliyon 2019, 5, e01684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, S.; Zhang, X.; Tan, G.; Tian, L.; Liu, D.; Liu, Y.; Yang, X.; Pan, W. A novel pH-induced thermosensitive hydrogel composed of carboxymethyl chitosan and poloxamer cross-linked by glutaraldehyde for ophthalmic drug delivery. Carbohydr. Polym. 2017, 155, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Tan, H. Alginate-Based Biomaterials for Regenerative Medicine Applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef]
- Jeon, O.; Bouhadir, K.H.; Mansour, J.M.; Alsberg, E. Photocrosslinked alginate hydrogels with tunable biodegradation rates and mechanical properties. Biomaterials 2009, 30, 2724–2734. [Google Scholar] [CrossRef]
- Nazemi, Z.; Nourbakhsh, M.S.; Kiani, S.; Daemi, H.; Ashtiani, M.K.; Baharv, H. Effect of chemical composition and sulfated modification of alginate in the development of delivery systems based on electrostatic interactions for small molecule drugs. Mater. Lett. 2020, 263, 127235. [Google Scholar] [CrossRef]
- Zhang, B.; Yan, Y.; Shen, Q.; Ma, D.; Huang, L.; Cai, X.; Tan, S. A colon targeted drug delivery system based on alginate modificated graphene oxide for colorectal liver metastasis. Mater. Sci. Eng. C 2017, 79, 185–190. [Google Scholar] [CrossRef]
- Katuwavila, N.P.; Perera, A.D.L.C.; Samarakoon, S.R.; Soysa, P.; Karunaratne, V.; Amaratunga, G.A.J.; Karunaratne, D.N. Chitosan-Alginate Nanoparticle System Efficiently Delivers Doxorubicin to Mcf-7 Cells. J. Nanomater. 2016, 2016, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Lu, Z.; Wu, H.; Li, W.; Zheng, L.; Zhao, J. Collagen-alginate as bioink for three-dimensional (3D) cell printing based cartilage tissue engineering. Mater. Sci. Eng. C 2017, 83, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.S.; Marrero-Berrios, I.; Perez, I.; Maguire, T.; Radhakrishnan, P.; Manchikalapati, D.; Yarmush, J. Alginate-liposomal construct for bupivacaine delivery and MSC function regulation. Drug Deliv. Transl. Res. 2018, 8, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Liberski, A.; Latif, N.; Raynaud, C.; Bollensdorff, C.; Yacoub, M. Alginate for cardiac regeneration: From seaweed to clinical trials. Glob. Cardiol. Sci. Pract. 2016, 2016, e201604. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, M.; Halperin-sternfeld, M.; Grinberg, I.; Adler-abramovich, L. Injectable Alginate-Peptide Composite Hydrogel as a Scaffold for Bone Tissue Regeneration. Nanomaterials 2019, 9, 497. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Lam, M.T. Alginate application for heart and cardiovascular diseases. In Springer Series in Biomaterials Science and Engineering; Springer: Singapore, 2018; Volume 11, pp. 185–212. [Google Scholar]
- Venkatesan, J.; Nithya, R.; Sudha, P.N.; Kim, S.-K. Role of Alginate in Bone Tissue Engineering. Adv. Food Nutr. Res. 2014, 73, 45–57. [Google Scholar] [CrossRef]
- Gomez, C.G.; Lambrecht, M.V.P.; Lozano, J.E.; Rinaudo, M.; Villar, M.A. Influence of the extraction–purification conditions on final properties of alginates obtained from brown algae (Macrocystis pyrifera). Int. J. Biol. Macromol. 2009, 44, 365–371. [Google Scholar] [CrossRef]
- Rehm, B. Bacterial polymers: Biosynthesis, modifications and applications. Nat. Rev. Microbiol. 2010, 8, 578–592. [Google Scholar] [CrossRef]
- Tipton, P.A. Synthesis of alginate in bacteria. In Comprehensive Natural Products; Liu, H.W., Mander, L., Eds.; Elsevier: Oxford, UK, 2010; pp. 423–441. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, J.; Ao, Q. Preparation of alginate-based biomaterials and their applications in biomedicine. Mar. Drugs 2021, 19, 264. [Google Scholar] [CrossRef]
- Hu, C.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Ions-induced gelation of alginate: Mechanisms and applications. Int. J. Biol. Macromol. 2021, 177, 578–588. [Google Scholar] [CrossRef]
- Donati, I.; Paoletti, S. Material properties of alginates. In Alginates: Biology and Applications; Rehm, H.A.B., Ed.; Springer: Dordrecht, The Netherlands, 2009; Volume 13, pp. 1–53. [Google Scholar]
- Fu, S.; Thacker, A.; Sperger, D.M.; Boni, R.L.; Buckner, I.S.; Velankar, S.; Block, L.H. Relevance of rheological properties of sodium alginate in solution to calcium alginate gel properties. Aaps Pharmscitech. 2011, 12, 453–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ménard, M.; Dusseault, J.; Langlois, G.; Baille, W.E.; Tam, S.K.; Yahia, L.H.; Hallé, J.P. Role of protein contaminants in the immunogenicity of alginates. J. Biomed. Mater. Res. B 2010, 93, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Rezaa Mohammadi, M.; Rodrigez, S.; Cao, R.; Alexander, M.; Lakey, J.R.T. Immune response to subcutaneous implants of alginate microcapsules. Mater. Today Proc. 2018, 5, 15580–15585. [Google Scholar] [CrossRef]
- Kuo, C.K.; Ma, P.X. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: Part 1. Structure, gelation rate and mechanical properties. Biomaterials 2001, 22, 511–521. [Google Scholar] [CrossRef]
- Donati, I.; Holtan, S.; Mørch, Y.A.; Borgogna, M.; Dentini, M.; Skjåk-Bræk, G. New hypothesis on the role of alternating sequences in calcium-alginate gels. Biomacromolecules 2005, 6, 1031–1040. [Google Scholar] [CrossRef]
- Borgogna, M.; Skjåk-Bræk, G.; Paoletti, S.; Donati, I. On the initial binding of alginate by calcium ions. The tilted egg-box hypothesis. J. Phys. Chem. B 2013, 117, 7277–7282. [Google Scholar] [CrossRef]
- Cao, L.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Egg-box model-based gelation of alginate and pectin: A review. Carbohydr. Polym. 2020, 242, 116389. [Google Scholar] [CrossRef]
- Otterlei, M.; Ostgaard, K.; Skjåk-Bræk, G.; Smidsrød, O.; Soon-Shiong, P.; Espevik, T. Induction of cytokine production from human monocytes stimulated with alginate. J. Immunother. 1991, 10, 286–291. [Google Scholar] [CrossRef]
- Kulseng, B.; Skjåk-Bræk, G.; Ryan, L.; Andersson, A.; King, A.; Faxvaag, A.; Espevik, T. TRANSPLANTATION OF ALGINATE MICROCAPSULES: Generation of Antibodies Against Alginates and Encapsulated Porcine Islet-Like Cell Clusters. Transplantation 1999, 67, 978–984. [Google Scholar] [CrossRef]
- Okolie, C.L.; Mason, B.; Mohan, A.; Pitts, N.; Udenigwe, C.C. Extraction technology impacts on the structure-function relationship between sodium alginate extracts and their in vitro prebiotic activity. Food Biosci. 2020, 37, 100672. [Google Scholar] [CrossRef]
- Liu, J.; Yang, S.; Li, X.; Yan, Q.; Reaney, M.J.T.; Jiang, Z. Alginate oligosaccharides: Production, biological activities, and potential applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1859–1881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Progr. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beaumont, M.; Tran, R.; Vera, G.; Niedrist, D.; Rousset, A.; Pierre, R.; Shastri, V.P.; Forget, A. Hydrogel-Forming Algae Polysaccharides: From Seaweed to Biomedical Applications. Biomacromolecules 2021, 22, 1027–1052. [Google Scholar] [CrossRef] [PubMed]
- Choukaife, H.; Doolaanea, A.A.; Alfatama, M. Alginate nanoformulation: Influence of process and selected variables. Pharmaceuticals 2020, 13, 335. [Google Scholar] [CrossRef]
- Vissers, A.M.; Caligiani, A.; Sforza, S.; Vincken, J.P.; Gruppen, H. Phlorotannin Composition of Laminaria digitata. Phytochem. Anal. 2017, 28, 487–495. [Google Scholar] [CrossRef]
- Hu, S.; de Vos, P. Polymeric Approaches to Reduce Tissue Responses Against Devices Applied for Islet-Cell Encapsulation. Front. Bioeng. Biotechnol. 2019, 7, 351–358. [Google Scholar] [CrossRef]
- Torres, M.L.; Fernandez, J.M.; Dellatorre, F.G.; Cortizo, A.M.; Oberti, T.G. Purification of alginate improves its biocompatibility and eliminates cytotoxicity in matrix for bone tissue engineering. Algal Res. 2019, 40, 101499. [Google Scholar] [CrossRef]
- Vining, K.; Mooney, D. Mechanical forces direct stem cell behaviour in development and regeneration. Nat. Rev. Mol. Cell Biol. 2017, 18, 728–742. [Google Scholar] [CrossRef]
- Giri, T.K.; Thakur, D.; Alexander, A.; Ajazuddin; Badwaik, H.; Tripathi, D.K. Alginate based hydrogel as a potential biopolymeric carrier for drug delivery and cell delivery systems: Present status and applications. Curr. Drug Deliv. 2012, 9, 539–555. [Google Scholar] [CrossRef]
- Bidarra, S.J.; Barrias, C.C.; Granja, P.L. Injectable alginate hydrogels for cell delivery in tissue engineering. Acta Biomater. 2014, 10, 1646–1662. [Google Scholar] [CrossRef]
- Pawar, S.N.; Edgar, K.J. Alginate derivatization: A review of chemistry, properties and applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef] [PubMed]
- Curley, C.J.; Dolan, E.B.; Otten, M.; Hinderer, S.; Duffy, G.P.; Murphy, B.P. An injectable alginate/extra cellular matrix (ECM) hydrogel towards acellular treatment of heart failure. Drug Deliv. Transl. Res. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Farokhi, M.; Jonidi Shariatzadeh, F.; Solouk, A.; Mirzadeh, H. Alginate Based Scaffolds for Cartilage Tissue Engineering: A Review. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 230–247. [Google Scholar] [CrossRef]
- Skaugrud, Ø.; Hagen, A.; Borgersen, B.; Dornish, M. Biomedical and Pharmaceutical Applications of Alginate and Chitosan. Biotechnol. Genet. Eng. Rev. 1999, 16, 23–40. [Google Scholar] [CrossRef] [Green Version]
- Kostenko, A.; Swioklo, S.; Connon, C.J. Alginate in corneal tissue engineering. Biomed. Mater. 2022, 17, 022004. [Google Scholar] [CrossRef]
- Rastogi, P.; Kandasubramanian, B. Review of alginate-based hydrogel bioprinting for application in tissue engineering. Biofabrication 2019, 11, 042001. [Google Scholar] [CrossRef]
- Axpe, E.; Oyen, M.L. Applications of alginate-based bioinks in 3D bioprinting. Int. J. Mol. Sci. 2016, 17, 1976. [Google Scholar] [CrossRef] [Green Version]
- Strange, D.G.; Tonsomboon, K.; Oyen, M.L. Mechanical behaviour of electrospun fibre-reinforced hydrogels. J. Mater. Sci. Mater. Med. 2014, 25, 681–690. [Google Scholar] [CrossRef]
- Stafiej, P.; Küng, F.; Kruse, F.E.; Schubert, D.W.; Fuchsluger, T. Mechanical and Optical Properties of PCL Nanofiber Reinforced Alginate Hydrogels for Application in Corneal Wound Healing. Biomater. Med. Appl. 2018, 2, 1000118. [Google Scholar] [CrossRef]
- Tonsomboon, K.; Oyen, M. Composite electrospun gelatin fiber-alginate gel scaffolds for mechanically robust tissue engineered cornea. J. Mech. Behav. Biomed. Mater. 2013, 21, 185–194. [Google Scholar] [CrossRef]
- Isaacson, A.; Swioklo, S.; Connon, C.J. 3D bioprinting of a corneal stroma equivalent. Exp. Eye Res. 2018, 173, 188–193. [Google Scholar] [CrossRef]
- Yan, J.; Chen, F.; Amsden, B.G. Cell sheets prepared via gel-sol transition of calcium RGD-alginate. Acta Biomater. 2016, 30, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Farasatkia, A.; Kharaziha, M. Robust and double-layer micro-patterned bioadhesive based on silk nanofibril/GelMA-alginate for stroma tissue engineering. Int. J. Biol. Macromol. 2021, 183, 1013–1025. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Liu, K.; Li, T.; Zhang, W.; Dong, Y.; Lv, J.; Wang, W.; Sun, J.; Li, M.; Wang, M.; et al. An in situ hydrogel based on carboxymethyl chitosan and sodium alginate dialdehyde for corneal wound healing after alkali burn. J. Biomed. Mater. Res. Part A 2019, 107, 742–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Z.; Su, X.; Xu, Y.; Kong, B.; Sun, W.; Mi, S. Bioprinting three-dimensional cell-laden tissue constructs with controllable degradation. Sci. Rep. 2016, 6, 24474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Y.; Liu, W.; Han, B.; Yang, C.; Ma, Q.; Song, F.; Bi, Q. An in situ formed biodegradable hydrogel for reconstruction of the corneal endothelium. Coll. Surf. B Biointerfaces 2011, 82, 1–7. [Google Scholar] [CrossRef]
- Wright, B.; De Bank, P.A.; Luetchford, K.A.; Acosta, F.R.; Connon, C.J. Oxidized alginate hydrogels as niche environments for corneal epithelial cells. J. Biomed. Mater. Res. Part A 2014, 102, 3393–3400. [Google Scholar] [CrossRef] [Green Version]
- Campos, D.F.; Blaeser, A.; Korsten, A.; Neuss, S.; Jäkel, J.; Vogt, M.; Fischer, H. The Stiffness and Structure of Three-Dimensional Printed Hydrogels Direct the Differentiation of Mesenchymal Stromal Cells Toward Adipogenic and Osteogenic Lineages. Tissue Eng. Part A 2015, 21, 740–756. [Google Scholar] [CrossRef]
- Wu, J.; Du, Y.; Watkins, S.C.; Funderburgh, J.L.; Wagner, W.R. The engineering of organized human corneal tissue through the spatial guidance of corneal stromal stem cells. Biomaterials 2012, 33, 1343–1352. [Google Scholar] [CrossRef] [Green Version]
- Hecht, H.; Srebnik, S. Structural Characterization of Sodium Alginate and Calcium Alginate. Biomacromolecules 2016, 17, 2160–2167. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, X. Alginate hydrogel dressings for advanced wound management. Int. J. Biol. Macromol. 2020, 162, 1414–1428. [Google Scholar] [CrossRef]
- Wicklein, V.J.; Singer, B.B.; Scheibel, T.; Salehi, S. Nanoengineered biomaterials for corneal regeneration. In Nanoengineered Biomaterials for Regenerative Medicine; Mozafari, M., Rajadas, J., Kaplan, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 379–415. [Google Scholar] [CrossRef]
- Okutan, N.; Terzi, P.; Altay, F. Affecting parameters on electrospinning process and characterization of electrospun gelatin nanofibers. Food Hydrocoll. 2014, 39, 19–26. [Google Scholar] [CrossRef]
- Kong, B.; Chen, Y.; Liu, R.; Liu, X.; Liu, C.; Shao, Z.; Xiong, L.; Liu, X.; Sun, W.; Mi, S. Fiber Reinforced GelMA Hydrogel to Induce the Regeneration of Corneal Stroma. Nat. Commun. 2020, 11, 1435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaurasia, S.; Lim, R.; Lakshminarayanan, R.; Mohan, R. Nanomedicine Approaches for Corneal Diseases. J. Funct. Biomater. 2015, 6, 277–298. [Google Scholar] [CrossRef] [Green Version]
- Khare, A.; Grover, K.; Pawar, P.; Singh, I. Mucoadhesive polymers for enhancing retention in ocular drug delivery: A critical review. Rev. Adhes. Adhes. 2014, 2, 467–502. [Google Scholar] [CrossRef]
- Sarker, B.; Rompf, J.; Silva, R.; Lang, N.; Detsch, R.; Kaschta, J.; Fabry, B.; Boccaccini, A.R. Alginate-based hydrogels with improved adhesive properties for cell encapsulation. Int. J. Biol. Macromol. 2015, 78, 72–78. [Google Scholar] [CrossRef]
- Grigore, A.; Sarker, B.; Fabry, B.; Boccaccini, A.R.; Detsch, R. Behavior of Encapsulated MG-63 Cells in RGD and Gelatine-Modified Alginate Hydrogels. Tissue Eng. A 2014, 20, 2140–2150. [Google Scholar] [CrossRef]
- Shachar, M.; Tsur-Gang, O.; Dvir, T.; Leor, J.; Cohen, S. The effect of immobilized RGD peptide in alginate scaffolds on cardiac tissue engineering. Acta Biomater. 2011, 7, 152–162. [Google Scholar] [CrossRef]
- Van Nieuwenhove, I.V.; Salamon, A.; Peters, K.; Graulus, G.J.; Martins, J.C.; Frankel, D.; Kersemans, K.; De Vos, F.; Van Vlierberghe, S.; Dubruel, P. Gelatin- and starch-based hydrogels. Part A: Hydrogel development, characterization and coating. Carbohydr. Polym. 2016, 152, 129–139. [Google Scholar] [CrossRef] [Green Version]
- Dehghani, S.; Rasoulianboroujeni, M.; Ghasemi, H.; Keshel, S.H.; Nozarian, Z.; Hashemian, M.N.; Zarei-Ghanavati, M.; Latifi, G.; Ghaffari, R.; Cui, Z.; et al. 3D-Printed membrane as an alternative to amniotic membrane for ocular surface/conjunctival defect reconstruction: An in vitro & in vivo study. Biomaterials 2018, 174, 95–112. [Google Scholar] [CrossRef]
- Tayebi, L.; Rasoulianboroujeni, M.; Moharamzadeh, K.; Almela, T.K.D.; Cui, Z.; Ye, H. 3D-printed membrane for guided tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 84, 148–158. [Google Scholar] [CrossRef] [PubMed]
- D’Avanzo, N.; Bruno, M.C.; Giudice, A.; Mancuso, A.; de Gaetano, F.; Cristiano, M.C.; Paolino, D.; Fresta, M. Influence of materials properties on bio-physical features and effectiveness of 3D-scaffolds for periodontal regeneration. Molecules 2021, 26, 1643. [Google Scholar] [CrossRef]
- Rowley, J.A.; Madlambayan, G.; Mooney, D.J. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials 1999, 20, 45–53. [Google Scholar] [CrossRef]
- Rowley, J.A.; Mooney, D.J. Alginate Type and RGD Density Control Myoblast Phenotype. J. Biomed. Mater. Res. 2002, 60, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, N.; Shimizu, T.; Yamato, M.; Okano, T. Tissue engineering based on cell sheet technology. Adv. Mater. 2007, 19, 3089–3099. [Google Scholar] [CrossRef]
- Spotnitz, W.D. Fibrin Sealant: The Only Approved Hemostat, Sealant, and Adhesive-a Laboratory and Clinical Perspective. ISRN Surg. 2014, 2014, 203943. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Brugnano, J.; Sun, S.; Vase, A.; Orwin, E. The development of a tissue-engineered cornea, Biomaterials and culture methods. Pediatr. Res. 2008, 63, 535–544. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Wang, W.; Yu, J.; Yu, X.; Zheng, Q.; Peng, F.; He, Z.; Zhao, W.; Zhang, Z.; Li, X.; et al. Combination of dexamethasone and Avastin® by supramolecular hydrogel attenuates the inflammatory corneal neovascularization in rat alkali burn model. Colloids Surf. B 2017, 159, 241–250. [Google Scholar] [CrossRef]
- Tsai, C.Y.; Woung, L.C.; Yen, J.C.; Tseng, P.C.; Chiou, S.H.; Sung, Y.J.; Liu, K.T.; Cheng, Y.H. Thermosensitive chitosan-based hydrogels for sustained release of ferulic acid on corneal wound healing. Carbohydr. Polym. 2016, 135, 308–315. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Jayakrishnan, A. Self-cross-linking biopolymers as injectable in situ forming biodegradable scaffolds. Biomaterials 2005, 26, 3941–3951. [Google Scholar] [CrossRef]
- Kim, W.S.; Mooney, D.J.; Arany, P.R.; Lee, K.; Huebsch, N.; Kim, J. Adipose tissue engineering using injectable, oxidised alginate hydrogels. Tissue Eng. A 2012, 18, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Bonino, C.A.; Krebs, M.D.; Saquing, C.D.; Jeong, S.I.; Shearer, K.L.; Alsberg, E.; Khan, S.A. Electrospinning alginate-based nanofibers: From blends to crosslinked low molecular weight alginate-only systems. Carbohydr. Polym. 2011, 85, 111–119. [Google Scholar] [CrossRef]
- Paul, K.; Linh, N.T.B.; Kim, B.; Sarkar, S.K.; Choi, H.J.; Bae, S.H.; Min, Y.K.; Lee, B.T. Effect of Rat Bone Marrow Derived−Stem Cell Delivery from Serum-Loaded Oxidized Alginate−Gelatin−Biphasic Calcium Phosphate Hydrogel for Bone Tissue Regeneration Using a Nude Mouse Critical-Sized Calvarial Defect Model. J. Bioact. Compat. Polym. 2015, 30, 188–208. [Google Scholar] [CrossRef]
- Hao, X.; Silva, E.A.; Månsson-Broberg, A.; Grinnemo, K.H.; Siddiqui, A.J.; Dellgren, G.; Sylvén, C. Angiogenic effects of sequential release of VEGF-A165 and PDGF-BB with alginate hydrogels after myocardial infarction. Cardiovasc. Res. 2007, 75, 178–185. [Google Scholar] [CrossRef] [Green Version]




| Combined Materials | Target | Research Model | ||||
|---|---|---|---|---|---|---|
| Manufacturing Technique | Characterization Steps | Experimental Studies | Advantages Achieved | Ref. | ||
| Alg-PLC | - | Electrospinning |
| - |
| [146] |
| Cornea wound healing | Electrospinning |
| - |
| [147] | |
| Alg-Gel | Entire cornea | Electrospinning |
|
|
| [148] |
| Alg-GelMA | Corneal stroma | 3D Bioprinting |
|
|
| [149] |
| Alg-RGD | Corneal epithelium | Cell sheets |
|
|
| [150] |
| Alg-SNF-GelMA | Corneal stroma | Micropatterned membranes |
|
|
| [151] |
| OA-CMCTS | Corneal alkali burns | In situ forming hydrogel |
|
|
| [152] |
| Alg-Coll-Gel | Corneal epithelium | 3D bioprinting |
|
|
| [153] |
| OA-CTS | Corneal endothelium | In situ forming hydrogel | - |
|
| [154] |
| OA-Coll | Corneal epithelium |
|
|
| [155] | |
| Properties | Noteworthy Considerations |
|---|---|
| Strength and stiffness | Mechanical properties may be tunable as a function of the intended aim. The blend with electrospun fibers of both synthetic and natural polymers reinforce alginate-based materials, without compromising their transparency [147,148]. The use of oxidized alginate can lead to softer matrices [155]. The natural shape of the cornea has to be maintained for constructs [149]. |
| Degradation time | The degradation rate of alginate composites can be modulated from three days to around two weeks by changing the molar ratios between alginate and chelating agents (e.g., sodium citrate) [153]. Hydrogels of oxidized alginate can be degraded in vivo after 30 days [154]. The rate of degradation seems to be directly proportional to the corneal epithelial cell viability [155]. |
| Crosslinking methods | Ionic crosslinking (with CaCl2) is the most-common method, but it can slow down the degradation rate [150,153,166]. The chemical crosslinking of gel blends (with EDC-NHS) allows the finer control of the hydrogels’ physical characteristics, but it could affect cell survival [148]. Blending with photo-crosslinkable polymers ensures transparency but requires a long time for dialysis [151]. The self-crosslinking of oxidized alginate is obtainable according to the functional group of the other blending components [152] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarsitano, M.; Cristiano, M.C.; Fresta, M.; Paolino, D.; Rafaniello, C. Alginate-Based Composites for Corneal Regeneration: The Optimization of a Biomaterial to Overcome Its Limits. Gels 2022, 8, 431. https://doi.org/10.3390/gels8070431
Tarsitano M, Cristiano MC, Fresta M, Paolino D, Rafaniello C. Alginate-Based Composites for Corneal Regeneration: The Optimization of a Biomaterial to Overcome Its Limits. Gels. 2022; 8(7):431. https://doi.org/10.3390/gels8070431
Chicago/Turabian StyleTarsitano, Martine, Maria Chiara Cristiano, Massimo Fresta, Donatella Paolino, and Concetta Rafaniello. 2022. "Alginate-Based Composites for Corneal Regeneration: The Optimization of a Biomaterial to Overcome Its Limits" Gels 8, no. 7: 431. https://doi.org/10.3390/gels8070431