Neutralization and Salt Effect on the Structure and Mechanical Properties of Polyacrylic Acid Gels under Equivolume Conditions
Abstract
:1. Introduction
2. Theory
2.1. Osmotic Pressure of Gels
2.2. Osmotic Pressure Due to Mixing
2.3. Osmotic Pressure Due to Imbalanced Mobile Ion Concentrations
2.4. Osmotic Pressure Due to the Network Elasticity
3. Results and Discussion
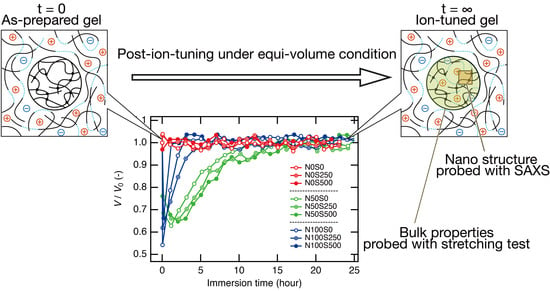
3.1. Volume Change during the Post-Ion-Tuning
3.2. Structural Analysis of the Post-Ion-Tuned Equivolume Gels
3.3. Mechanical Properties of the Post-Ion-Tuned Equivolume PAA Gels
4. Conclusions
5. Materials and Methods
5.1. Sample Preparation
5.2. Swelling of Prepared Gel
5.3. Observation of Swelling with a Microscope
5.4. Small-Angle X-ray Scattering (SAXS)
5.5. Tensile Tests
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
- Chemical potential equality
- 2.
- Electroneutrality
References
- Kwon, H.J.; Yasuda, K.; Gong, J.P.; Ohmiya, Y. Polyelectrolyte hydrogels for replacement and regeneration of biological tissues. Macromol. Res. 2014, 22, 227–235. [Google Scholar] [CrossRef]
- Howse, J.R.; Topham, P.; Crook, C.J.; Gleeson, A.J.; Bras, W.; Jones, R.A.L.; Ryan, A.J. Reciprocating power generation in a chemically driven synthetic muscle. Nano Lett. 2006, 6, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Topham, P.D.; Howse, J.R.; Fernyhough, C.M.; Ryan, A.J. The Performance of Poly(Styrene)-Block-Poly(2-Vinyl Pyridine)-Block-Poly(Styrene) triblock copolymers as PH-Driven actuators. Soft Matter 2007, 3, 1506–1507. [Google Scholar] [CrossRef]
- Iijima, M.; Yoshimura, M.; Tsuchiya, T.; Tsukada, M.; Ichikawa, H.; Fukumori, Y.; Kamiya, H. Direct measurement of interactions between stimulation-responsive drug delivery vehicles and artificial mucin layers by colloid probe atomic force microscopy. Langmuir 2008, 24, 3987–3992. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.Z.; Prebeg, Z.; Kurjaković, N. A PH-dependent colon targeted oral drug delivery system using methacrylic acid copolymers. I. manipulation of drug release using eudragit L100-55 and eudragit S100 combinations. J. Control. Release Off. J. Control. Release Soc. 1999, 58, 215–222. [Google Scholar] [CrossRef]
- Lee, H.-R.; Woo, J.; Han, S.H.; Lim, S.-M.; Lim, S.; Kang, Y.-W.; Song, W.J.; Park, J.-M.; Chung, T.D.; Joo, Y.-C.; et al. Stretchable ionic diode from copolyelectrolyte hydrogels with methacrylated polysaccharides. Adv. Funct. Mater. 2018, 29, 1806909. [Google Scholar] [CrossRef]
- Kim, K.B.; Han, J.-H.; Kim, H.C.; Chung, T.D. Polyelectrolyte junction field effect transistor based on microfluidic chip. Appl. Phys. Lett. 2010, 96, 143506. [Google Scholar] [CrossRef]
- Lim, S.-M.; Yoo, H.; Oh, M.-A.; Han, S.H.; Lee, H.-R.; Chung, T.D.; Joo, Y.-C.; Sun, J.-Y. Ion-to-Ion amplification through an open-junction ionic diode. Proc. Natl. Acad. Sci. USA 2019, 116, 13807–13815. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Katashima, T.; Li, X.; Mitsukami, Y.; Yokoyama, Y.; Sakumichi, N.; Chung, U.; Shibayama, M.; Sakai, T. Swelling behaviors of hydrogels with alternating neutral/highly charged sequences. Macromolecules 2020. [Google Scholar] [CrossRef]
- Tang, J.; Katashima, T.; Li, X.; Mitsukami, Y.; Yokoyama, Y.; Chung, U.; Shibayama, M.; Sakai, T. Effect of nonlinear elasticity on the swelling behaviors of highly swollen polyelectrolyte gels. Gels 2021, 7, 25. [Google Scholar] [CrossRef]
- Walker, A.; Vratsanos, M.; Kozawa, S.; Askew, T.; Hemmendinger, K.; McGrail, B.; Bedford, N.; Wnek, G. Enhanced elasticity in poly(Acrylic Acid) gels viasynthesis in the presence of high concentrations of select salts. Soft Matter 2019, 15, 7596–7604. [Google Scholar] [CrossRef]
- Schosseler, F.; Ilmain, F.; Candau, S.J. Structure and properties of partially neutralized poly(Acrylic Acid) gels. Macromolecules 1991, 24, 225–234. [Google Scholar] [CrossRef]
- Schosseler, F.; Moussaïd, A.; Munch, J.P.; Candau, S.J. Weakly charged polyelectrolyte gels: Temperature and salt effects on the statics and the dynamics. J. Phys. II 1991, 1, 1197–1219. [Google Scholar] [CrossRef]
- Shibayama, M.; Tanaka, T.; Han, C.C. Small-angle neutron scattering study on weakly charged temperature sensitive polymer gels. J. Chem. Phys. 1992, 97, 6842–6854. [Google Scholar] [CrossRef]
- Tamburic, S.; Craig, D.Q.M. An investigation into the rheological, dielectric and mucoadhesive properties of poly(Acrylic Acid) gel systems. J. Control. Release 1995, 37, 59–68. [Google Scholar] [CrossRef]
- Nisato, G.; Skouri, R.; Schosseler, F.; Munch, J.-P.; Candau, S.J. Elastic behaviour of salt-free polyelectrolyte gels. Faraday Discuss. 1995, 101, 133. [Google Scholar] [CrossRef]
- Skouri, R.; Schosseler, F.; Munch, J.P.; Candau, S.J. Swelling and elastic properties of polyelectrolyte gels. Macromolecules 1995, 28, 197–210. [Google Scholar] [CrossRef]
- Benguigui, L. Comparison between the elasticity of polyacrylamide and polyacrylic gels. J. Phys. II 1995, 5, 437–443. [Google Scholar] [CrossRef]
- Nisato, G.; Schosseler, F.; Candau, S.J. Swelling equilibrium properties of partially charged gels: The effect of salt on the shear modulus. Polym. Gels Netw. 1996, 4, 481–498. [Google Scholar] [CrossRef]
- Shibayama, M.; Ikkai, F.; Inamoto, S.; Nomura, S.; Han, C.C. PH and salt concentration dependence of the microstructure of poly(N-isopropylacrylamide-Co-acrylic Acid) gels. J. Chem. Phys. 1996, 105, 4358–4366. [Google Scholar] [CrossRef]
- Horkay, F.; Tasaki, I.; Basser, P.J. Osmotic swelling of polyacrylate hydrogels in physiological salt solutions. Biomacromolecules 2000, 1, 84–90. [Google Scholar] [CrossRef]
- Yin, D.-W.; Horkay, F.; Douglas, J.F.; Pablo, J.J. Molecular simulation of the swelling of polyelectrolyte gels by monovalent and divalent counterions. J. Chem. Phys. 2008, 129, 154902–154912. [Google Scholar] [CrossRef] [Green Version]
- Horkay, F.; Basser, P.J. Ionic and PH effects on the osmotic properties and structure of polyelectrolyte gels. J. Polym. Sci. Part B Polym. Phys. 2008, 46, 2803–2810. [Google Scholar] [CrossRef] [Green Version]
- Yin, D.-W.; Cruz, M.O.; Pablo, J.J. Swelling and collapse of polyelectrolyte gels in equilibrium with monovalent and divalent electrolyte solutions. J. Chem. Phys. 2009, 131, 194907. [Google Scholar] [CrossRef] [Green Version]
- Longo, G.S.; Cruz, M.O.; Szleifer, I. Molecular theory of weak polyelectrolyte gels: The role of PH and salt concentration. Macromolecules 2011, 44, 147–158. [Google Scholar] [CrossRef]
- Morozova, S.; Muthukumar, M. Elasticity at swelling equilibrium of ultrasoft polyelectrolyte gels: Comparisons of theory and experiments. Macromolecules 2017, 50, 2456–2466. [Google Scholar] [CrossRef]
- Morishima, K.; Li, X.; Oshima, K.; Mitsukami, Y.; Shibayama, M. Small-angle scattering study of tetra-poly(Acrylic Acid) gels. J. Chem. Phys. 2018, 149, 163301–163309. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Muthukumar, M. Interplay between microscopic and macroscopic properties of charged hydrogels. Macromolecules 2020, 53, 90–101. [Google Scholar] [CrossRef]
- Flory, P.J. Principles of Polymer Chemistry; Cornell University Press: Ithaca, NY, USA, 1953. [Google Scholar]
- Bastide, J.; Candau, S.; Leibler, L. Osmotic deswelling of gels by polymer solutions. Macromolecules 1981, 14, 719–726. [Google Scholar] [CrossRef]
- Horkay, F.; Nishi, K.; Shibayama, M. Decisive test of the ideal behavior of tetra-PEG gels. J. Chem. Phys. 2017, 146, 164905–164909. [Google Scholar] [CrossRef] [PubMed]
- Dixler, D.S.; Ander, P. Self-diffusion coefficients of sodium ion in aqueous sodium polyacrylate solutions containing sodium chloride. J. Phys. Chem. 1973, 77, 2684–2687. [Google Scholar] [CrossRef]
- Tanaka, T.; Hocker, L.O.; Benedek, G.B. Spectrum of light scattered from a viscoelastic gel. J. Chem. Phys. 1973, 59, 5151–5159. [Google Scholar] [CrossRef]
- Bunzl, K. Kinetics of ion exchange in a stirred-flow cell. J. Chem. Soc. Faraday Trans. 1993, 89, 107–112. [Google Scholar] [CrossRef]
- Candau, S.; Bastide, J.; Delsanti, M. Polymer networks. Adv. Polym. Sci. 1982, 44. [Google Scholar] [CrossRef]
- Mendes, E.; Lindner, P.; Buzier, M.; Boué, F.; Bastide, J. Experimental evidence for inhomogeneons swelling and deformation in statistical gels. Phys. Rev. Lett. 1991, 66, 1595–1598. [Google Scholar] [CrossRef]
- Takata, S.; Norisuye, T.; Shibayama, M. Small-angle neutron-scattering study on preparation temperature dependence of thermosensitive gels. Macromolecules 2002, 35, 4779–4784. [Google Scholar] [CrossRef]
- Roe, R.-J. Methods of X-Ray and Neutron Scattering in Polymer Science; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Nierlich, M.; Williams, C.E.; Boué, F.; Cotton, J.P.; Daoud, M.; Famoux, B.; Jannink, G.; Picot, C.; Moan, M.; Wolff, C.; et al. Small angle neutron scattering by semi-dilute solutions of polyelectrolyte. J. Phys. 1979, 40, 701–704. [Google Scholar] [CrossRef]
- Moinard, D.; Taton, D.; Gnanou, Y.; Rochas, C.; Borsali, R. SAXS from four-arm polyelectrolyte stars in semi-dilute solutions. Macromol. Chem. Phys. 2003, 204, 89–97. [Google Scholar] [CrossRef]
- Chremos, A.; Horkay, F. Disappearance of the polyelectrolyte peak in salt-free solutions. Phys. Rev. E 2020, 102, 012611. [Google Scholar] [CrossRef]
- Nishida, K.; Kaji, K.; Kanaya, T. Charge density dependence of correlation length due to electrostatic repulsion in polyelectrolyte solutions. Macromolecules 1995, 28, 2472–2475. [Google Scholar] [CrossRef]
- Ise, N.; Okubo, T.; Yamamoto, K.; Kawai, H.; Hashimoto, T.; Fujimura, M.; Hiragi, Y. Ordered structure in dilute solutions of ionic biopolymers. 2. small-angle X-ray scattering study of sodium polyacrylate solution. J. Am. Chem. Soc. 1980, 102, 7901–7906. [Google Scholar] [CrossRef]
- Manning, G.S. Limiting laws and counterion condensation in polyelectrolyte solutions I. colligative properties. J. Chem. Phys. 1969, 51, 924–933. [Google Scholar] [CrossRef]
- Reed, W.F.; Ghosh, S.; Medjahdi, G.; Francois, J. Dependence of Polyelectrolyte apparent persistence lengths, viscosity, and diffusion on ionic strength and linear charge density. Macromolecules 1991, 24, 6189–6198. [Google Scholar] [CrossRef]
- Rubinstein, M.; Colby, R.H.; Dobrynin, A.V.; Joanny, J.-F. Elastic modulus and equilibrium swelling of polyelectrolyte gels. Macromolecules 1996, 29, 398–406. [Google Scholar] [CrossRef]
- Truzzolillo, D.; Bordi, F.; Cametti, C.; Sennato, S. Counterion condensation of differently flexible polyelectrolytes in aqueous solutions in the dilute and semidilute regime. Phys. Rev. E 2009, 79, 011804. [Google Scholar] [CrossRef] [PubMed]
- Kłos, J.; Pakula, T. Monte carlo simulations of a polyelectrolyte chain with added salt: Effect of temperature and salt valence. J. Chem. Phys. 2005, 123, 24903. [Google Scholar] [CrossRef] [PubMed]









| S0 | S250 | S500 | |
|---|---|---|---|
| N0 | 20,970 ± 3080 | 20,690 ± 3660 | 22,860 ± 3480 |
| N50 | 14,140 ± 2810 | 17,870 ± 2150 | 17,180 ± 2810 |
| N100 | 16,020 ± 2370 | 16,590 ± 2110 | 16,410 ± 1930 |
| S0 | S250 | S500 | |
|---|---|---|---|
| N0 | 4.0 ± 1.2 | 3.9 ± 1.5 | 3.3 ± 1.5 |
| N50 | 2.9 ± 1.1 | 3.5 ± 0.7 | 4.1 ± 1.5 |
| N100 | 3.7 ± 1.4 | 3.2 ± 1.2 | 3.0 ± 1.1 |
| Theory | Experimental | ||||||
|---|---|---|---|---|---|---|---|
| S0 | S250 | S500 | S0 | S250 | S500 | ||
| N0 | 1 | 1.52 | 1.76 | N0 | 1 | 0.986 | 1.09 |
| N50 | 0.384 | 0.434 | 0.472 | N50 | 0.674 | 0.852 | 0.819 |
| N100 | 0.384 | 0.434 | 0.472 | N100 | 0.764 | 0.791 | 0.783 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsuji, Y.; Shibayama, M.; Li, X. Neutralization and Salt Effect on the Structure and Mechanical Properties of Polyacrylic Acid Gels under Equivolume Conditions. Gels 2021, 7, 69. https://doi.org/10.3390/gels7020069
Tsuji Y, Shibayama M, Li X. Neutralization and Salt Effect on the Structure and Mechanical Properties of Polyacrylic Acid Gels under Equivolume Conditions. Gels. 2021; 7(2):69. https://doi.org/10.3390/gels7020069
Chicago/Turabian StyleTsuji, Yui, Mitsuhiro Shibayama, and Xiang Li. 2021. "Neutralization and Salt Effect on the Structure and Mechanical Properties of Polyacrylic Acid Gels under Equivolume Conditions" Gels 7, no. 2: 69. https://doi.org/10.3390/gels7020069