Sustainable, Highly Efficient and Superhydrophobic Fluorinated Silica Functionalized Chitosan Aerogel for Gravity-Driven Oil/Water Separation
Abstract
:1. Introduction
2. Results and Discussion
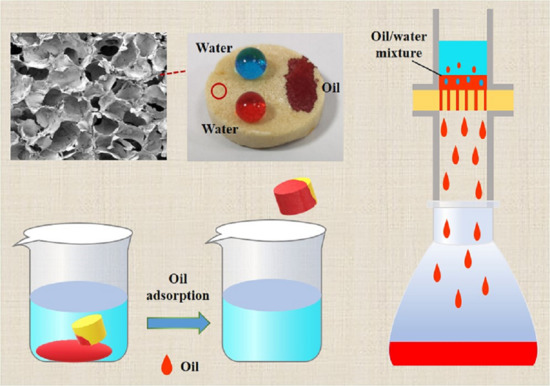
2.1. SEM Morphologies
2.2. XPS and FTIR Analysis
2.3. Mechanical Properties
2.4. Surface Wettability
2.5. Self-Cleaning Behavior of F-CS Aerogel
2.6. Oil Adsorption Behavior of F-CS Aerogel
2.7. Oil/Water Separation Performance of F-CS Aerogel
3. Conclusions
4. Experimental Section
4.1. Materials
4.2. Synthesis of Fluorinated Silica Nanoparticles
4.3. Preparation of the F-CS Aerogel
4.4. Characterization
4.5. Oil/water Separation Performance
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duman, O.; Diker, C.Ö.; Tunç, S. Development of highly hydrophobic and superoleophilic fluoro organothiol-coated car-bonized melamine sponge/rGO composite absorbent material for the efficient and selective absorption of oily substances from aqueous environments. J. Environ. Chem. Eng. 2021, 9, 105093. [Google Scholar] [CrossRef]
- Khosravi, M.; Azizian, S. Preparation of superhydrophobic and superoleophilic nanostructured layer on steel mesh for oil-water separation. Sep. Purif. Technol. 2017, 172, 366–373. [Google Scholar] [CrossRef]
- Karki, H.P.; Kafle, L.; Ojha, D.P.; Song, J.H.; Kim, H.J. Cellulose/polyacrylonitrile electrospun composite fiber for effec-tive separation of the surfactant-free oil-in-water mixture under a versatile condition. Sep. Purif. Technol. 2019, 210, 913–919. [Google Scholar] [CrossRef]
- Chen, X.; Liang, Y.N.; Tang, X.-Z.; Shen, W.; Hu, X. Additive-free poly (vinylidene fluoride) aerogel for oil/water separa-tion and rapid oil absorption. Chem. Eng. J. 2017, 308, 18–26. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, T.; Qiu, F.; Yue, X.; Yang, D.; Li, P.; Zhu, Y. Facile one-step fabrication of highly hydrophobic, renewable and mechanically flexible sponge with dynamic coating for efficient oil/water separation. J. Taiwan Inst. Chem. Eng. 2019, 95, 515–524. [Google Scholar] [CrossRef]
- Zhou, C.; Chen, Z.; Yang, H.; Hou, K.; Zeng, X.; Zheng, Y.; Cheng, J. Nature-Inspired Strategy toward Superhydrophobic Fabrics for Versatile Oil/Water Separation. ACS Appl. Mater. Interfaces 2017, 9, 9184–9194. [Google Scholar] [CrossRef]
- Wang, L.; Xiao, X.; Liu, E.; Yu, S.; Yin, X.; Wang, J.; Zhu, G.; Li, Q.; Li, J. Fabrication of superhydrophobic needle-like Ca-P coating with anti-fouling and anti-corrosion properties on AZ31 magnesium alloy. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 620, 126568. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, F.; Gao, Y. Superhydrophobic and oleophobic dual-function coating with durablity and self-healing proper-ty based on a waterborne solution. Appl. Mater. Today 2021, 22, 100970. [Google Scholar] [CrossRef]
- Wang, J.; Han, F.; Chen, Y.; Wang, H. A pair of MnO2 nanocrystal coatings with inverse wettability on metal meshes for efficient oil/water separation. Sep. Purif. Technol. 2019, 209, 119–127. [Google Scholar] [CrossRef]
- Guo, H.; Yang, J.; Xu, T.; Zhao, W.; Zhang, J.; Zhu, Y.; Wen, C.; Li, Q.; Sui, X.; Zhang, L. A Robust Cotton Textile-Based Mate-rial for High-Flux Oil-Water Separation. ACS Appl. Mater. Interfaces 2019, 11, 13704–13713. [Google Scholar] [CrossRef]
- Lu, Y.; Yuan, W. Superhydrophobic/Superoleophilic and Reinforced Ethyl Cellulose Sponges for Oil/Water Separation: Synergistic Strategies of Cross-linking, Carbon Nanotube Composite, and Nanosilica Modification. ACS Appl. Mater. Interfaces 2017, 9, 29167–29176. [Google Scholar] [CrossRef]
- Wang, R.; Shou, D.; Lv, O.; Kong, Y.; Deng, L.; Shen, J. pH-Controlled drug delivery with hybrid aerogel of chitosan, car-boxymethyl cellulose and graphene oxide as the carrier. Int. J. Biol. Macromol. 2017, 103, 248–253. [Google Scholar] [CrossRef]
- Batista, M.; Gonçalves, V.; Gaspar, F.; Nogueira, I.; Matias, A.; Gurikov, P. Novel alginate-chitosan aerogel fibres for potential wound healing applications. Int. J. Biol. Macromol. 2020, 156, 773–782. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhang, X.; Wang, A.; Hu, X.; Deng, L.; Lou, L.; Shen, H. The synthesis and simulations of solvent-responsive bi-layer hydrogel. Polymer 2020, 204, 122801. [Google Scholar] [CrossRef]
- Wu, J.; Li, H.; Lai, X.; Chen, Z.; Zeng, X. Conductive and superhydrophobic F-rGO@CNTs/chitosan aerogel for piezoresis-tive pressure sensor. Chem. Eng. J. 2020, 386, 123998. [Google Scholar] [CrossRef]
- Cao, N.; Lyu, Q.; Li, J.; Wang, Y.; Yang, B.; Szunerits, S.; Boukherroub, R. Facile synthesis of fluorinated polydopa-mine/chitosan/reduced graphene oxide composite aerogel for efficient oil/water separation. Chem. Eng. J. 2017, 326, 17–28. [Google Scholar] [CrossRef]
- Qi, C.; Zhao, L.; Lin, Y.; Wu, D. Graphene oxide/chitosan sponge as a novel filtering material for the removal of dye from water. J. Colloid Interface Sci. 2018, 517, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Gao, J.; Liu, C.; Chen, Y.; Zhong, G.; Hodges, C.; Chen, M.; Zhang, H. Preparation and UV aging of nano-SiO2/fluorinated polyacrylate polyurethane hydrophobic composite coating. Prog. Org. Coatings 2020, 141, 105556. [Google Scholar] [CrossRef]
- Saadatbakhsh, M.; Jamali Asl, S.; Kiani, M.J.; Nouri, N.M. Slip length measurement of pdms/hydrophobic silica superhy-drophobic coating for drag reduction application. Surf. Coat. Techno. 2020, 404, 126428. [Google Scholar] [CrossRef]
- Wan, S.; Cong, Y.; Jiang, D.; Dong, Z.-H. Weathering barrier enhancement of printed circuit board by fluorinated silica based superhydrophobic coating. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 628–638. [Google Scholar] [CrossRef]
- Li, Z.; Zhong, L.; Zhang, T.; Qiu, F.; Yue, X.; Yang, D. Sustainable, Flexible, and Superhydrophobic Functionalized Cellulose Aerogel for Selective and Versatile Oil/Water Separation. ACS Sustain. Chem. Eng. 2019, 7, 9984–9994. [Google Scholar] [CrossRef]
- Chen, C.; Weng, D.; Mahmood, A.; Chen, S.; Wang, J. Separation Mechanism and Construction of Surfaces with Special Wettability for Oil/Water Separation. ACS Appl. Mater. Interfaces 2019, 11, 11006–11027. [Google Scholar] [CrossRef]
- Guo, C.; Ding, H.; Xie, M.; Zhang, H.; Hong, X.; Sun, L.; Ding, F. Multifunctional superamphiphobic fluorinated silica with a core-shell structure for anti-fouling and anti-corrosion applications. Colloids Surf. A Physicochem. Eng. Asp. 2021, 615, 126155. [Google Scholar] [CrossRef]
- Basu, B.J.; Kumar, V.D.; Anandan, C. Surface studies on superhydrophobic and oleophobic polydimethylsiloxane–silica nanocomposite coating system. Appl. Surf. Sci. 2012, 261, 807–814. [Google Scholar] [CrossRef]
- Lakshmi, R.V.; Bera, P.; Anandan, C.; Basu, B.J. Effect of the size of silica nanoparticles on wettability and surface chemis-try of sol–gel superhydrophobic and oleophobic nanocomposite coatings. Appl. Surf. Sci. 2014, 320, 780–786. [Google Scholar] [CrossRef]
- Saengkaew, J.; Le, D.; Samart, C.; Sawada, H.; Nishida, M.; Chanlek, N.; Kongparakul, S.; Kiatkamjornwong, S. Superhy-drophobic coating from fluoroalkylsilane modified natural rubber encapsulated SiO2 composites for self-driven oil/water separation. Appl. Surf. Sci. 2018, 462, 164–174. [Google Scholar] [CrossRef]
- Beppu, M.; Vieira, R.; Aimoli, C.; Santana, C. Crosslinking of chitosan membranes using glutaraldehyde: Effect on ion permeability and water absorption. J. Membr. Sci. 2007, 301, 126–130. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, Z.; Sheng, L.; Ma, M.; Xu, Q.; Jin, Y. Structure-property of crosslinked chitosan/silica composite films modified by genipin and glutaraldehyde under alkaline conditions. Carbohydr. Polym. 2019, 215, 348–357. [Google Scholar] [CrossRef]
- Wu, J.; Su, C.; Jiang, L.; Ye, S.; Liu, X.; Shao, W. Green and Facile Preparation of Chitosan Sponges as Potential Wound Dressings. ACS Sustain. Chem. Eng. 2018, 6, 9145–9152. [Google Scholar] [CrossRef]
- Mishra, A.; Pandey, V.K.; Shankar, B.S.; Melo, J.S. Spray drying as an efficient route for synthesis of silica nanoparti-cles-sodium alginate biohybrid drug carrier of doxorubicin. Colloids Surf. B Biointerfaces 2021, 197, 111445. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Lai, X.; Li, H.; Zeng, X. Effective improvement of anti-tracking of addition-cure liquid silicone rubber via charge dissipation of fluorosilane-grafted silica. Polym. Degrad. Stab. 2019, 167, 250–258. [Google Scholar] [CrossRef]
- Yeerken, T.; Yu, W.; Feng, J.; Xia, Q.; Liu, H. Durable superamphiphobic aramid fabrics modified by PTFE and FAS for chemical protective clothing. Prog. Org. Coat. 2019, 135, 41–50. [Google Scholar] [CrossRef]
- Pan, Z.; Guan, Y.; Liu, Y.; Cheng, F. Facile fabrication of hydrophobic and underwater superoleophilic elastic and mechani-cal robust graphene/PDMS sponge for oil/water separation. Sep. Purif. Technol. 2021, 261, 118273. [Google Scholar] [CrossRef]
- Jiang, L.; Wen, Y.; Zhu, Z.; Liu, X.; Shao, W. A Double cross-linked strategy to construct graphene aerogels with highly effi-cient methylene blue adsorption performance. Chemosphere 2021, 265, 129169. [Google Scholar] [CrossRef]
- Wang, H.; Gao, F.; Ren, R.; Wang, Z.; Yue, R.; Wei, J.; Wang, X.; Kong, Z.; Zhang, H.; Zhang, X. Caffeic acid polymer rapidly modified sponge with excellent anti-oil-adhesion property and efficient separation of oil-in-water emulsions. J. Hazard. Mater. 2021, 404, 124197. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhu, L.; Liu, X.; Wu, L.; Dai, K.; Liu, C.; Shen, C.; Guo, X.; Zheng, G.; Guo, Z. Superhydrophobic Shish-kebab Mem-brane with Self-Cleaning and Oil/Water Separation Properties. ACS Sustain. Chem. Eng. 2018, 6, 9866–9875. [Google Scholar] [CrossRef]
- Karatum, O.; Steiner, I.S.A.; Griffin, J.S.; Shi, W.; Plata, D.L. Flexible, Mechanically Durable Aerogel Composites for Oil Capture and Recovery. ACS Appl. Mater. Interfaces 2016, 8, 215–224. [Google Scholar] [CrossRef]
- Gu, H.; Li, G.; Li, P.; Liu, H.; Chadyagondo, T.T.; Li, N.; Xiong, J. Superhydrophobic and breathable SiO2/polyurethane po-rous membrane for durable water repellent application and oil-water separation. Appl. Surf. Sci. 2020, 512, 144837. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, L.; Wang, H.; Bian, Z. Preparation of MCC/MC Silica Sponge and Its Oil/Water Separation Apparatus Appli-cation. Ind. Eng. Chem. Res. 2017, 56, 5795–5801. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, M.; Liu, Z.; Kang, M.; Huang, C.; Fu, G. Fabrication of highly durable and robust superhydropho-bic-superoleophilic nanofibrous membranes based on a fluorine-free system for efficient oil/water separation. J. Membr. Sci. 2019, 570–571, 303–313. [Google Scholar] [CrossRef]
- Gao, J.; Huang, X.; Xue, H.; Tang, L.; Li, R.K. Facile preparation of hybrid microspheres for super-hydrophobic coating and oil-water separation. Chem. Eng. J. 2017, 326, 443–453. [Google Scholar] [CrossRef]
- Yao, H.; Lu, X.; Xin, Z.; Zhang, H.; Li, X. A durable bio-based polybenzoxazine/SiO2 modified fabric with superhydropho-bicity and superoleophilicity for oil/water separation. Sep. Purif. Technol. 2019, 229, 115792. [Google Scholar] [CrossRef]
- Tang, X.; Si, Y.; Ge, J.; Ding, B.; Liu, L.; Zheng, G.; Luo, W.; Yu, J. In situ polymerized superhydrophobic and superoleophilic nanofibrous membranes for gravity driven oil–water separation. Nanoscale 2013, 5, 11657–11664. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Wen, Q.; Peng, Y.; Guo, Z. Simple one-pot approach toward robust and boiling-water resistant superhydrophobic cotton fabric and the application in oil/water separation. J. Mater. Chem. A 2017, 5, 21866–21874. [Google Scholar] [CrossRef]
- Xiong, C.; Quan, Z.; Zhang, H.; Wang, L.; Qin, X.; Wang, R.; Yu, J. Hierarchically tunable structure of polystyrene-based microfiber membranes for separation and selective adsorption of oil-water. Appl. Surf. Sci. 2020, 532, 147400. [Google Scholar] [CrossRef]
- Ruan, X.; Xu, T.; Chen, D.; Ruan, Z.; Hu, H. Superhydrophobic paper with mussel-inspired polydimethylsiloxane–silica nanoparticle coatings for effective oil/water separation. RSC Adv. 2020, 10, 8008–8015. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]







| Oil | Flux (L·m−2·h−1) | Ref | |
|---|---|---|---|
| SNP/PBZ/PI | dichloromethane | ~4800 (gravity) | Ma et al., 2019 [40] |
| PVDF/SiO2 | chloroform | ~2050 (gravity) | Gao et al., 2017 [41] |
| PC-a/SiO2 0.6 | dichloromethane | ~8470 (gravity) | Yao et al., 2019 [42] |
| PC-a/SiO2 0.6 | chloroform | 9320 | Yao et al., 2019 [42] |
| FPMIA-1/SNP-2 | dichloromethane | 3311 (gravity) | Tang et al., 2013 [43] |
| PDA@SiO2 coated fabric | diesel oil | ~4000 (gravity) | Guo et al., 2017 [44] |
| PS-CA-SiO2 | petroleum ether | ~2500 (gravity) | Xiong et al., 2020 [45] |
| PDMS–SiO2/PDA/paper | chloroform | ~4600 (gravity) | Ruan et al., 2020 [46] |
| F-CS | dichloromethane | 17,081 (gravity) | This work |
| F-CS | chloroform | 20,401 (gravity) | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Z.; Jiang, L.; Liu, J.; He, S.; Shao, W. Sustainable, Highly Efficient and Superhydrophobic Fluorinated Silica Functionalized Chitosan Aerogel for Gravity-Driven Oil/Water Separation. Gels 2021, 7, 66. https://doi.org/10.3390/gels7020066
Zhu Z, Jiang L, Liu J, He S, Shao W. Sustainable, Highly Efficient and Superhydrophobic Fluorinated Silica Functionalized Chitosan Aerogel for Gravity-Driven Oil/Water Separation. Gels. 2021; 7(2):66. https://doi.org/10.3390/gels7020066
Chicago/Turabian StyleZhu, Zhongjie, Lei Jiang, Jia Liu, Sirui He, and Wei Shao. 2021. "Sustainable, Highly Efficient and Superhydrophobic Fluorinated Silica Functionalized Chitosan Aerogel for Gravity-Driven Oil/Water Separation" Gels 7, no. 2: 66. https://doi.org/10.3390/gels7020066