Addition of Anionic Polysaccharide Stabilizers Modulates In Vitro Digestive Proteolysis of a Chocolate Milk Drink in Adults and Children
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Characterization of Stabilizers
2.2.2. Preparation and Characterization of CMDs
2.2.3. In Vitro Adult’s or Child’s Digestion of CMD with and without Stabilizers
2.2.4. Monitoring Stabilizer Effect on In Vitro Digestive Proteolysis of CMD
3. Results and Discussion
3.1. Characterization of Stabilizers and CMDs
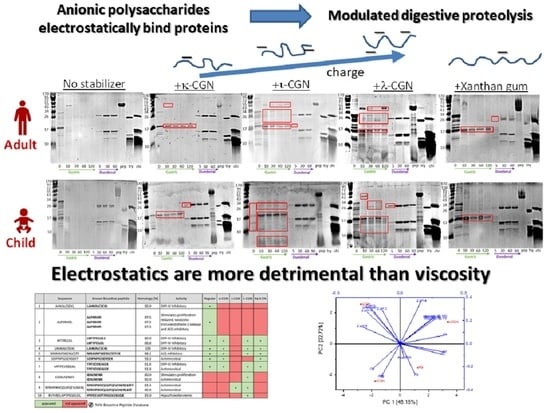
3.2. Monitoring Stabilizer Effects on In Vitro Digestive Proteolysis of CMD
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

Appendix B
| Sequence | Known Bioactive Peptide | Activity | Homology % | References | R | &κ | &ι | &λ | &X |
|---|---|---|---|---|---|---|---|---|---|
| ARHPHPHLSF | HPHPHLSF | ACE-inhibitory | 80 | [58,75] | + | + | + | + | + |
| AVPYPQRDMPIQAF | VPYPQRDMPIQAFL | Antimicrobial | 92.9 | [68] | + | + | + | + | + |
| DELQDKIHPF | TEDELQDKIHPF | Antimicrobial | 83.3 | [76] | + | + | + | + | + |
| DIQKVAGTW | IQKVAGTW | DPP-IV Inhibitory, ACE-inhibitory, Antimicrobial, ACE-inhibitory | 88.9 | [76,77] | + | + | + | + | + |
| IQKVAGTW | 88.9 | ||||||||
| GLDIQKVAGT | 80 | ||||||||
| LDIQKVAGTW | 80 | ||||||||
| ELKPTPEGDL | LKPTPEGDLE | DPP-IV Inhibitory DPP-IV Inhibitory | 90 | [55] | + | + | + | + | + |
| LKPTPEGDL | 90 | ||||||||
| FSDKIAKY | FSDKIAK | Antimicrobial ACE-inhibitory | 87.5 | [78,79] | + | + | + | + | + |
| FSDKIAK | 87.5 | ||||||||
| FTKKTKLTEEEKNRL | TKKTKLTEEEKNRL | Antimicrobial | 93.3 | [80] | + | + | + | + | + |
| IIAEKTKIPAVF | IIAEKTKIPAVF | Antimicrobial | 100 | [76] | + | + | + | + | + |
| IQPKTKVIPYVRYL | WIQPKTKVIPYVRYL | Antimicrobial Antimicrobial | 93.3 | [80] | + | + | + | + | + |
| IQPKTKVIPYVR | 85.7 | ||||||||
| KTKLTEEEKNRL | TKKTKLTEEEKNRL | Antimicrobial | 85.7 | [80] | + | + | + | + | + |
| KTKLTEEEKNRLNF | TKKTKLTEEEKNRL | Antimicrobial | 85.7 | [80] | + | + | + | + | + |
| LKPTPEGDL | LKPTPEGDLE | DPP-IV Inhibitory DPP-IV Inhibitory | 90 | [55] | + | + | + | + | + |
| LKPTPEGDL | 100 | ||||||||
| LVYPFPGPIPNSL | LVYPFPGPIPNSLPQN LVYPFPGPIPNSLPQ | ACE-inhibitory ACE-inhibitory | 81.3 | [81,82] | + | + | + | + | + |
| 86.7 | |||||||||
| MAIPPKKNQDKTEIPTINT | MAIPPKKNQDKTEIPTINT MAIPPKKDQDKTEVPAINT | Antimicrobial Antimicrobial | 100 | [76,83] | + | + | + | + | + |
| 68.4 | |||||||||
| MAIPPKKNQDKTEIPTINT | MAIPPKKNQDKTEIPTINT MAIPPKKDQDKTEVPAINT | Antimicrobial Antimicrobial | 100 | [76,83] | + | + | + | + | + |
| 68.4 | |||||||||
| PQNIPPLTQT | LPQNIPPLT | DPP-IV Inhibitory ACE-inhibitory | 80 | [84,85] | + | + | + | + | + |
| NIPPLTQTPV | 80 | ||||||||
| PQNIPPLTQTP | NIPPLTQTPV | ACE-inhibitory | 81.8 | [84] | + | + | + | + | + |
| PTVMFPPQSVL | SPTVMFPPQSVL | DPP-IV Inhibitory | 91.7 | [86] | + | + | + | + | + |
| PVLGPVRGPF | EPVLGPVRGP | Cytomodulatory ACE-inhibitory ACE-inhibitory | 90 | [87,88,89,90] | + | + | + | + | + |
| VLGPVRGPFP | 90 | ||||||||
| EPVLGPVRGPFP | 83.3 | ||||||||
| PVLGPVRGPFPIIV | YQEPVLGPVRGPFPIIV | Immunomodulatory Antithrombin Antimicrobial ACE-inhibitory Antimicrobial ACE-inhibitory | 82.4 | [66,76,81,91,92,93] | + | + | + | + | + |
| YQEPVLGPVRGPFPIIV | 82.4 | ||||||||
| YQEPVLGPVRGPFPIIV | 82.4 | ||||||||
| YQEPVLGPVRGPFPIIV | 82.4 | ||||||||
| YQEPVLGPVRGPFPI | 80 | ||||||||
| QEPVLGPVRGPFPIIV | 87.5 | ||||||||
| RHPHPHLSF | HPHPHLSF | ACE-inhibitory | 88.9 | [58,75] | + | + | + | + | + |
| RPKHPIKHQGL | RPKHPIKHQ | ACE-inhibitory | 81.8 | [94] | + | + | + | + | + |
| SDIPNPIGSENSE | SDIPNPIGSENSEK | Antimicrobial | 92.9 | [61] | + | + | + | + | + |
| SDIPNPIGSENSEK | SDIPNPIGSENSEK | Antimicrobial | 100 | [61] | + | + | + | + | + |
| SDIPNPIGSENSEKTTM | SDIPNPIGSENSEK | Antimicrobial | 82.4 | [61] | + | + | + | + | + |
| SDIPNPIGSENSEKTTM | SDIPNPIGSENSEK | Antimicrobial | 82.4 | [61] | + | + | + | + | + |
| SDKIAKY | FSDKIAK | Antimicrobial ACE-inhibitory | 85.7 | [78,79] | + | + | + | + | + |
| FSDKIAK | 85.7 | ||||||||
| SLSQSKVLPVPQ | SQSKVLPVPQ | ACE-inhibitory | 83.3 | [87] | + | + | + | + | + |
| SQSKVLPVPQK | SQSKVLPVPQ | ACE-inhibitory ACE-inhibitory | 90.9 | [87,93] | + | + | + | + | + |
| SKVLPVPQ | 72.7 | ||||||||
| TKKTKLTEEEKNRL | TKKTKLTEEEKNRL | Antimicrobial | 100 | [80] | + | + | + | + | + |
| TKKTKLTEEEKNRLNF | TKKTKLTEEEKNRL | Antimicrobial | 87.5 | [80] | + | + | + | + | + |
| VEELKPTPEGDL | VEELKPTPEGNLE | Antimicrobial | 76.9 | [76] | + | + | + | + | + |
| VLDTDYKK | VLDTDYK | ACE-inhibitory | 87.5 | [81] | + | + | + | + | + |
| VYPFPGPIPN | VYPFPGPIP | prolyl endopeptidase-inhibitory PEP-inhibitory prolyl endopeptidase-inhibitory PEP-inhibitory DPP-IV Inhibitory ACE-inhibitory Antioxidant ACE-inhibitory ACE-inhibitory ACE-inhibitory ACE-inhibitory | 90 | [86,94,95,96,97,98,99,100] | + | + | + | + | + |
| VYPFPGPIP | 90 | ||||||||
| YPFPGPIPN | 90 | ||||||||
| YPFPGPIPN | 90 | ||||||||
| VYPFPGPIPN | 100 | ||||||||
| VYPFPGPIPN | 100 | ||||||||
| LVYPFTGPIPN | 90.9 | ||||||||
| LVYPFPGPIP | 90 | ||||||||
| VYPFPGPI | 80 | ||||||||
| VYPFPGPI | 80 | ||||||||
| YPFPGPIPN | 90 | ||||||||
| YPFPGPIPN | 90 | ||||||||
| VYPFPGPIPN | 100 | ||||||||
| VYPFPGPIPN | 100 | ||||||||
| LVYPFTGPIPN | 90.9 | ||||||||
| LVYPFPGPIP | |||||||||
| LVYPFPGPI | |||||||||
| VYPFPGPIPNS | VYPFPGPIP | prolyl endopeptidase-inhibitory PEP-inhibitory DPP-IV Inhibitory ACE-inhibitory Antioxidant ACE-inhibitory ACE-inhibitory ACE-inhibitory | 81.8 | [94,95,96,99,100,101] | + | + | + | + | + |
| VYPFPGPIP | 81.8 | ||||||||
| YPFPGPIPN | 81.8 | ||||||||
| YPFPGPIPN | 81.8 | ||||||||
| VYPFPGPIPN | 90.9 | ||||||||
| VYPFPGPIPN | 90.9 | ||||||||
| LVYPFTGPIPN | 90.9 | ||||||||
| LVYPFPGPIP | 81.8 | ||||||||
| VYPFPGPIPNSL | VYPFPGPIPN | Antioxidant ACE-inhibitory ACE-inhibitory | 83.3 | [82,96] | + | + | + | + | + |
| VYPFPGPIPN | 83.3 | ||||||||
| LVYPFPGPIPNSLPQ | 80 | ||||||||
| YQEPVLGPVRGPF | YQEPVLGPVRGPFPI | Antimicrobial ACE-inhibitory ACE-inhibitory | 86.7 | [66,76,87,102] | + | + | + | + | + |
| YQEPVLGPVRG | 84.6 | ||||||||
| EPVLGPVRGPFP | 84.6 | ||||||||
| YQEPVLGPVRGPFPIIV | YQEPVLGPVRGPFPIIV | Immunomodulatory Antithrombin Antimicrobial ACE-inhibitory Antimicrobial ACE-inhibitory ACE-inhibitory | 100 | [66,76,91,92,93,103] | + | + | + | + | + |
| YQEPVLGPVRGPFPIIV | 100 | ||||||||
| YQEPVLGPVRGPFPIIV | 100 | ||||||||
| YQEPVLGPVRGPFPIIV | 100 | ||||||||
| YQEPVLGPVRGPFPI | 88.2 | ||||||||
| QEPVLGPVRGPFPIIV | 94.1 | ||||||||
| LLYQEPVLGPVRGPFPIIV | 89.4 | ||||||||
| YQKFPQY | YQKFPQY | Antioxidant ACE-inhibitory | 100 | [104,105] | + | + | + | + | + |
| YQKFPQY | 100 | ||||||||
| YQKFPQYL | YQKFPQY | Antioxidant ACE-inhibitory | 87.5 | [104,105] | + | + | + | + | + |
| YQKFPQY | 87.5 | ||||||||
| YQQKPVAL | YYQQKPVA | Antimicrobial | 87.5 | [78] | + | + | + | + | + |
| YVEELKPTPEGDL | VEELKPTPEGNLE | Antimicrobial | 76.9 | [76] | + | + | + | + | + |
References
- Bengoechea, C.; Peinado, I.; McClements, D.J. Formation of protein nanoparticles by controlled heat treatment of lactoferrin: Factors affecting particle characteristics. Food Hydrocoll. 2011, 25, 1354–1360. [Google Scholar] [CrossRef]
- Bengoechea, C.; Jones, O.G.; Guerrero, A.; McClements, D.J. Formation and characterization of lactoferrin/pectin electrostatic complexes: Impact of composition, pH and thermal treatment. Food Hydrocoll. 2011, 25, 1227–1232. [Google Scholar] [CrossRef]
- David-Birman, T.; Mackie, A.; Lesmes, U. Impact of dietary fibers on the properties and proteolytic digestibility of lactoferrin nano-particles. Food Hydrocoll. 2013, 31, 33–41. [Google Scholar] [CrossRef]
- Galazka, V.B.; Smith, D.; Ledward, D.A.; Dickinson, E. Complexes of bovine serum albumin with sulphated polysaccharides: Effects of pH, ionic strength and high pressure treatment. Food Chem. 1999, 64, 303–310. [Google Scholar] [CrossRef]
- Jones, O.G.; Lesmes, U.; Dubin, P.; McClements, D.J. Effect of polysaccharide charge on formation and properties of biopolymer nanoparticles created by heat treatment of β-lactoglobulin-pectin complexes. Food Hydrocoll. 2010, 24, 374–383. [Google Scholar] [CrossRef]
- Laneuville, S.I.; Turgeon, S.L.; Sanchez, C.; Paquin, P. Gelation of Native β-Lactoglobulin Induced by Electrostatic Attractive Interaction with Xanthan Gum. Langmuir 2006. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, F.; Carvajal, M.T.; Harris, M.T. Interactions between bovine serum albumin and alginate: An evaluation of alginate as protein carrier. J. Colloid Interface Sci. 2009, 332, 345–353. [Google Scholar] [CrossRef]
- Doublier, J.L.; Garnier, C.; Renard, D.; Sanchez, C. Protein-polysaccharide interactions. Curr. Opin. Colloid Interface Sci. 2000, 5, 202–214. [Google Scholar] [CrossRef]
- Jones, O.G.; McClements, D.J. Recent progress in biopolymer nanoparticle and microparticle formation by heat-treating electrostatic protein-polysaccharide complexes. Adv. Colloid Interface Sci. 2011, 167, 49–62. [Google Scholar] [CrossRef]
- Li, J.M.; Nie, S.P. The functional and nutritional aspects of hydrocolloids in foods. Food Hydrocoll. 2014, 53, 46–61. [Google Scholar] [CrossRef]
- van de Velde, F.; de Hoog, E.H.A.; Oosterveld, A.; Tromp, R.H. Protein-Polysaccharide Interactions to Alter Texture. Annu. Rev. Food Sci. Technol. 2015, 6, 371–388. [Google Scholar] [CrossRef] [PubMed]
- David, S.; Wojciechowska, A.; Portmann, R.; Shpigelman, A.; Lesmes, U. The impact of food-grade carrageenans and consumer age on the in vitro proteolysis of whey proteins. Food Res. Int. 2020, 130, 108964. [Google Scholar] [CrossRef] [PubMed]
- Fahoum, L.; Moscovici, A.; David, S.; Shaoul, R.; Rozen, G.; Meyron-Holtz, E.G.; Lesmes, U. Digestive fate of dietary carrageenan: Evidence of interference with digestive proteolysis and disruption of gut epithelial function. Mol. Nutr. Food Res. 2017, 61, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.; Matthias, T. Changes in intestinal tight junction permeability associated with industrial food additives explain the rising incidence of autoimmune disease. Autoimmun. Rev. 2015, 14, 479–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tharakan, A.; Norton, I.T.; Fryer, P.J.; Bakalis, S. Mass transfer and nutrient absorption in a simulated model of small intestine. J. Food Sci. 2010, 75, E339–E346. [Google Scholar] [CrossRef]
- Lopes, B.d.M.; Lessa, V.L.; Silva, B.M.; Filho, M.A.d.S.C.; Schnitzler, E.; Lacerda, L.G. Xanthan gum: Properties, production conditions, quality and economic perspective. J. Food Nutr. Res. 2015, 54, 185–194. [Google Scholar]
- Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Lambré, C.; Leblanc, J.; et al. Re-evaluation of xanthan gum (E 415) as a food additive. EFSA J. 2017, 15. [Google Scholar] [CrossRef] [Green Version]
- Necas, J.; Bartosikova, L. Carrageenan: A review. Vet. Med. (Praha) 2013, 58, 187–205. [Google Scholar] [CrossRef] [Green Version]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Kuhnle, G.G.; et al. Re-evaluation of carrageenan (E 407) and processed Eucheuma seaweed (E 407a) as food additives. EFSA J. 2018, 16. [Google Scholar] [CrossRef]
- Campo, V.L.; Kawano, D.F.; Silva, D.B.d.; Carvalho, I. Carrageenans: Biological properties, chemical modifications and structural analysis—A review. Carbohydr. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Drohan, D.D.; Tziboula, A.; McNulty, D.; McNulty, D. Milk protein-carrageenan interactions. Food Hydrocoll. 1997, 11, 101–107. [Google Scholar] [CrossRef]
- Hemar, Y.; Tamehana, M.; Munro, P.A.; Singh, H. Viscosity, microstructure and phase behavior of aqueous mixtures of commercial milk protein products and xanthan gum. Food Hydrocoll. 2001, 15, 565–574. [Google Scholar] [CrossRef]
- De Kruif, C.G.; Tuinier, R. Polysaccharide protein interactions. Food Hydrocoll. 2001, 15, 555–563. [Google Scholar] [CrossRef]
- Schorsch, C.; Jones, M.G.; Norton, I.T. Phase behaviour of pure micellar casein/κ-carrageenan systems in milk salt ultrafiltrate. Food Hydrocoll. 2000, 14, 347–358. [Google Scholar] [CrossRef]
- Chassaing, B.; Koren, O.; Goodrich, J.K.; Poole, A.C.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015, 519, 92–96. [Google Scholar] [CrossRef] [Green Version]
- David, S.; Shani Levi, C.; Fahoum, L.; Ungar, Y.; Meyron-Holtz, E.G.; Shpigelman, A.; Lesmes, U. Revisiting the carrageenan controversy: Do we really understand the digestive fate and safety of carrageenan in our foods? Food Funct. 2018, 9, 1344–1352. [Google Scholar] [CrossRef]
- McKim, J.M.; Willoughby, J.A.; Blakemore, W.R.; Weiner, M.L. Clarifying the confusion between poligeenan, degraded carrageenan, and carrageenan: A review of the chemistry, nomenclature, and in vivo toxicology by the oral route. Crit. Rev. Food Sci. Nutr. 2018, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Gouseti, O.; Jaime-Fonseca, M.R.; Fryer, P.J.; Mills, C.; Wickham, M.S.J.; Bakalis, S. Hydrocolloids in human digestion: Dynamic in-vitro assessment of the effect of food formulation on mass transfer. Food Hydrocoll. 2014, 42, 378–385. [Google Scholar] [CrossRef] [Green Version]
- Anderson, W.; Baillie, A.J. Carrageenans and the proteolytic activity of human gastric secretion. J. Pharm. Pharmacol. 1967, 19, 720–728. [Google Scholar] [CrossRef]
- Baillie, A.J.; Anderson, W. Macroanionic Inhibition of Peptic Activity by High and Low Molecular Weight Macroanions. Nature 1968, 218, 770–771. [Google Scholar] [CrossRef]
- Bourlieu, C.; Ménard, O.; Bouzerzour, K.; Mandalari, G.; Macierzanka, A.; Mackie, A.R.; Dupont, D. Specificity of infant digestive conditions: Some clues for developing relevant in vitro models. Crit. Rev. Food Sci. Nutr. 2014, 54, 1427–1457. [Google Scholar] [CrossRef]
- Dupont, D.; Mandalari, G.; Molle, D.; Jardin, J.; Léonil, J.; Faulks, R.M.; Wickham, M.S.J.; Mills, E.N.C.; Mackie, A.R. Comparative resistance of food proteins to adult and infant in vitro digestion models. Mol. Nutr. Food Res. 2010, 54, 767–780. [Google Scholar] [CrossRef] [PubMed]
- Levi, C.S.; Lesmes, U. Bi-compartmental elderly or adult dynamic digestion models applied to interrogate protein digestibility. Food Funct. 2014, 5, 2402–2409. [Google Scholar] [CrossRef] [PubMed]
- Menard, O.; Cattenoz, T.; Guillemin, H.; Souchon, I.; Deglaire, A.; Dupont, D.; Picque, D.; Ménard, O.; Cattenoz, T.; Guillemin, H.; et al. Validation of a new in vitro dynamic system to simulate infant digestion. Food Chem. 2014, 145, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Rémond, D.; Shahar, D.R.; Gille, D.; Pinto, P.; Kachal, J.; Peyron, M.-A.; Dos Santos, C.N.; Walther, B.; Bordoni, A.; Dupont, D.; et al. Understanding the gastrointestinal tract of the elderly to develop dietary solutions that prevent malnutrition. Oncotarget 2015, 6, 13858–13898. [Google Scholar] [CrossRef] [Green Version]
- David, S.; Fahoum, L.; Rozen, G.; Shaoul, R.; Shpigelman, A.; Meyron-Holtz, E.G.; Lesmes, U. Reply to the Comment on “Revisiting the carrageenan controversy: Do we really understand the digestive fate and safety of carrageenan in our foods?” by M. Weiner and J. McKim, Food Funct., 2019, 10: DOI: 10.1039/C8FO01282B. Food Funct. 2019, 10, 1763–1766. [Google Scholar] [CrossRef]
- Shani-Levi, C.; Levi-Tal, S.; Lesmes, U. Comparative performance of milk proteins and their emulsions under dynamic in vitro adult and infant gastric digestion. Food Hydrocoll. 2013, 32, 349–357. [Google Scholar] [CrossRef]
- Lecacheux, D.; Panaras, R.; Brigand, G.; Martin, G. Molecular weight distribution of carrageenans by size exclusion chromatography and low angle laser light scattering. Carbohydr. Polym. 1985, 5, 423–440. [Google Scholar] [CrossRef]
- Viebke, C.; Borgström, J.; Piculell, L. Characterisation of kappa- and iota-carrageenan coils and helices by MALLS/GPC. Carbohydr. Polym. 1995, 27, 145–154. [Google Scholar] [CrossRef]
- Hanson, A.L.; Metzger, L.E. Evaluation of increased vitamin D fortification in high-temperature, short-time-processed 2% milk, UHT-processed 2% fat chocolate milk, and low-fat strawberry yogurt. J. Dairy Sci. 2010, 93, 801–807. [Google Scholar] [CrossRef] [Green Version]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, S.D.; Beverly, R.L.; Qu, Y.; Dallas, D.C. Milk bioactive peptide database: A comprehensive database of milk protein-derived bioactive peptides and novel visualization. Food Chem. 2017, 232, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Moscovici, A.M.; Joubran, Y.; Briard-Bion, V.; MacKie, A.; Dupont, D.; Lesmes, U. The impact of the Maillard reaction on the in vitro proteolytic breakdown of bovine lactoferrin in adults and infants. Food Funct. 2014, 5, 1898. [Google Scholar] [CrossRef] [PubMed]
- Mooney, C.; Haslam, N.J.; Pollastri, G.; Shields, D.C. Towards the Improved Discovery and Design of Functional Peptides: Common Features of Diverse Classes Permit Generalized Prediction of Bioactivity. PLoS ONE 2012, 7, e45012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tasneem, M.; Siddique, F.; Ahmad, A.; Farooq, U. Stabilizers: Indispensable Substances in Dairy Products of High Rheology. Crit. Rev. Food Sci. Nutr. 2014, 54, 869–879. [Google Scholar] [CrossRef]
- Dickinson, E. Stability and rheological implications of electrostatic milk protein - Polysaccharide interactions. Trends Food Sci. Technol. 1998, 9, 347–354. [Google Scholar] [CrossRef]
- Meyer, S.; Berrut, S.; Goodenough, T.I.J.; Rajendram, V.S.; Pinfield, V.J.; Povey, M.J.W. A comparative study of ultrasound and laser light diffraction techniques for particle size determination in dairy beverages. Meas. Sci. Technol. 2006, 17, 289. [Google Scholar] [CrossRef]
- Langendorff, V.; Cuvelier, G.; Michon, C.; Launay, B.; Parker, A.; De, C.G. Effects of carrageenan type on the behaviour of carrageenan/milk mixtures. Food Hydrocoll. 2000, 14, 273–280. [Google Scholar] [CrossRef]
- Glicerina, V.; Balestra, F.; Dalla Rosa, M.; Romani, S. Effect of manufacturing process on the microstructural and rheological properties of milk chocolate. J. Food Eng. 2015, 145, 45–50. [Google Scholar] [CrossRef]
- Iglauer, S.; Wu, Y.; Shuler, P.; Tang, Y.; Goddard, W.A. Dilute iota- and kappa-Carrageenan solutions with high viscosities in high salinity brines. J. Pet. Sci. Eng. 2011, 75, 304–311. [Google Scholar] [CrossRef]
- Laneuville, S.I.; Turgeon, S.L.; Paquin, P. Changes in the physical properties of xanthan gum induced by a dynamic high-pressure treatment. Carbohydr. Polym. 2013, 92, 2327–2336. [Google Scholar] [CrossRef] [PubMed]
- Mine, N.; Santos, P.H.S.; Campanella, O. Mechanically modified xanthan gum: Rheology and polydispersity aspects. Carbohydr. Polym. 2015, 134, 475–484. [Google Scholar] [CrossRef]
- Tárrega, A.; Martínez, M.; Vélez- Ruiz, J.F.; Fiszman, S. Hydrocolloids as a tool for modulating the expected satiety of milk-based snacks. Food Hydrocoll. 2014, 39, 51–57. [Google Scholar] [CrossRef]
- Stanley, N.F. Carrageenans. In Food Gels; Springer: Dordrecht, The Netherlands, 1990; pp. 79–119. [Google Scholar]
- Lacroix, I.M.E.; Li-Chan, E.C.Y. Isolation and characterization of peptides with dipeptidyl peptidase-IV inhibitory activity from pepsin-treated bovine whey proteins. Peptides 2014, 54, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Jacquot, A.; Gauthier, S.F.; Drouin, R.; Boutin, Y. Proliferative effects of synthetic peptides from β-lactoglobulin and α-lactalbumin on murine splenocytes. Int. Dairy J. 2010, 20, 514–521. [Google Scholar] [CrossRef]
- Maes, W.; Van Camp, J.; Vermeirssen, V.; Hemeryck, M.; Ketelslegers, J.M.; Schrezenmeir, J.; Van Oostveldt, P.; Huyghebaert, A. Influence of the lactokinin Ala-Leu-Pro-Met-His-Ile-Arg (ALPMHIR) on the release of endothelin-1 by endothelial cells. Regul. Pept. 2004, 118, 105–109. [Google Scholar] [CrossRef]
- Mullally, M.M.; Meisel, H.; Fitzgerald, R.J. Identification of a novel angiotensin-I-converting enzyme inhibitory peptides corresponding to a tryptic fragment of bovine β-lactoglobulin. FEBS Lett. 1997, 402, 99–101. [Google Scholar] [CrossRef] [Green Version]
- Yamada, A.; Sakurai, T.; Ochi, D.; Mitsuyama, E.; Yamauchi, K.; Abe, F. Antihypertensive effect of the bovine casein-derived peptide Met-Lys-Pro. Food Chem. 2015, 172, 441–446. [Google Scholar] [CrossRef]
- Tu, M.; Wang, C.; Chen, C.; Zhang, R.; Liu, H.; Lu, W.; Jiang, L.; Du, M. Identification of a novel ACE-inhibitory peptide from casein and evaluation of the inhibitory mechanisms. Food Chem. 2018, 256, 98–104. [Google Scholar] [CrossRef]
- Hayes, M.; Ross, R.P.; Fitzgerald, G.F.; Hill, C.; Stanton, C. Casein-derived antimicrobial peptides generated by Lactobacillus acidophilus DPC6026. Appl. Environ. Microbiol. 2006, 72, 2260–2264. [Google Scholar] [CrossRef] [Green Version]
- Demers-Mathieu, V.; Gauthier, S.F.; Britten, M.; Fliss, I.; Robitaille, G.; Jean, J. Antibacterial activity of peptides extracted from tryptic hydrolyzate of whey protein by nanofiltration. Int. Dairy J. 2013, 28, 94–101. [Google Scholar] [CrossRef]
- Power, O.; Fernández, A.; Norris, R.; Riera, F.A.; FitzGerald, R.J. Selective enrichment of bioactive properties during ultrafiltration of a tryptic digest of β-lactoglobulin. J. Funct. Foods 2014, 9, 38–47. [Google Scholar] [CrossRef]
- Silveira, S.T.; Martínez-Maqueda, D.; Recio, I.; Hernández-Ledesma, B. Dipeptidyl peptidase-IV inhibitory peptides generated by tryptic hydrolysis of a whey protein concentrate rich in β-lactoglobulin. Food Chem. 2013, 141, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Sedaghati, M.; Ezzatpanah, H.; Mashhadiakbar Boojar, M.; Tajabadi Ebrahimi, M.; Aminafshar, M. Plasmin-digest of β-lactoglobulin with antibacterial properties. Food Agric. Immunol. 2015, 26, 218–230. [Google Scholar] [CrossRef] [Green Version]
- Birkemo, G.A.; O’Sullivan, O.; Ross, R.P.; Hill, C. Antimicrobial activity of two peptides casecidin 15 and 17, found naturally in bovine colostrum. J. Appl. Microbiol. 2009, 106, 233–240. [Google Scholar] [CrossRef]
- Lahov, E.; Regelson, W. Antibacterial and immunostimulating casein-derived substances from milk: Casecidin, isracidin peptides. Food Chem. Toxicol. 1996, 34, 131–145. [Google Scholar] [CrossRef]
- Liu, Y.; Eichler, J.; Pischetsrieder, M. Virtual screening of a milk peptide database for the identification of food-derived antimicrobial peptides. Mol. Nutr. Food Res. 2015, 59, 2243–2254. [Google Scholar] [CrossRef]
- Nagaoka, S.; Futamura, Y.; Miwa, K.; Awano, T.; Yamauchi, K.; Kanamaru, Y.; Tadashi, K.; Kuwata, T. Identification of Novel Hypocholesterolemic Peptides Derived from Bovine Milk β-Lactoglobulin. Biochem. Biophys. Res. Commun. 2001, 281, 11–17. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Z.; Vardhanabhuti, B. Effect of charge density of polysaccharides on self-assembled intragastric gelation of whey protein/polysaccharide under simulated gastric conditions. Food Funct. 2014, 5, 1829–1838. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera: A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Ye, A.; Lin, Q.; Han, J.; Singh, H. Gastric digestion of milk protein ingredients: Study using an in vitro dynamic model. J. Dairy Sci. 2018, 101, 6842–6852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, A.; Cui, J.; Dalgleish, D.; Singh, H. The formation and breakdown of structured clots from whole milk during gastric digestion. Food Funct. 2016, 7, 4259–4266. [Google Scholar] [CrossRef]
- Bohn, T.; Carriere, F.; Day, L.; Deglaire, A.; Egger, L.; Freitas, D.; Golding, M.; Le Feunteun, S.; Macierzanka, A.; Menard, O.; et al. Correlation between in vitro and in vivo data on food digestion. What can we predict with static in vitro digestion models? Crit. Rev. Food Sci. Nutr. 2018, 58, 2239–2261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miguel, M.; Gómez-Ruiz, J.; Recio, I.; Aleixandre, A. Changes in arterial blood pressure after single oral administration of milk-casein-derived peptides in spontaneously hypertensive rats. Mol. Nutr. Food Res. 2010, 54, 1422–1427. [Google Scholar] [CrossRef] [PubMed]
- Almaas, H.; Eriksen, E.; Sekse, C.; Comi, I.; Flengsrud, R.; Holm, H.; Jensen, E.; Jacobsen, M.; Langsrud, T.; Vegarud, G.E. Antibacterial peptides derived from caprine whey proteins, by digestion with human gastrointestinal juice. Br. J. Nutr. 2011, 106, 896–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacroix, I.M.E.; Meng, G.; Cheung, I.W.Y.; Li-Chan, E.C.Y. Do whey protein-derived peptides have dual dipeptidyl-peptidase IV and angiotensin I-converting enzyme inhibitory activities? J. Funct. Foods 2016, 21, 87–96. [Google Scholar] [CrossRef] [Green Version]
- López-Expósito, I.; Minervini, F.; Amigo, L.; Recio, I. Identification of antibacterial peptides from bovine κ-casein. J. Food Prot. 2006, 69, 2992–2997. [Google Scholar] [CrossRef]
- López-Expósito, I.; Quirós, A.; Amigo, L.; Recio, I. Casein hydrolysates as a source of antimicrobial, antioxidant and antihypertensive peptides. Lait 2007, 87, 241–249. [Google Scholar] [CrossRef]
- Sistla, S. Structure-activity relationships of αs-casein peptides with multifunctional biological activities. Mol. Cell. Biochem. 2013, 384, 29–38. [Google Scholar] [CrossRef]
- Pihlanto-leppa, A.; Koskinen, I.; Piilola, K.; Tupasela, T.; Korhonen, H. Angiotensin I-converting enzyme inhibitory properties of whey protein digests: Concentration and characterization of active peptides. J. Dairy Res. 2018, 67, 53–64. [Google Scholar] [CrossRef]
- Smacchi, E.; Gobbetti, M. Peptides from several Italian cheeses inhibitory to proteolytic enzymes of lactic acid bacteria, pseudomonas fluorescens ATCC 948 and to the angiotensin I-converting enzyme. Enzyme Microb. Technol. 1998, 22, 687–694. [Google Scholar] [CrossRef]
- Robitaille, G.; Lapointe, C.; Leclerc, D.; Britten, M. Effect of pepsin-treated bovine and goat caseinomacropeptide on Escherichia coli and Lactobacillus rhamnosus in acidic conditions. J. Dairy Sci. 2012, 95, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gobbetti, M.; Ferranti, P.; Smacchi, E.; Goffredi, F.; Addeo, F. Production of angiotensin-I-converting-enzyme-inhibitory peptides in fermented milks started by Lactobacillus delbrueckii subsp. bulgaricus SS1 and Lactococcus lactis subsp. cremoris FT4. Appl. Environ. Microbiol. 2000, 66, 3898–3904. [Google Scholar] [CrossRef] [Green Version]
- Nongonierma, A.B.; Fitzgerald, R.J. Structure activity relationship modelling of milk protein-derived peptides with dipeptidyl peptidase IV (DPP-IV) inhibitory activity. Peptides 2016, 79, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Chen, R.; Ma, H.; Chen, S. Isolation and Identification of Dipeptidyl Peptidase IV-Inhibitory Peptides from Trypsin/Chymotrypsin-Treated Goat Milk Casein Hydrolysates by 2D-TLC and LC-MS/MS. J. Agric. Food Chem. 2015, 63, 8819–8828. [Google Scholar] [CrossRef]
- Hayes, M.; Stanton, C.; Slattery, H.; O’Sullivan, O.; Hill, C.; Fitzgerald, G.F.; Ross, R.P. Casein fermentate of Lactobacillus animalis DPC6134 contains a range of novel propeptide angiotensin-converting enzyme inhibitors. Appl. Environ. Microbiol. 2007, 73, 4658–4667. [Google Scholar] [CrossRef] [Green Version]
- Quirós, A.; Ramos, M.; Muguerza, B.; Delgado, M.A.; Miguel, M.; Aleixandre, A.; Recio, I. Identification of novel antihypertensive peptides in milk fermented with Enterococcus faecalis. Int. Dairy J. 2007, 17, 33–41. [Google Scholar] [CrossRef]
- Miguel, M.; Recio, I.; Ramos, M.; Delgado, M.A.; Aleixandre, M.A. Antihypertensive effect of peptides obtained from Enterococcus faecalis-fermented milk in rats. J. Dairy Sci. 2006, 89, 3352–3359. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Zhou, F.; Wang, L.; Fengling, B.; Dziugan, P.; Walczak, P.; Zhang, B. Characterization of a bioactive peptide with cytomodulatory effect released from casein. Eur. Food Res. Technol. 2014, 238, 315–322. [Google Scholar] [CrossRef]
- Sandreé, C.; Gleizes, A.; Forestier, F.o.; Gorges-Kergot, R.; Chilmonczyk, S.; Léonil, J.l.; Moreau, M.-C.; Labarre, C. A Peptide Derived from Bovine β-Casein Modulates Functional Properties of Bone Marrow-Derived Macrophages from Germfree and Human Flora-Associated Mice. J. Nutr. 2001, 131, 2936–2942. [Google Scholar] [CrossRef]
- Rojas-Ronquillo, R.; Cruz-Guerrero, A.; Flores-Nájera, A.; Rodríguez-Serrano, G.; Gómez-Ruiz, L.; Reyes-Grajeda, J.P.; Jiménez-Guzmán, J.; García-Garibay, M. Antithrombotic and angiotensin-converting enzyme inhibitory properties of peptides released from bovine casein by Lactobacillus casei Shirota. Int. Dairy J. 2012, 26, 147–154. [Google Scholar] [CrossRef]
- Yamamoto, N.; Akino, A.; Takano, T. Antihypertensive Effect of the Peptides Derived from Casein by an Extracellular Proteinase from Lactobacillus helveticus CP790. J. Dairy Sci. 1994, 77, 917–922. [Google Scholar] [CrossRef]
- Saito, T.; Nakamura, T.; Kitazawa, H.; Kawai, Y.; Itoh, T. Isolation and structural analysis of antihypertensive peptides that exist naturally in Gouda cheese. J. Dairy Sci. 2000, 83, 1434–1440. [Google Scholar] [CrossRef]
- Asano, M.; Nio, N.; Ariyoshi, Y. Inhibition of Prolyl Endopeptidase by Synthetic β-Casein Peptides and Their Derivatives with a C-Terminal Prolinol or Prolinal. Biosci. Biotechnol. Biochem. 1992, 56, 976–977. [Google Scholar] [CrossRef] [PubMed]
- Eisele, T.; Stressler, T.; Kranz, B.; Fischer, L. Bioactive peptides generated in an enzyme membrane reactor using Bacillus lentus alkaline peptidase. Eur. Food Res. Technol. 2013, 236, 483–490. [Google Scholar] [CrossRef] [Green Version]
- Minervini, F.; Algaron, F.; Rizzello, C.G.; Fox, P.F.; Monnet, V.; Gobbetti, M. Angiotensin I-converting-enzyme-inhibitory and antibacterial peptides from Lactobacillus helveticus PR4 proteinase-hydrolyzed caseins of milk from six species. Appl. Environ. Microbiol. 2003, 69, 5297–5305. [Google Scholar] [CrossRef] [Green Version]
- Otte, J.; Shalaby, S.M.A.; Zakora, M.; Nielsen, M.S. Fractionation and identification of ACE-inhibitory peptides from α-lactalbumin and β-casein produced by thermolysin-catalysed hydrolysis. Int. Dairy J. 2007, 17, 1460–1472. [Google Scholar] [CrossRef]
- Pihlanto, A.; Virtanen, T.; Korhonen, H. Angiotensin I converting enzyme (ACE) inhibitory activity and antihypertensive effect of fermented milk. Int. Dairy J. 2010, 20, 3–10. [Google Scholar] [CrossRef]
- Quirós, A.; Hernández-Ledesma, B.; Ramos, M.; Amigo, L.; Recio, I. Angiotensin-converting enzyme inhibitory activity of peptides derived from caprine kefir. J. Dairy Sci. 2005, 88, 3480–3487. [Google Scholar] [CrossRef] [Green Version]
- Uenishi, H.; Kabuki, T.; Seto, Y.; Serizawa, A.; Nakajima, H. Isolation and identification of casein-derived dipeptidyl-peptidase 4 (DPP-4)-inhibitory peptide LPQNIPPL from gouda-type cheese and its effect on plasma glucose in rats. Int. Dairy J. 2012, 22, 24–30. [Google Scholar] [CrossRef]
- Sagardia, I.; Iloro, I.; Elortza, F.; Bald, C. Quantitative structure-activity relationship based screening of bioactive peptides identified in ripened cheese. Int. Dairy J. 2013, 33, 184–190. [Google Scholar] [CrossRef]
- Lu, Y.; Govindasamy-Lucey, S.; Lucey, J.A. Angiotensin-I-converting enzyme-inhibitory peptides in commercial Wisconsin Cheddar cheeses of different ages. J. Dairy Sci. 2016, 99, 41–52. [Google Scholar] [CrossRef] [PubMed]
- del Mar Contreras, M.; Sanchez, D.; Sevilla, M.Á.; Recio, I.; Amigo, L. Resistance of casein-derived bioactive peptides to simulated gastrointestinal digestion. Int. Dairy J. 2013, 32, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Silva, S.V.; Pihlanto, A.; Malcata, F.X. Bioactive peptides in ovine and caprine cheeselike systems prepared with proteases from Cynara cardunculus. J. Dairy Sci. 2006, 89, 3336–3344. [Google Scholar] [CrossRef] [Green Version]





| Ca | Fe | K | Mg | Na | S | |
|---|---|---|---|---|---|---|
| κ-CGN | 0.130 ± 0.007 | Non detected | 62.04 ± 3.573 | 0.144 ± 0.005 | 3.850 ± 0.014 | 46.75 ± 1.040 |
| ι-CGN | 0.820 ± 0.031 | 0.004 ± 0.000 | 23.31 ± 1.043 | 0.327 ± 0.068 | 22.61 ± 1.527 | 74.85 ± 2.200 |
| λ-CGN | 3.905 ± 0.249 | Non detected | 7.535 ± 0.481 | 1.913 ± 0.439 | 17.11 ± 1.514 | 58.90 ± 0.000 |
| XG | 6.820 ± 0.140 | Non detected | 0.480 ± 0.170 | 0.070 ± 0.000 | 3.630 ± 0.130 | 0.950 ± 0.060 |
| Volumes Used from Stock Solutions | |||||
|---|---|---|---|---|---|
| Compound | Stock Solutions [g/L] | Saliva [mL] | SGF [mL] | SDF [mL] | SBF [mL] |
| KCl | 46.72 | 20.00 | 56.00 | 10.80 | 10.80 |
| KH2PO4 | 68.00 | 40.00 | 1.800 | 1.600 | 35.80 |
| NaHCO3 | 84.00 | 8.000 | 26.00 | 87.00 | 19.00 |
| NaCl | 120.0 | 2.000 | 20.00 | 15.08 | 16.00 |
| MgCl2(H2O)6 | 30.00 | 2.000 | 4.000 | 2.200 | 2.200 |
| NH4Cl | 27.28 | --- | 2.000 | --- | --- |
| NaH2PO4(H2O)2 | 166.0 | --- | --- | --- | 20.00 |
| Urea | 22.50 | 10.00 | 0.600 | 4.800 | 10.40 |
| DDW | 918.0 | 889.6 | 878.5 | 885.8 | |
| pH adjustment | 6.800 | 1.300 | 8.100 | 8.200 | |
| CaCl2(H2O)2 2 M [µL per mL of simulated fluid] | 0.273 | 0.180 | 1.200 | 1.850 | |
| Peptide # | Sequence | Known Bioactive Peptide | Activity | %Homology | References | R | &κ | &ι | &λ | &X |
|---|---|---|---|---|---|---|---|---|---|---|
| P1 | AHKALCSEKL | LAHKALCSEKL | DPP-IV Inhibitory | 90.0 | [55] | + | ||||
| P2 | ALPMHIRL | ALPMHIR ALPMHIR ALPMHIR | stimulates proliferation Reduced vasoconstrictorendothelin-1 release ACE-inhibitory | 87.5 87.5 87.5 | [56,57,58,59] | + | ||||
| P3 | KPTPEGDL | LKPTPEGDLE LKPTPEGDL | DPP-IV Inhibitory DPP-IV Inhibitory | 80.0 88.8 | [55] | + | + | + | + | |
| P4 | LAHKALCSEKL | LAHKALCSEKL | DPP-IV Inhibitory | 100 | [55] | + | + | + | + | |
| P5 | NMAINPSKENLCSTF | NMAINPSKENLCSTFCK | ACE-inhibitory | 88.2 | [60] | + | + | + | + | |
| P6 | SDIPNPIGSENSEKT | SDIPNPIGSENSEK | Antimicrobial | 93.3 | [61] | + | + | + | ||
| P7 | VRTPEVDDEAL | TPEVDDEALEK TPEVDDEALEK | DPP-IV Inhibitory Antimicrobial | 81.8 81.8 | [62,63,64] | + | + | + | + | |
| Total number | 7 | 5 | ND | 4 | 5 |
| Peptide # | Sequence | Known Bioactive Peptide | Activity | %Homology | References | R | &κ | &ι | &λ | &X |
|---|---|---|---|---|---|---|---|---|---|---|
| P8 | KIDALNENKV | IDALNENK IDALNENK | stimulates proliferation antimicrobial | 80.0 80.0 | [56,62,65] | + | ||||
| P9 | RPKHPIKHQGLPQEVLNENL | RPKHPIKHQGLPQEVLNENLLRFF RPKHPIKHQGLPQEVLNENLLRF | Antimicrobial Antimicrobial | 83.3 86.9 | [66,67,68] | + | + | |||
| P10 | RVYVEELKPTPEGDLEIL | VYVEELKPTPEGDLEILLQK | Hypocholesterolemic | 85.0 | [69] | + |
| Sequence | Score | R | &κ | &ι | &λ | &X |
|---|---|---|---|---|---|---|
| DRTPPFYCLCPEGF | 0.88 | + | + | + | + | + |
| DSWPCVMGR | 0.92 | + | ||||
| FGKNGKNCPDKFCL | 0.88 | + | + | + | + | + |
| FGSPPGQRDLL | 0.80 | + | + | + | + | + |
| FSQSCAPGADPKSRL | 0.82 | + | + | + | + | + |
| GGVSLPEWVCTTF | 0.84 | + | + | + | + | + |
| VRETCGCCDCEKRCGAL | 0.82 | + | + | + | + | + |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
David, S.; Magram Klaiman, M.; Shpigelman, A.; Lesmes, U. Addition of Anionic Polysaccharide Stabilizers Modulates In Vitro Digestive Proteolysis of a Chocolate Milk Drink in Adults and Children. Foods 2020, 9, 1253. https://doi.org/10.3390/foods9091253
David S, Magram Klaiman M, Shpigelman A, Lesmes U. Addition of Anionic Polysaccharide Stabilizers Modulates In Vitro Digestive Proteolysis of a Chocolate Milk Drink in Adults and Children. Foods. 2020; 9(9):1253. https://doi.org/10.3390/foods9091253
Chicago/Turabian StyleDavid, Shlomit, Maya Magram Klaiman, Avi Shpigelman, and Uri Lesmes. 2020. "Addition of Anionic Polysaccharide Stabilizers Modulates In Vitro Digestive Proteolysis of a Chocolate Milk Drink in Adults and Children" Foods 9, no. 9: 1253. https://doi.org/10.3390/foods9091253