Non-Destructive Technologies for Detecting Insect Infestation in Fruits and Vegetables under Postharvest Conditions: A Critical Review
Abstract
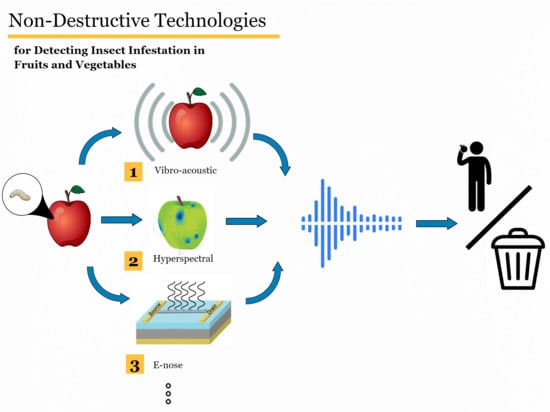
:1. Introduction
2. Traditional Manual Methods
3. Noninvasive Methods
3.1. Spectroscopic Techniques
3.2. Visible Light Sensing
3.3. Imaging Techniques
3.3.1. Hyperspectral Imaging Systems
3.3.2. X-ray Imaging
3.3.3. Magnetic Resonance Imaging (MRI)
3.3.4. Thermal Imaging
4. Acoustic Techniques for Insect Infestation Detection
5. E-Nose and E-Tongue
6. Critical Comparison between Different Noninvasive Methods
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Padayachee, A.; Day, L.; Howell, K.; Gidley, M.J. Complexity and health functionality of plant cell wall fibers from fruits and vegetables. J. Crit. Rev. Food Sci. 2017, 57, 59–81. [Google Scholar] [CrossRef] [PubMed]
- Gherini, A. Gen-Z Is About to Outnumber Millennials. Here’s How That Will Affect the Business World. Available online: https://www.inc.com/anne-gherini/gen-z-is-about-to-outnumber-millennials-heres-how-that-will-affect-business-world.html (accessed on 22 August 2018).
- Lu, Y.; Huang, Y.; Lu, R. Innovative Hyperspectral Imaging-Based Techniques for Quality Evaluation of Fruits and Vegetables: A Review. Appl. Sci. 2017, 7, 189. [Google Scholar] [CrossRef]
- Suktanarak, S.; Teerachaichayut, S. Non-destructive quality assessment of hens’ eggs using hyperspectral images. J. Food Eng. 2017, 215, 97–103. [Google Scholar] [CrossRef]
- Badii, K.; Billah, M.; Afreh-Nuamah, K.; Obeng-Ofori, D.; Nyarko, G. Review of the pest status, economic impact and management of fruit-infesting flies (Diptera: Tephritidae) in Africa. Afr. J. Agric. Res. 2015, 10, 1488–1498. [Google Scholar] [CrossRef] [Green Version]
- Pimentel, D.; Zuniga, R.; Morrison, D. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol. Econ. 2005, 52, 273–288. [Google Scholar] [CrossRef]
- Gulati, P.; Weier, S.A.; Santra, D.; Subbiah, J.; Rose, D.J. Effects of feed moisture and extruder screw speed and temperature on physical characteristics and antioxidant activity of extruded proso millet (P Anicum Miliaceum) flour. Int. J. Food Sci. 2016, 51, 114–122. [Google Scholar] [CrossRef]
- Liu, H.; Lee, S.-H.; Chahl, J.S. A review of recent sensing technologies to detect invertebrates on crops. Precis. Agric. 2017, 18, 635–666. [Google Scholar] [CrossRef]
- Greenwood, P. American Horticultural Society Pests & Diseases: Pests and Diseases; Dorling Kindersley: London, UK, 2000. [Google Scholar]
- Rady, A.; Ekramirad, N.; Adedeji, A.; Li, M.; Alimardani, R. Hyperspectral imaging for detection of codling moth infestation in GoldRush apples. Postharvest Biol. Technol. 2017, 129, 37–44. [Google Scholar] [CrossRef]
- USDA Annual Report. Available online: https://www.aphis.usda.gov/publications/plant_health/report-ppq-2017.pdf (accessed on 10 May 2018).
- USDA Annual Report. Available online: https://www.aphis.usda.gov/publications/plant_health/report-ppq-2016.pdf (accessed on 10 March 2017).
- Moscetti, R.; Haff, R.P.; Stella, E.; Contini, M.; Monarca, D.; Cecchini, M.; Massantini, R. Feasibility of NIR spectroscopy to detect olive fruit infested by Bactrocera oleae. Postharvest Biol. Technol. 2015, 99, 58–62. [Google Scholar] [CrossRef]
- Peshlov, B.N.; Dowell, F.E.; Drummond, F.A.; Donahue, D.W. Comparison of three near infrared spectrophotometers for infestation detection in wild blueberries using multivariate calibration models. J. Near Infrared Spectrosc. 2009, 17, 203–212. [Google Scholar] [CrossRef]
- Saranwong, S.; Haff, R.P.; Thanapase, W.; Janhiran, A.; Kasemsumran, S.; Kawano, S. A feasibility study using simplified near infrared imaging to detect fruit fly larvae in intact fruit. J. Near Infrared Spectrosc. 2011, 19, 55–60. [Google Scholar] [CrossRef]
- Wang, J.; Nakano, K.; Ohashi, S.; Takizawa, K.; He, J. Comparison of different modes of visible and near-infrared spectroscopy for detecting internal insect infestation in jujubes. J. Food Eng. 2010, 101, 78–84. [Google Scholar] [CrossRef]
- Li, M.; Ekramirad, N.; Rady, A.M.; Adedeji, A. Application of Acoustic Emission and Machine Learning to Detect Codling Moth Infested Apples. Trans. ASABE (Am. Soc. Agric. Biol. Eng.) 2018, 61. [Google Scholar] [CrossRef]
- Liljedahl, L.; Abbott, J. Changes in Sonic Resonance of ‘Delicious’ and ‘Golden Delicious’ Apples Undergoing Accelerated Ripening. Trans. ASAE 1994, 37, 907–912. [Google Scholar] [CrossRef]
- Mankin, R.W.; Hagstrum, D.W.; Smith, M.T.; Roda, A.L.; Kairo, M.T.K. Perspective and Promise: A Century of Insect Acoustic Detection and Monitoring. Am. Entomol. 2011, 57, 30–44. [Google Scholar] [CrossRef]
- Blasco, J.; Munera, S.; Aleixos, N.; Cubero, S.; Molto, E. Machine Vision-Based Measurement Systems for Fruit and Vegetable Quality Control in Postharvest. In Measurement, Modeling and Automation in Advanced Food Processing; Springer: Cham, Switzerland, 2017; pp. 71–91. [Google Scholar]
- Cen, H.; Lu, R.; Ariana, D.P.; Mendoza, F. Hyperspectral Imaging-Based Classification and Wavebands Selection for Internal Defect Detection of Pickling Cucumbers. Food Bioprocess Technol. 2013, 7, 1689–1700. [Google Scholar] [CrossRef]
- Zhang, L.; McCarthy, M.J. Assessment of pomegranate postharvest quality using nuclear magnetic resonance. Postharvest Biol. Technol. 2013, 77, 59–66. [Google Scholar] [CrossRef]
- Chuang, C.-L.; Ouyang, C.-S.; Lin, T.-T.; Yang, M.-M.; Yang, E.-C.; Huang, T.-W.; Kuei, C.-F.; Luke, A.; Jiang, J.-A. Automatic X-ray quarantine scanner and pest infestation detector for agricultural products. Computers 2011, 77, 41–59. [Google Scholar] [CrossRef]
- Haff, R.P.; Toyofuku, N. X-ray detection of defects and contaminants in the food industry. Sens. Instrum. Food Qual. Saf. 2008, 2, 262–273. [Google Scholar] [CrossRef]
- Burns, D.A.; Ciurczak, E.W. Handbook of Near-Infrared Analysis; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Nicolaï, B.M.; Defraeye, T.; De Ketelaere, B.; Herremans, E.; Hertog, M.L.; Saeys, W.; Torricelli, A.; Vandendriessche, T.; Verboven, P. Nondestructive measurement of fruit and vegetable quality. Annu. Rev. Food Sci. Technol. 2014, 5, 285–312. [Google Scholar] [CrossRef] [Green Version]
- Rajendran, S. Detection of insect infestation in stored foods. Adv. Food Nutr. Res. 2005, 49, 163–232. [Google Scholar]
- Sun, D.-W. Hyperspectral Imaging for Food Quality Analysis and Control; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Mehle, N.; Trdan, S. Traditional and modern methods for the identification of thrips (Thysanoptera) species. J. Pest Sci. 2012, 85, 179–190. [Google Scholar] [CrossRef]
- Lakshmi, S.; Pandey, A.; Ravi, N.; Chauhan, O.; Gopalan, N.; Sharma, R. Non-destructive quality monitoring of fresh fruits and vegetables. Def. Life Sci. J. 2017, 2, 103–110. [Google Scholar]
- Xing, J.; Guyer, D.; Ariana, D.; Lu, R. Determining optimal wavebands using genetic algorithm for detection of internal insect infestation in tart cherry. Sens. Instrum. Food Qual. Saf. 2008, 2, 161–167. [Google Scholar] [CrossRef]
- Burks, C.; Dowell, F.; Xie, F. Measuring fig quality using near-infrared spectroscopy. J. Stored Prod. Res. 2000, 36, 289–296. [Google Scholar] [CrossRef]
- Sirisomboon, P.; Hashimoto, Y.; Tanaka, M. Study on non-destructive evaluation methods for defect pods for green soybean processing by near-infrared spectroscopy. J. Food Eng. 2009, 93, 502–512. [Google Scholar] [CrossRef]
- Moscetti, R.; Haff, R.P.; Saranwong, S.; Monarca, D.; Cecchini, M.; Massantini, R. Nondestructive detection of insect infested chestnuts based on NIR spectroscopy. Postharvest Biol. Technol. 2014, 87, 88–94. [Google Scholar] [CrossRef]
- Singh, C.B.; Jayas, D.S.; Paliwal, J.; White, N.D. Identification of insect-damaged wheat kernels using short-wave near-infrared hyperspectral and digital colour imaging. Comput. Electron. Agric. 2010, 73, 118–125. [Google Scholar] [CrossRef]
- Jamshidi, B. Ability of near-infrared spectroscopy for non-destructive detection of internal insect infestation in fruits: Meta-analysis of spectral ranges and optical measurement modes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 225, 117479. [Google Scholar] [CrossRef]
- Jamshidi, B.; Mohajerani, E.; Farazmand, H.; Mahmoudi, A.; Hemmati, A. Pattern recognition-based optical technique for non-destructive detection of Ectomyelois ceratoniae infestation in pomegranates during hidden activity of the larvae. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 206, 552–557. [Google Scholar] [CrossRef]
- Chen, P.; Sun, Z. A review of non-destructive methods for quality evaluation and sorting of agricultural products. J. Agric. Eng. Res. 1991, 49, 85–98. [Google Scholar] [CrossRef]
- Patel, K.K.; Kar, A.; Jha, S.; Khan, M. Machine vision system: A tool for quality inspection of food and agricultural products. J. Food Sci. Technol. 2012, 49, 123–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blasco, J.; Aleixos, N.; Gómez, J.; Moltó, E. Citrus sorting by identification of the most common defects using multispectral computer vision. J. Food Eng. 2007, 83, 384–393. [Google Scholar] [CrossRef]
- Blasco, J.; Aleixos, N.; Moltó, E. Computer vision detection of peel defects in citrus by means of a region oriented segmentation algorithm. J. Food Eng. 2007, 81, 535–543. [Google Scholar] [CrossRef]
- Blasco, J.; Aleixos, N.; Gómez-Sanchis, J.; Moltó, E. Recognition and classification of external skin damage in citrus fruits using multispectral data and morphological features. Biosyst. Eng. 2009, 103, 137–145. [Google Scholar] [CrossRef]
- López-García, F.; Andreu-García, G.; Blasco, J.; Aleixos, N.; Valiente, J.-M. Automatic detection of skin defects in citrus fruits using a multivariate image analysis approach. Comput. Electron. Agric. 2010, 71, 189–197. [Google Scholar] [CrossRef]
- Lòpez, F.; Prats-Montalbán, J.; Ferrer, A.; González, J.M. Defect Detection in Random Colour Textures Using the MIA T2 Defect Maps. In Proceedings of the International Conference Image Analysis and Recognition, Póvoa de Varzim, Portugal, 18–20 September 2006; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2006; pp. 752–763. [Google Scholar] [CrossRef]
- Blasco, J.; Cubero, S.; Moltó, E. Quality evaluation of citrus fruits. In Computer Vision Technology for Food Quality Evaluation; Elsevier: Amsterdam, The Netherlands, 2016; pp. 305–325. [Google Scholar]
- Pearson, T.; Doster, M.; Michailides, T. Automated detection of pistachio defects by machine vision. Appl. Eng. Agric. 2001, 17, 729. [Google Scholar] [CrossRef]
- Cubero, S.; Aleixos, N.; Moltó, E.; Gómez-Sanchis, J.; Blasco, J. Advances in machine vision applications for automatic inspection and quality evaluation of fruits and vegetables. Food 2011, 4, 487–504. [Google Scholar] [CrossRef]
- Ruiz-Altisent, M.; Ruiz-Garcia, L.; Moreda, G.; Lu, R.; Hernandez-Sanchez, N.; Correa, E.; Diezma, B.; Nicolaï, B.; García-Ramos, J. Sensors for product characterization and quality of specialty crops—A review. Comput. Electron. Agric. 2010, 74, 176–194. [Google Scholar] [CrossRef] [Green Version]
- Del Fiore, A.; Reverberi, M.; Ricelli, A.; Pinzari, F.; Serranti, S.; Fabbri, A.; Bonifazi, G.; Fanelli, C. Early detection of toxigenic fungi on maize by hyperspectral imaging analysis. Int. J. Food Microbiol. 2010, 144, 64–71. [Google Scholar] [CrossRef]
- Ekramirad, N.; Adedeji, A.A.; Alimardani, R. A review of non-destructive methods for detection of insect infestation in fruits and vegetables. Innov. Food Res. 2016, 2, 6–12. [Google Scholar]
- Lorente, D.; Aleixos, N.; Gómez-Sanchís, J.; Cubero, S.; García-Navarrete, O.; Blasco, J. Recent Advances and Applications of Hyperspectral Imaging for Fruit and Vegetable Quality Assessment. Food Bioprocess Technol. 2011, 5, 1121–1142. [Google Scholar] [CrossRef]
- Pu, Y.Y.; Feng, Y.Z.; Sun, D.W. Recent progress of hyperspectral imaging on quality and safety inspection of fruits and vegetables: A review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 176–188. [Google Scholar] [CrossRef]
- Rady, A.; Adedeji, A. Assessing different processed meats for adulterants using visible-near-infrared spectroscopy. Meat Sci. 2018, 136, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Wan, X.; Zhang, M.; Zhu, Q. Detection of insect-damaged vegetable soybeans using hyperspectral transmittance image. J. Food Eng. 2013, 116, 45–49. [Google Scholar] [CrossRef]
- Kaliramesh, S.; Chelladurai, V.; Jayas, D.; Alagusundaram, K.; White, N.; Fields, P. Detection of infestation by Callosobruchus maculatus in mung bean using near-infrared hyperspectral imaging. J. Stored Prod. Res. 2013, 52, 107–111. [Google Scholar] [CrossRef]
- Hamidisepehr, A.; Sama, M.P.; Turner, A.P.; Wendroth, O.O. A method for reflectance index wavelength selection from moisture-controlled soil and crop residue samples. Trans. ASABE 2017, 60, 1479–1487. [Google Scholar] [CrossRef]
- Wang, J.; Nakano, K.; Ohashi, S.; Kubota, Y.; Takizawa, K.; Sasaki, Y. Detection of external insect infestations in jujube fruit using hyperspectral reflectance imaging. Biosyst. Eng. 2011, 108, 345–351. [Google Scholar] [CrossRef]
- Mireei, S.A.; Amini-Pozveh, S.; Nazeri, M. Selecting optimal wavelengths for detection of insect infested tomatoes based on SIMCA-aided CFS algorithm. Postharvest Biol. Technol. 2017, 123, 22–32. [Google Scholar] [CrossRef]
- Li, J.; Rao, X.; Ying, Y. Detection of common defects on oranges using hyperspectral reflectance imaging. Comput. Electron. Agric. 2011, 78, 38–48. [Google Scholar] [CrossRef]
- Qin, J.; Burks, T.F.; Ritenour, M.A.; Bonn, W.G. Detection of citrus canker using hyperspectral reflectance imaging with spectral information divergence. J. Food Eng. 2009, 93, 183–191. [Google Scholar] [CrossRef]
- Liu, G.; He, J.; Wang, S.; Luo, Y.; Wang, W.; Wu, L.; Si, Z.; He, X. Application of Near-Infrared Hyperspectral Imaging for Detection of External Insect Infestations on Jujube Fruit. Int. J. Food Prop. 2015, 19, 41–51. [Google Scholar] [CrossRef]
- Haff, R.P.; Saranwong, S.; Thanapase, W.; Janhiran, A.; Kasemsumran, S.; Kawano, S. Automatic image analysis and spot classification for detection of fruit fly infestation in hyperspectral images of mangoes. J. Near Infrared Spectrosc. 2013, 86, 23–28. [Google Scholar] [CrossRef]
- Yang, C.-C.; Kim, M.S.; Millner, P.; Chao, K.; Cho, B.-K.; Mo, C.; Lee, H.; Chan, D.E. Development of multispectral imaging algorithm for detection of frass on mature red tomatoes. Postharvest Biol. Technol. 2014, 93, 1–8. [Google Scholar] [CrossRef]
- Ma, Y.; Huang, M.; Yang, B.; Zhu, Q. Automatic threshold method and optimal wavelength selection for insect-damaged vegetable soybean detection using hyperspectral images. Comput. Electron. Agric. 2014, 106, 102–110. [Google Scholar] [CrossRef]
- Li, H.; Chen, Q.; Zhao, J.; Wu, M. Nondestructive detection of total volatile basic nitrogen (TVB-N) content in pork meat by integrating hyperspectral imaging and colorimetric sensor combined with a nonlinear data fusion. Lwt-Food Sci. Technol. 2015, 63, 268–274. [Google Scholar] [CrossRef]
- Li, H.; Sun, X.; Pan, W.; Kutsanedzie, F.; Zhao, J.; Chen, Q. Feasibility study on nondestructively sensing meat’s freshness using light scattering imaging technique. Meat Sci. 2016, 119, 102–109. [Google Scholar] [CrossRef]
- Kubat, M. An Introduction to Machine Learning; Springer Publishing Company, Incorporated: New York, NY, USA, 2015. [Google Scholar]
- Pourkhak, B.; Mireei, S.A.; Sadeghi, M.; Hemmat, A. Multi-sensor data fusion in the nondestructive measurement of kiwifruit texture. Measurement 2017, 101, 157–165. [Google Scholar] [CrossRef]
- Adedeji, A.A.; Ngadi, M.O. 3-D Imaging of Deep-Fat Fried Chicken Nuggets Breading Coating Using X-Ray Micro-CT. Food Process Eng. 2009, 5. [Google Scholar] [CrossRef]
- Adedeji, A. Microstructural characterization of deep-fat fried breaded chicken nuggets using X-ray micro-computed tomography. Food Process Eng. 2011, 34. [Google Scholar] [CrossRef]
- Ammari, H. An Introduction to Mathematics of Emerging Biomedical Imaging; Springer: Berlin/Heidelberg, Germany, 2008; Volume 62. [Google Scholar]
- Kotwaliwale, N.; Singh, K.; Kalne, A.; Jha, S.N.; Seth, N.; Kar, A. X-ray imaging methods for internal quality evaluation of agricultural produce. J. Food Sci. Technol. 2014, 51, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Nylund, R.; Lutz, J. Separation of hollow heart potato tubers by means of size grading, specific gravity, and x-ray examination. Am. Potato J. 1950, 27, 214–222. [Google Scholar] [CrossRef]
- Follett, P.A.; Armstrong, J.W. Revised irradiation doses to control melon fly, Mediterranean fruit fly, and oriental fruit fly (Diptera: Tephritidae) and a generic dose for tephritid fruit flies. J. Econ. Entomol. 2004, 97, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- Lammertyn, J.; Dresselaers, T.; Van Hecke, P.; Jancsók, P.; Wevers, M.; Nicolai, B. MRI and X-ray CT study of spatial distribution of core breakdown in ‘Conference’pears. Magn. Reson. Imaging 2003, 21, 805–815. [Google Scholar] [CrossRef]
- Al-Mezeini, N.; Manickavasagan, A.; Al-Yahyai, R.; Al-Wahaibi, A.; Al-Raeesi, A.; Khriji, L. X-ray Imaging of Stored Dates to Detect Infestation by Saw-Toothed Beetles. Int. J. Fruit Sci. 2015, 16. [Google Scholar] [CrossRef]
- Veena, T.; Chidanand, D.; Alagusundaram, K. Quality analysis of mango fruit with fruit fly insect by non-destructive soft X-ray method. Int. J. Agric. Sci. Res. 2015, 5, 37–45. [Google Scholar]
- Haff, R.P.; Jackson, E.S.; Moscetti, R.; Massantini, R. Detection of fruit-fly infestation in olives using x-ray imaging: Algorithm development and prospects. Am. J. Agric. Sci. Technol. 2015, 4, 1–8. [Google Scholar] [CrossRef]
- Haff, R.P.; Pearson, T.C. An automatic algorithm for detection of infestations in X-ray images of agricultural products. Sens. Instrum. Food Qual. Saf. 2007, 1, 143–150. [Google Scholar] [CrossRef]
- Hansen, J.D.; Schlaman, D.W.; Haff, R.P.; Yee, W.L. Potential postharvest use of radiography to detect internal pests in deciduous tree fruits. J. Entomol. Sci. 2005, 40, 255–262. [Google Scholar] [CrossRef]
- Velasco, L.; Medina, C. Soft X-ray imaging for non-destructive detection of the mango pulp weevil (Stenochetus frigidus (Fabr.) infestation in fresh greencarabao’mango fruits. Philipp. Agric. Sci. 2004, 87, 160–164. [Google Scholar]
- Schatzki, T.; Haff, R.; Young, R.; Can, I.; Le, L.; Toyofuku, N. Defect detection in apples by means of X-ray imaging. Trans. ASAE 1997, 40, 1407–1415. [Google Scholar] [CrossRef]
- Keagy, P.M.; Parvin, B.; Schatzki, T.F. Machine recognition of navel orange worm damage in X-ray images of pistachio nuts. Lwt-Food Sci. Technol. 1996, 29, 140–145. [Google Scholar] [CrossRef] [Green Version]
- Sim, A.; Parvin, B.; Keagy, P. Invariant representation and hierarchical network for inspection of nuts from X-ray images. Int. J. Imaging Syst. Technol. 1996, 7, 231–237. [Google Scholar] [CrossRef]
- Thomas, P.; Kannan, A.; Degwekar, V.; Ramamurthy, M. Non-destructive detection of seed weevil-infested mango fruits by X-ray imaging. Postharvest Biol. Technol. 1995, 5, 161–165. [Google Scholar] [CrossRef]
- Haishi, T.; Koizumi, H.; Arai, T.; Koizumi, M.; Kano, H. Rapid detection of infestation of apple fruits by the peach fruit moth, Carposina sasakii Matsumura, larvae using a 0.2-T dedicated magnetic resonance imaging apparatus. Appl. Magn. Reson. 2011, 41, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, M.T.R. Transmisión Optica e Imagen en Visible e Infrarrojo en Frutas. Ensayo de Equipos Comerciales. Ph.D. Thesis, Universidad Politécnica de Madrid, Madrid, Spain, 2008. [Google Scholar]
- Hansen, J.D.; Carlton, R.; Adams, S.; Lacey, L.A. Infrared detection of internal feeders of deciduous tree fruits. J. Entomol. Sci. 2008, 43, 52–56. [Google Scholar] [CrossRef]
- Chelladurai, V.; Karuppiah, K.; Jayas, D.; Fields, P.; White, N. Detection of Callosobruchus maculatus (F.) infestation in soybean using soft X-ray and NIR hyperspectral imaging techniques. J. Stored Prod. Res. 2014, 57, 43–48. [Google Scholar] [CrossRef]
- Torres, I.D.A. Estudio, Aplicación y Propuesta de Automatización del Procesamiento de Imágenes Por Resonancia Magnética Para la Evaluación y Detección de Defectos Internos de Calidad en Cítricos y Melocotones. Ph.D. Thesis, Universitat Politècnica de València, Valencia, Spain, 2006. [Google Scholar]
- O’Donnell, C.P.; Fagan, C.; Cullen, P.J. Process Analytical Technology for the Food Industry; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Chen, Y.; Yang, W.; Li, M.; Hao, Z.; Zhou, P.; Sun, H. Research on pest image processing method based on Android thermal infrared lens. IFAC-PapersOnLine 2018, 51, 173–178. [Google Scholar] [CrossRef]
- Ishimwe, R.; Abutaleb, K.; Ahmed, F. Applications of thermal imaging in agriculture—A review. Adv. Remote Sens. 2014, 3, 128. [Google Scholar] [CrossRef] [Green Version]
- Zdunek, A.; Bednarczyk, J. Effect of mannitol treatment on ultrasound emission during texture profile analysis of potato and apple tissue. J. Texture Stud. 2006, 37, 339–359. [Google Scholar] [CrossRef]
- Soroker, V.; Nakache, Y.; Landau, U.; Mizrach, A.; Hetzroni, A.; Gerling, D. Note: Utilization of sounding methodology to detect infestation by Rhynchophorus ferrugineus on palm offshoots. Phytoparasitica 2004, 32, 6–8. [Google Scholar]
- Banga, K.S.; Kotwaliwale, N.; Mohapatra, D.; Giri, S.K.; Babu, V.B. Bioacoustic detection of Callosobruchus chinensis and Callosobruchus maculatus in bulk stored chickpea (Cicer arietinum) and green gram (Vigna radiata). Food Control 2019, 104, 278–287. [Google Scholar] [CrossRef]
- Cox, T.J. The Acoustic Emissions Produced by Escherichia Coli During the Growth Cycle. Master’s Thesis, Animal and Food Sciences, Agriculture, Food and Environment, Lexington, KY, USA, 2014. [Google Scholar]
- Muravin, B. Acoustic emission science and technology. J. Build. Infrastruct. Eng. Isr. Assoc. Eng. Archit. 2009, 1, 4–5. [Google Scholar]
- Yang, S.F.; Xue, L.; Zhao, J.M. Detecting system of crop disease stress based on acoustic emission and virtual technology. Appl. Mech. Mater. 2014, 556, 3331. [Google Scholar] [CrossRef]
- Meng, L. Acoustic Emission of Lactococcus lactis ssp. lactis C2 Infested with Three Bacteriophages c2, sk1 and ml3. Master’s Thesis, Animal and Food Sciences, Agriculture, Food and Environment, Lexington, KY, USA, 2016. [Google Scholar]
- Ghosh, D.; Stencel, J.M.; Hicks, C.D.; Payne, F.; Ozevin, D. Acoustic Emission Signal of Lactococcus lactis before and after Inhibition with NaN 3 and Infection with Bacteriophage c2. ISRN Microbiol. 2013, 2013, 257313. [Google Scholar] [CrossRef]
- Mao, J.; Yu, Y.; Rao, X.; Wang, J. Firmness prediction and modeling by optimizing acoustic device for watermelons. J. Food Eng. 2016, 168, 1–6. [Google Scholar] [CrossRef]
- Swietlicka, I.; Muszynski, S.; Marzec, A. Extruded bread classification on the basis of acoustic emission signal with application of artificial neural networks. Int. Agrophysics 2015, 29. [Google Scholar] [CrossRef]
- Zdunek, A.; Cybulska, J.; Konopacka, D.; Rutkowski, K. Evaluation of apple texture with contact acoustic emission detector: A study on performance of calibration models. J. Food Eng. 2011, 106, 80–87. [Google Scholar] [CrossRef]
- Ekramirad, N.; Chadwick, A.P.; Villanueva, R.T.; Donohue, K.D.; Adedeji, A.A. Low Frequency Signal Patterns for Codling Moth Larvae Activity in Apples. 2020. Available online: https://elibrary.asabe.org/abstract.asp?aid=51527 (accessed on 25 June 2020).
- Mankin, R.; Moore, A. Acoustic Detection of Oryctes rhinoceros (Coleoptera: Scarabaeidae: Dynastinae) and Nasutitermes luzonicus (Isoptera: Termitidae) in Palm Trees in Urban Guam. J. Econ. Entomol. 2010, 103, 1135–1143. [Google Scholar] [CrossRef]
- Hagstrum, D.W.; Flinn, P.W.; Shuman, D. Automated monitoring using acoustical sensors for insects in farm-stored wheat. J. Econ. Entomol. 1996, 89, 211–217. [Google Scholar] [CrossRef]
- Mankin, R.; Hubbard, J.; Flanders, K. Acoustic Indicators for Mapping Infestation Probabilities of Soil Invertebrates. J. Econ. Entomol. 2007, 100, 790–800. [Google Scholar] [CrossRef]
- Webb, C.; Litzkow, J.C.; Slaughter, D. A computerized acoustical larval detection system. Appl. Eng. Agric. 1988, 4, 268–274. [Google Scholar]
- Zdunek, A.; Konstankiewicz, K. Acoustic emission in investigation of plant tissue micro-cracking. Trans. ASAE 2004, 47, 1171. [Google Scholar] [CrossRef]
- Mankin, R.W.; Samson, P.R.; Chandler, K.J. Acoustic Detection of Melolonthine Larvae in Australian Sugarcane. J. Econ. Entomol. 2009, 102, 1523–1535. [Google Scholar] [CrossRef] [PubMed]
- Mankin, R.; Mizrach, A.; Hetzroni, A.; Levsky, S.; Nakache, Y.; Soroker, V. Temporal and Spectral features of Sounds of wood-boring Beetle Larvae: Identifiable patterns of Activity enable improved discrimination from background noise. Fla. Entomol. 2009, 91, 241–248. [Google Scholar] [CrossRef]
- Jalinas, J.; Güerri-Agulló, B.; Dosunmu, O.G.; Lopez Llorca, L.V.; Mankin, R.W. Acoustic activity cycles of Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae) early instars after Beauveria bassiana (Hypocreales: Clavicipitaceae) treatments. Ann. Entomol. Soc. Am. 2017, 110, 551–557. [Google Scholar] [CrossRef]
- Mankin, R.; Hodges, R.; Nagle, J.H.; Schal, C.; Pereira, R.; Koehler, P. Acoustic Indicators for Targeted Detection of Stored Product and Urban Insect Pests by Inexpensive Infrared, Acoustic, and Vibrational Detection of Movement. J. Econ. Entomol. 2010, 103, 1636–1646. [Google Scholar] [CrossRef]
- Zdunek, A.; Konopacka, D.; Jesionkowska, K. Crispness and crunchiness judgment of apples based on contact acoustic emission. J. Texture Stud. 2010, 41, 75–91. [Google Scholar] [CrossRef]
- Omkar, S.; Karanth, R. Rule extraction for classification of acoustic emission signals using ant colony optimisation. Eng. Appl. Artif. Intell. 2008, 21, 1381–1388. [Google Scholar] [CrossRef]
- Marzec, A.; Lewicki, P.P.; Ranachowski, Z. Influence of water activity on acoustic emission of flat extruded bread. J. Food Eng. 2007, 79, 410–422. [Google Scholar] [CrossRef]
- Flynn, T.; Salloum, H.; Hull-Sanders, H.; Sedunov, A.; Sedunov, N.; Sinelnikov, Y.; Sutin, A.; Masters, D. Acoustic methods of invasive species detection in agriculture shipments. In Proceedings of the 2016 IEEE Symposium on Technologies for Homeland Security (HST), Waltham, MA, USA, 10–11 May 2016; pp. 1–5. [Google Scholar]
- Pinhas, J.; Soroker, V.; Hetzroni, A.; Mizrach, A.; Teicher, M.; Goldberger, J. Automatic acoustic detection of the red palm weevil. Comput. Electron. Agric. 2008, 63, 131–139. [Google Scholar] [CrossRef]
- Trifa, V.M.; Kirschel, A.N.; Taylor, C.E.; Vallejo, E.E. Automated species recognition of antbirds in a Mexican rainforest using hidden Markov models. J. Acoust. Soc. Am. 2008, 123, 2424–2431. [Google Scholar] [CrossRef] [Green Version]
- Yu, D.; Li, J. Recent progresses in deep learning based acoustic models. IEEE/CAA J. Autom. Sin. 2017, 4, 396–409. [Google Scholar] [CrossRef]
- Appel, H.M.; Cocroft, R. Plants respond to leaf vibrations caused by insect herbivore chewing. Oecologia 2014, 175, 1257–1266. [Google Scholar] [CrossRef] [Green Version]
- Johansmann, M.; Siegmund, G.D.; Pineda, M. Targeting the Limits of Laser Doppler Vibrometry. In Proceedings of the IDEMA, Tokyo, Japan, 7–8 June 2005; pp. 1–12. [Google Scholar]
- Zorović, M.; Čokl, A. Laser vibrometry as a diagnostic tool for detecting wood-boring beetle larvae. J. Pest Sci. 2015, 88, 107–112. [Google Scholar] [CrossRef]
- Martin, P.; Rothberg, S.J. Pseudo-vibration sensitivities for commercial laser vibrometers. Mech. Syst. Signal Process. 2011, 25, 2753–2765. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, E.A.; Bai, J.; Plotto, A.; Dea, S. Electronic noses and tongues: Applications for the food and pharmaceutical industries. Sensors 2011, 11, 4744–4766. [Google Scholar] [CrossRef] [PubMed]
- Smyth, H.; Cozzolino, D. Instrumental methods (spectroscopy, electronic nose, and tongue) as tools to predict taste and aroma in beverages: Advantages and limitations. Chem. Rev. 2013, 113, 1429–1440. [Google Scholar] [CrossRef]
- Cui, S.; Ling, P.; Zhu, H.; Keener, H.M. Plant pest detection using an artificial nose system: A review. Sensors 2018, 18, 378. [Google Scholar] [CrossRef] [Green Version]
- Ghaffari, R.; Zhang, F.; Iliescu, D.; Hines, E.L.; Leeson, M.S.; Napier, R. Detection of Diseases and Volatile Discrimination of Plants: An Electronic Nose and Self-Organizing Maps Approach. In Intelligent Systems for Machine Olfaction: Tools and Methodologies; IGI Global: Hershey, PA, USA, 2011; pp. 214–230. [Google Scholar]
- Lan, Y.-B.; Zheng, X.-Z.; Westbrook, J.K.; Lopez, J.; Lacey, R.; Hoffmann, W.C. Identification of Stink Bugs Using an Electronic Nose. J. Bionic Eng. 2008, 5, 172–180. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, J. Discrimination of different types damage of rice plants by electronic nose. Biosyst. Eng. 2011, 109, 250–257. [Google Scholar] [CrossRef]
- Wang, P.; Zhuang, L.; Zou, Y.; Hsia, K.J. Future Trends of Bioinspired Smell and Taste Sensors. In Bioinspired Smell and Taste Sensors; Springer: Berlin/Heidelberg, Germany, 2015; pp. 309–324. [Google Scholar]
- Albert, K.J.; Lewis, N.S.; Schauer, C.L.; Sotzing, G.A.; Stitzel, S.E.; Vaid, T.P.; Walt, D.R. Cross-Reactive Chemical Sensor Arrays. Chem. Rev. 2000, 100, 2595–2626. [Google Scholar] [CrossRef] [PubMed]
- Askim, J.R.; Mahmoudi, M.; Suslick, K.S. Optical sensor arrays for chemical sensing: The optoelectronic nose. Chem. Soc. Rev. 2013, 42, 8649–8682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alagna, F.; Kallenbach, M.; Pompa, A.; De Marchis, F.; Rao, R.; Baldwin, I.T.; Bonaventure, G.; Baldoni, L. Olive fruits infested with olive fly larvae respond with an ethylene burst and the emission of specific volatiles. J. Integr. Plant Biol. 2016, 58, 413–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Wang, J. Detection of age and insect damage incurred by wheat, with an electronic nose. J. Stored Prod. Res. 2007, 43, 489–495. [Google Scholar] [CrossRef]
- Wen, T.; Zheng, L.; Dong, S.; Gong, Z.; Sang, M.; Long, X.; Luo, M.; Peng, H. Rapid detection and classification of citrus fruits infestation by Bactrocera dorsalis (Hendel) based on electronic nose. Postharvest Biol. Technol. 2019, 147, 156–165. [Google Scholar] [CrossRef]
- Mitsuno, H.; Sakurai, T.; Namiki, S.; Mitsuhashi, H.; Kanzaki, R. Novel cell-based odorant sensor elements based on insect odorant receptors. Biosens. Bioelectron. 2015, 65, 287–294. [Google Scholar] [CrossRef]
- Pasquini, C. Near infrared spectroscopy: Fundamentals, practical aspects and analytical applications. J. Braz. Chem. Soc. 2003, 14, 198–219. [Google Scholar] [CrossRef] [Green Version]
- El-Mesery, H.S.; Mao, H.; Abomohra, A.E.-F. Applications of non-destructive technologies for agricultural and food products quality inspection. Sensors 2019, 19, 846. [Google Scholar] [CrossRef] [Green Version]
- Gomes, J.F.S.; Leta, F.R. Applications of computer vision techniques in the agriculture and food industry: A review. Eur. Food Res. 2012, 235, 989–1000. [Google Scholar] [CrossRef]
- Hussain, A.; Pu, H.; Sun, D.-W. Innovative nondestructive imaging techniques for ripening and maturity of fruits–a review of recent applications. Trends Food Sci. 2018, 72, 144–152. [Google Scholar] [CrossRef]
- Jiang, J.-A.; Chang, H.-Y.; Wu, K.-H.; Ouyang, C.-S.; Yang, M.-M.; Yang, E.-C.; Chen, T.-W.; Lin, T.-T. An adaptive image segmentation algorithm for X-ray quarantine inspection of selected fruits. Computers 2008, 60, 190–200. [Google Scholar] [CrossRef]
- Pathmanaban, P.; Gnanavel, B.; Anandan, S.S. Recent Application of imaging techniques for Fruit quality Assessment. Trends Food Sci. Technol. 2019, 94, 32–42. [Google Scholar] [CrossRef]
- Sutin, A.; Yakubovskiy, A.; Salloum, H.R.; Flynn, T.J.; Sedunov, N.; Nadel, H. Towards an Automated Acoustic Detection Algorithm for Wood-Boring Beetle Larvae (Coleoptera: Cerambycidae and Buprestidae). J. Econ. Entomol. 2019, 112, 1327–1336. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Liang, G.; Jiang, Z.; Wang, J. Advances in electronic nose development for application to agricultural products. Food Anal. Methods 2019, 12, 2226–2240. [Google Scholar] [CrossRef]
- Rady, A.; Giaretta, A.; Akinbode, A.; Ruwaya, M.; Dev, S. Pretreatment and freezing rate effect on physical, microstructural, and nutritional properties of fried sweet potato. Trans. ASABE 2019, 62, 45–59. [Google Scholar] [CrossRef]









| Sensor Type | Crop | Insect Type | Machine Learning Technique | Classification Results | Reference |
|---|---|---|---|---|---|
| RGB camera | Citrus | Scale insect (Coccoidea) | MIA | 92.8% | [43] |
| RGB camera | Citrus | Thrips (Thysanoptera), Scales, and Medfly (Ceratitis capitata) egg | BDA | 73–86% | [42] |
| RGB camera | Citrus | Medfly | BDA | NA | [45] |
| RGB camera | Citrus | Thrips, Scales, and Medfly egg | ROSA | 93.4–100% | [41] |
| RGB camera | Citrus | Thrips, Scales, and Medfly egg | LDA | 43.2–78.1% | [40] |
| Line scan cameras | Pistachio | Insect damage | DF | 74–91.8% | [46] |
| Sensor Type: Wavelength, nm | Crop | Imaging Mode | Insect | Machine Learning Technique | Classification Results | Reference |
|---|---|---|---|---|---|---|
| HSI: 400–900 | Apple | Reflectance | Codling moth | DT | Healthy: 81% Infested: 86% | [10] |
| HSI: 400–1000 | Citrus: Orange | Reflectance | N/A | PCA and BR | 100% | [59] |
| HSI: 450–930 | Citrus: Red Ruby Grapefruit | Reflectance | Leafminers | SID | 95.2% | [60] |
| HSI: 400–720 | Jujube | Reflectance | External insect | SDA | Healthy: 98% Infested: 94% | [57] |
| HSI: 900–1700 | Jujube | Reflectance | Carposina niponensis walsingham | BR | Healthy: 100% Infested: 93.1% | [61] |
| HSI: 400–1000 | Mango | Reflectance | Fruit fly | DA | Up to 99% | [62] |
| HSI: 400–1000 | Mango | Absorbance | Fruit fly | DA | Up to 99.1 % | [15] |
| HSI: 1000–1600 | Mung bean | Reflectance | Callosobru-chus maculatus | LDA and QDA | Healthy: 93.7% Infested: 75.5–95.7% | [55] |
| HSI: 740–1000 | Pickling cucumbers | Transmittance and Reflectance | Fruit fly | PLS-DA | 88–93% | [21] |
| HSI: 580–980 and 590–1550 | Tart cherry | Transmittance and Reflectance | Plum curculio | GA and PLS-DA | Healthy: 81.3% Infested: 95.8% | [31] |
| HSI: 460–800 | Tomatoes | Reflectance | Tomato hornworms frass | Detecting algorithm | Healthy: 86–95% Infested: 71–99% | [63] |
| HSI: 400–1100 | Tomatoes | Transmittance | Tuta absoluta (Meyrick) | ANN | 95% Classification accuracy | [58] |
| HSI: 400–1000 | Vegetable soybean | Transmittance | Etiella zinckenella Treitschke (moth) | SVDD | Healthy: 97.3% Infested: 87.5% | [54] |
| HSI: 400–1000 | Vegetable soybean | Transmittance | Pod borer (Maruca vitrata) | SVM | Healthy: 100% Infested: 91.7% | [64] |
| Sensor Type | Crop | Insect | Machine Learning Technique | Classification Results | Reference |
|---|---|---|---|---|---|
| X-ray machine | Dates | Saw-Toothed Beetles (Oryzaephilus surinamensis) | LDA and SDA | Healthy: 99% Infested: 100% | [76] |
| Soft X-ray machine in IICPT | Mango | Fruit fly | N/A | N/A | [77] |
| X-ray cabinet | Olives | Fruit fly | DA | Healthy: 90% Infested: 50–86% | [78] |
| X-ray images | Olives | Olive fly(Bactrocera oleae) | IDA | 50–88% | [79] |
| X-ray CT imaging and film X-ray | Apples Cherries | Codling moth and western cherry fruit fly (Rhagoletis indifferens) | N/A | N/A | [80] |
| Soft X-ray machine | Mango | Mango pulp weevil(Sternochetus frigidus) | N/A | N/A | [81] |
| Film and on-line scanning X-ray equipment | Apples | Codling moth | None—visual | 6–99% | [82] |
| X-ray machine | Pistachio | Navel orange worm(Amyelois transitella) | SDA | 40–67% | [83] |
| X-ray machine | Pistachio | Insect damage | ANN | 54–96% | [84] |
| X-ray machine | Mango | Seed weevil (Sternochetus mangiferae) | N/A | 100% | [85] |
| Low-field MRI equipment | Apples | Peach fruit moth (Carposina Sasakii Matsumura) | N/A | 100% | [86] |
| Low-field MRI equipment | Peaches | Fruit fly | N/A | Healthy: 58% Infested: 71% | [87] |
| IR thermal camera | Apples | Codling moth | Paired t-test | Significant at α = 1% | [88] |
| IR Thermal camera | Cowpea | Cowpea seed beetle (Callosobruchus Maculatus) | QDA | 80% | [89] |
| Method | Advantages | Disadvantages |
|---|---|---|
| Spectroscopy | No sample preparation needed, determining both chemical and physical characteristics, ease of use and suitable for on-line applications [16,139] | Large amount of samples/data and different chemometric methods are needed to build accurate models [140]. Does not provide spatial data. |
| Visible light sensing | Simple and cost-effective, accurate and suitable for on-line monitoring [61,141] | Only suitable for detecting external defects, sensitive to external lighting variations |
| HSI | Merges the advantage of a color vision system with that of spectroscopic system [142]. Provides both spectral and spatial features for accurate segmentation and identification of region of interest, it can detect internal defects [140] | HSI data are voluminous, contain huge redundant data that requires tedious analysis to upgrade to multispectral images by selecting useful wavelengths), its hardware is costly, different chemometric methods are required to extract useful information [140] |
| X-ray imaging | Can detect internal defects causing density differences, such as cavities | High costs, poor penetration in materials with high water content, and difficulty in effectively differentiating normal and infested tissues with similar densities [143] |
| MRI | No harmful ionizing radiation, high-resolution visual information of internal structure, it gives quality 2D and 3D images [144] | High costs, large dimensions, and heaviness [86,90] |
| Thermal imaging | Easy handling and portability [144] | Sensitivity to the environmental condition and relatively high costs to obtain high-resolution images [93] |
| Acoustic | Sensitive, efficient, and clear detection capabilities of various insects [145]. Inexpensive, automatic, and continuous monitoring [19] | Prone to background noise [19]. Incapable of detecting insect eggs |
| E- nose and E-tongue | Low-cost, rapid, and environmentally friendly testing [146] | Reported detection levels and accuracies are not very high [146] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adedeji, A.A.; Ekramirad, N.; Rady, A.; Hamidisepehr, A.; Donohue, K.D.; Villanueva, R.T.; Parrish, C.A.; Li, M. Non-Destructive Technologies for Detecting Insect Infestation in Fruits and Vegetables under Postharvest Conditions: A Critical Review. Foods 2020, 9, 927. https://doi.org/10.3390/foods9070927
Adedeji AA, Ekramirad N, Rady A, Hamidisepehr A, Donohue KD, Villanueva RT, Parrish CA, Li M. Non-Destructive Technologies for Detecting Insect Infestation in Fruits and Vegetables under Postharvest Conditions: A Critical Review. Foods. 2020; 9(7):927. https://doi.org/10.3390/foods9070927
Chicago/Turabian StyleAdedeji, Akinbode A., Nader Ekramirad, Ahmed Rady, Ali Hamidisepehr, Kevin D. Donohue, Raul T. Villanueva, Chadwick A. Parrish, and Mengxing Li. 2020. "Non-Destructive Technologies for Detecting Insect Infestation in Fruits and Vegetables under Postharvest Conditions: A Critical Review" Foods 9, no. 7: 927. https://doi.org/10.3390/foods9070927