Beverage and Food Fragrance Biotechnology, Novel Applications, Sensory and Sensor Techniques: An Overview
Abstract
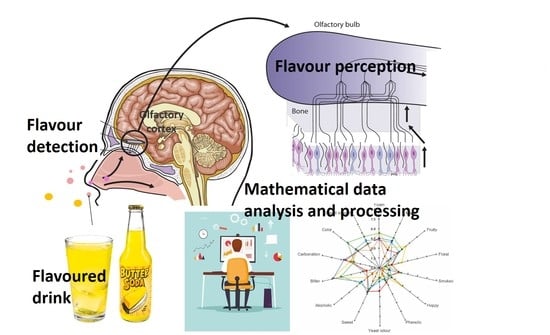
:1. Introduction
2. Functional Characterisation and Metabolic Engineering of Flavour Compounds Biosynthesis in Plants
3. Functional Characterisation and Metabolic Engineering of Flavour Compounds Biosynthesis in Microorganism Cells
4. Mechanisms of Olfaction and Ligand–Receptor Interaction
5. Recent Sensory Analysis Techniques
5.1. Overview of Sensory Techniques: ‘Product Understanding’ and ‘Consumer Understanding’
5.1.1. Traditional and Novel Single-Point Techniques
5.1.2. Time-Intensity Methods
5.2. Electronic Nose and Other Sensors
6. Recent Innovations in the Statistical Technique of Sensory Data Analysis
7. Final Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Dunkel, A.; Steinhaus, M.; Kotthoff, M.; Nowak, B.; Krautwurst, D.; Schieberle, P.; Hofmann, T. Nature’s Chemical Signatures in Human Olfaction: A Foodborne Perspective for Future Biotechnology. Angew. Chem. Int. Ed. 2014, 53, 7124–7143. [Google Scholar] [CrossRef]
- Ferrer, I.; Garcia-Esparcia, P.; Carmona, M.; Carro, E.; Aronica, E.; Kovacs, G.G.; Gustincich, S. Olfactory Receptors in Non-Chemosensory Organs: The Nervous System in Health and Disease. Front. Aging Neurosci. 2016, 8, 163. [Google Scholar] [CrossRef] [Green Version]
- Goto, T.; Salpekar, A.; Monk, M. Expression of a testis-specific member of the olfactory receptor gene family in human primordial germ cells. Mol. Hum. Reprod. 2001, 7, 553–558. [Google Scholar] [CrossRef]
- Hillier, L.; Lennon, G.; Becker, M.; Bonaldo, M.F.; Chiapelli, B.; Chissoe, S.; Dietrich, N.; Dubuque, T.; Favello, A.; Gish, W.; et al. Generation and analysis of 280,000 humans expressed sequence tags. Gen. Res. 1996, 6, 807–828. [Google Scholar] [CrossRef] [Green Version]
- Farbiszewski, R.; Kranc, R. Olfactory receptors and the mechanism of odor perception. Pol. Ann. Med. 2013, 20, 51–55. [Google Scholar] [CrossRef]
- Malnic, B.; Gonzalez-Kristeller, D.C.; Gutiyama, L.M. Odorant receptors. The Neurobiology of Olfaction. In Frontiers in Neuroscience; Menini, A., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2010. [Google Scholar]
- Rouquier, S.; Giorgi, D. Olfactory receptor gene repertoires in mammals. Mutat. Res. 2007, 616, 95–102. [Google Scholar] [CrossRef]
- Shepherd, G. The human sense of smell: Are we better than we think? PLoS Biol. 2004, 2, e146. [Google Scholar] [CrossRef] [Green Version]
- Godfrey, P.A.; Malnic, B.; Buck, L.B. The mouse olfactory receptor gene family. Proc. Natl. Acad. Sci. USA 2004, 101, 2156–2161. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Firestein, S. The olfactory receptor gene superfamily of the mouse. Nat. Neurosci. 2002, 5, 124–133. [Google Scholar] [CrossRef]
- Niimura, Y.; Nei, M. Extensive gains and losses of olfactory receptor genes in mammalian evolution. PLoS ONE 2007, 2, e708. [Google Scholar] [CrossRef]
- Ngai, J.; Dowling, M.M.; Buck, L.; Axel, R.; Chess, A. The family of genes encoding odorant receptors in the channel catfish. Cell 1993, 72, 657–666. [Google Scholar] [CrossRef]
- Freitag, J.; Ludwig, G.; Andreini, I.; Rossler, P.; Breer, H. Olfactory receptors in aquatic and terrestrial vertebrates. J. Comp. Physiol. 1998, 183, 635–650. [Google Scholar] [CrossRef]
- Glusman, G.; Yanai, I.; Rubin, I.; Lancet, D. The complete human olfactory subgenome. Gen. Res. 2001, 11, 685–702. [Google Scholar] [CrossRef] [Green Version]
- Shaaban, H.A.; Mahmoud, K.F.; Amin, A.A.; EL Banna, H.A. Application of Biotechnology to the Production of Natural Flavor and Fragrance Chemicals. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 2670–2717. Available online: https://www.researchgate.net/publication/310460244 (accessed on 1 September 2019).
- Braga, A.; Guerreiro, C.; Belo, I. Generation of Flavors and fragrances through biotransformation and de novo Synthesis. Food Bioproc. Tech. 2018, 11, 2217–2228. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Gupta, C.; Garg, A.P.; Prakash, D. A biotechnological approach to microbial based perfumes and flavours. J. Microbiol. Exp. 2015, 2, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Huccetogullari, D.; Luo, Z.W.; Lee, S.Y. Metabolic engineering of microorganisms for production of aromatic compounds. Microb. Cell Fact. 2019, 18, 41. [Google Scholar] [CrossRef]
- Murthy, H.N.; Georgiev, M.I.; Park, S.-Y.; Dandin, V.S.; Paek, K.-Y. The safety assessment of food ingredients derived from plant cell, tissue and organ cultures: A review. Food Chem. 2015, 176, 426–432. [Google Scholar] [CrossRef]
- Cataldo, V.F.; López, J.; Cárcamo, M.; Agosin, E. Chemical vs. biotechnological synthesis of C13-apocarotenoids: Current methods, applications, and perspectives. Appl. Microbiol. Biotechnol. 2016, 100, 5703–5718. [Google Scholar] [CrossRef]
- Schewe, H.; Mirata, M.A.; Schrader, J. Bioprocess Engineering for Microbial Synthesis and Conversion of Isoprenoids. Adv. Biochem. Eng. Biotechnol. 2015, 148, 251–286. [Google Scholar] [CrossRef]
- Sá, A.G.A.; Meneses, A.C.; Araújo, P.H.H.; Oliveira, D. A review on enzymatic synthesis of aromatic esters used as flavor ingredients for food, cosmetics and pharmaceuticals industries. Trends Food Sci. Technol. 2017, 69, 95–105. [Google Scholar] [CrossRef]
- Fletcher, E.; Krivoruchko, A.; Nielsen, J. Industrial systems biology and its impact on synthetic biology of yeast cell factories. Biotechnol. Bioeng. 2015, 113, 1164–1170. [Google Scholar] [CrossRef] [Green Version]
- Pham-Hoang, B.-N.; Phan-Thi, H.; Waché, Y. Can biological structures be natural and sustainable capsules? Front. Chem. 2015, 3, 36. [Google Scholar] [CrossRef] [Green Version]
- Siegmund, B. Biogenesis of aroma compounds: Flavour formation in fruits and vegetables. In Flavour Development, Analysis and Perception in Food and Beverages; Parker, J.K., Elmore, J.S., Methven, L., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition: Cambridge, UK, 2015; pp. 127–149. [Google Scholar] [CrossRef]
- Dudareva, D.; Pichersky, E. Metabolic engineering of plant volatiles. Curr. Opin. Biotechnol. 2008, 19, 181–189. [Google Scholar] [CrossRef]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function, and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef]
- Pech, J.C.; Latché, A.; Van der Rest, B. Genes involved in the biosynthesis of aroma volatiles and biotechnological applications. In Fruit and Vegetable Flavour. Recent Advances and Future Prospects; Brückner, B., Wyllie, S.G., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition: Cambridge, UK, 2008; pp. 254–271. [Google Scholar] [CrossRef]
- Aragüez, I.; Valpuesta, V. Metabolic engineering of aroma components in fruits. Biotechnol. J. 2013, 8, 1144–1158. [Google Scholar] [CrossRef]
- El Hadi, M.A.M.; Zhang, F.-J.; Wu, F.-F.; Zhou, C.-H.; Tao, J. Advances in fruit aroma volatile research. Molecules 2013, 18, 8200–8229. [Google Scholar] [CrossRef]
- Pierik, R.; Ballaré, C.L.; Dicke, M. Ecology of plant volatiles: Taking a plant community perspective. Plant Cell Environ. 2015, 37, 1845–1853. [Google Scholar] [CrossRef] [Green Version]
- Belitz, H.-D.; Grosch, W.; Schieberle, P. Food Chemistry, 3rd ed.; Translation from the fifth German edition by M.M. Burghagen; Springer: New York, NY, USA, 2004. [Google Scholar]
- Beekwilder, J.; Alvarez-Huerta, M.; Neef, E.; Verstappen, F.W.A.; Bouwmeester, H.J.; Aharoni, A. Functional characterization of enzymes forming volatile esters from strawberry and banana. Plant Physiol. 2004, 135, 1865–1878. [Google Scholar] [CrossRef] [Green Version]
- Kader, A.A. A perspective on postharvest horticulture (1978–2003). HortScience 2003, 38, 1004–1008. [Google Scholar] [CrossRef] [Green Version]
- Forney, C.F.; Kalt, W.; Jordan, M.A. The composition of strawberry aroma is influenced by cultivar, maturity, and storage. HortScience 2000, 35, 1022–1026. [Google Scholar] [CrossRef] [Green Version]
- Baietto, M.; Wilson, A.D. Electronic-nose applications for fruit identification, ripeness, and quality grading. Sensors 2015, 15, 899–931. [Google Scholar] [CrossRef]
- Valero, D.; Serrano, M. Postharvest Biology and Technology for Preserving Fruit Quality; CRC Press-Taylor & Francis: Boca Raton, FL, USA, 2010. [Google Scholar]
- Croteau, R.; Karp, F. Origin of natural odorants. In Perfumes: Art, Science, and Technology; Muller, P.M., Lamparsky, D., Eds.; Elsevier Applied Science: London, UK, 1991; pp. 101–126. [Google Scholar] [CrossRef]
- Galliard, T.; Matthew, J.A. Lipoxygenase-mediated cleavage of fatty acids to carbonyl fragments in tomato fruits. Phytochemistry 1977, 16, 339–343. [Google Scholar] [CrossRef]
- Stevens, M.A. Relationship between polyene-carotene content and volatile compound composition of tomatoes. J. Am. Soc. Hortic. Sci. 1970, 95, 461–464. [Google Scholar]
- Tholl, D. Biosynthesis and biological functions of terpenoids in plants. Adv. Biochem. Eng. Biotechnol. 2015, 148, 63–106. [Google Scholar] [CrossRef]
- Fortes, A.M.; Granell, A.; Pezzotti, M.; Bouzayen, M. Editorial: Molecular and Metabolic Mechanisms Associated with Fleshy Fruit Quality. Front. Plant Sci. 2017, 8, 1236. [Google Scholar] [CrossRef] [Green Version]
- Peled-Zehavi, H.; Oliva, M.; Xie, Q.; Tzin, V.; Oren-Shamir, M.; Aharoni, A.; Galili, G. Metabolic Engineering of the Phenylpropanoid and Its Primary, Precursor Pathway to Enhance the Flavor of Fruits and the Aroma of Flowers. Bioengineering 2015, 2, 204–212. [Google Scholar] [CrossRef]
- Aharoni, A.; Jongsma, M.A.; Kim, T.Y.; Ri, M.B.; Giri, A.P.; Verstappen, F.W.A.; Schwab, W.; Bouwmeester, H.J. Metabolic engineering of terpenoid biosynthesis in plants. Phytochem. Rev. 2006, 5, 49–58. [Google Scholar] [CrossRef] [Green Version]
- Farhi, M.; Marhevka, E.; Masci, T.; Marcos, E.; Eyal, Y.; Ovadis, M.; Abeliovich, H.; Vainstein, A. Harnessing yeast subcellular compartments for the production of plant terpenoids. Metab. Eng. 2011, 13, 474–481. [Google Scholar] [CrossRef]
- Longo, M.A.; Sanroman, M.A. Production of Food Aroma Compounds: Microbial and Enzymatic Methodologies. Food Technol. Biotechnol. 2006, 44, 335–353. [Google Scholar]
- Vandamme, E.J.; Soetaert, W. Bioflavours and fragrances via fermentation and biocatalysis. J. Chem. Technol. Biotechnol. 2002, 77, 1323–1332. [Google Scholar] [CrossRef]
- Luque de Castro, M.D.; Garcia-Ayuso, L.E. Soxhlet extraction of solid materials: An outdated technique with a promising innovative future. Anal. Chim. Acta 1998, 369, 1–10. [Google Scholar] [CrossRef]
- Nielsen, J.; Keasling, J.D. Engineering cellular metabolism. Cell 2016, 164, 1185–1197. [Google Scholar] [CrossRef] [Green Version]
- Janssens, L.; de Pooter, H.L.; Vandamme, E.J.; Schamp, N.M. Production of flavours by microorganisms. Process Biochem. 1992, 27, 195–215. [Google Scholar] [CrossRef]
- Krings, U.; Berger, R.G. Biotechnological production of flavours and fragrances. Appl. Microbiol. Biotechnol. 1998, 49, 1–8. [Google Scholar] [CrossRef]
- Aguedo, M.; Ly, M.H.; Belo, I.; Teixeira, J.A.; Belin, J.M.; Waché, Y. The use of enzymes and microorganisms for the production of aroma compounds from lipids. Food Technol. Biotechnol. 2004, 42, 327–336. [Google Scholar]
- Sun, W.; Zhao, Y.-J.; Li, C. De Novo Synthesis of Plant Natural Products in Yeast. In Yeasts in Biotechnology; Thalita, P.B., Ed.; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar] [CrossRef] [Green Version]
- Bicas, J.L.; Molina, G.; Cavalcante Barros, F.F.; Pastore, G.M. White biotechnology for sustainable chemistry. RSC Green Chem. 2015. [Google Scholar] [CrossRef]
- Bution, M.L.; Molina, G.; Abrahão, M.R.E.; Pastore, G.M. Genetic and metabolic engineering of microorganisms for the development of new flavor compounds from terpenic substrates. Crit. Rev. Biotechnol. 2015, 35, 313–325. [Google Scholar] [CrossRef]
- Zwenger, S.; Basu, C. Plant terpenoids: Applications and future potentials. Biotechnol. Mol. Biol. Rev. 2008, 3, 1–7. [Google Scholar]
- Liao, P.; Hemmerlin, A.; Bach, T.J.; Chye, M.L. The potential of the mevalonate pathway for enhance disoprenoid production. Biotechnol. Adv. 2016, 34, 697–713. [Google Scholar] [CrossRef]
- Albrecht, M.; Misawa, N.; Sandmann, G. Metabolic engineering of the terpenoid biosynthetic pathway of Escherichia coli for production of the carotenoids β-carotene and zeaxanthin. Biotechnol. Lett. 1999, 21, 791–795. [Google Scholar] [CrossRef]
- Kim, S.-W.; Keasling, J.D. Metabolic engineering of the nonmevalonate isopentenyl diphosphate synthesis pathway in Escherichia coli enhances lycopene production. Biotechnol. Bioeng. 2001, 72, 408–415. [Google Scholar] [CrossRef]
- Leonard, E.; Ajikumar, P.K.; Thayer, K.; Xiao, W.-H.; Mo, J.D.; Tidor, B.; Stephanopoulos, G.; Prather, K.L.J. Combining metabolic and protein engineering of a terpenoid biosynthetic pathway for overproduction and selectivity control. Proc. Natl. Acad. Sci. USA 2010, 107, 13654–13659. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.F.; Wang, G. Strategies of isoprenoids production in engineered bacteria. J. Appl. Microbiol. 2016, 121, 932–940. [Google Scholar] [CrossRef] [Green Version]
- Ward, V.C.A.; Chatzivasileiou, A.O.; Stephanopoulos, G. Metabolic engineering of Escherichia coli for the production of isoprenoids. FEMS Microbiol. Lett. 2018, 365, 10. [Google Scholar] [CrossRef] [Green Version]
- Paramasivan, K.; Mutturi, S. Progress in terpene synthesis strategies through engineering of Saccharomyces cerevisiae. Crit. Rev. Biotechnol. 2017, 37, 974–989. [Google Scholar] [CrossRef]
- Ignea, C.; Pontini, M.; Maffei, M.E.; Makris, A.M.; Kampranis, S.C. Engineering monoterpene production in yeast using a synthetic dominant negative geranyl diphosphate synthase. ACS Synth. Biol. 2014, 3, 298–306. [Google Scholar] [CrossRef]
- Vickers, C.E.; Williams, T.C.; Peng, B.; Cherry, J. Recent advances in synthetic biology for engineering isoprenoid production in yeast. Curr. Opin. Chem. Biol. 2017, 40, 47–56. [Google Scholar] [CrossRef]
- Wriessnegger, T.; Pichler, H. Yeast metabolic engineering—Targeting sterol metabolism and terpenoid formation. Prog. Lipid. Res. 2013, 52, 277–293. [Google Scholar] [CrossRef]
- Denby, C.M.; Li, R.A.; Vu, V.T.; Costello, Z.; Lin, W.; Chan, L.J.G.; Williams, J.; Donaldson, B.; Bamforth, C.W.; Petzold, C.J.; et al. Industrial brewing yeast engineered for the production of primary flavor determinants in hopped beer. Nat. Commun. 2018, 9, 965. [Google Scholar] [CrossRef]
- Wyk, N.V.; Kroukamp, H.; Pretorius, I.S. The Smell of Synthetic Biology: Engineering Strategies for Aroma Compound Production in Yeast. Fermentation 2018, 4, 54. [Google Scholar] [CrossRef] [Green Version]
- Erb, T.J.; Jones, P.R.; Bar-Even, A. Synthetic metabolism: Metabolic engineering meets enzyme design. Curr. Opin. Chem. Biol. 2017, 37, 56–62. [Google Scholar] [CrossRef]
- Smanski, M.J.; Zhou, H.; Claesen, J.; Shen, B.; Fischbach, M.A.; Voigt, C.A. Synthetic biology to access and expand nature’s chemical diversity. Nat. Rev. Microbiol. 2016, 14, 135–149. [Google Scholar] [CrossRef] [Green Version]
- Chemler, J.A.; Koffas, M.A. Metabolic engineering for plant natural product biosynthesis in microbes. Curr Opin. Biotechnol. 2008, 19, 597–605. [Google Scholar] [CrossRef]
- Siddiqui, M.S.; Thodey, K.; Trenchard, I.; Smolke, C.D. Advancing secondary metabolite biosynthesis in yeast with synthetic biology tools. FEMS Yeast Res. 2012, 12, 144–170. [Google Scholar] [CrossRef]
- Cravens, A.; Payne, J.; Smolke, C.D. Synthetic biology strategies for microbial biosynthesis of plant natural products. Nat. Commun. 2019, 10, 2142. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; DeLoache, W.C.; Dueber, J.E. Spatial organization of enzymes for metabolic engineering. Metab. Eng. 2012, 14, 242–251. [Google Scholar] [CrossRef]
- Zhao, C.; Gao, X.; Liu, X.; Wang, Y.; Yang, S.; Wang, F.; Re, Y. Enhancing biosynthesis of a ginsenoside precursor by self-assembly of two key enzymes in Pichia pastoris. J. Agric. Food Chem. 2016, 64, 3380–3385. [Google Scholar] [CrossRef]
- Xu, W.; Klumbys, E.; Ang, L.E.; Zhao, H. Emerging molecular biology tools and strategies for engineering natural product biosynthesis. Metab. Eng. Commun. 2020, 10, e00108. [Google Scholar] [CrossRef]
- Istifli, E.S.; Hüsunet, M.T.; Ila, H.B. Cell Division, Cytotoxicity, and the Assays Used in the Detection of Cytotoxicity, Cytotoxicity-Definition, Identification, and Cytotoxic Compounds. Intech. Open 2019. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.-M.; Vo, P.N.L.; Na, D. Advancement of Metabolic Engineering Assisted by Synthetic Biology. Catalysts 2018, 8, 619. [Google Scholar] [CrossRef] [Green Version]
- Anal, A.K. Quality Ingredients and Safety Concerns for Traditional Fermented Foods and Beverages from Asia. A Review. Fermentation 2019, 5, 8. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Fung, D.Y.C. Alkaline-Fermented Foods: A Review with Emphasis on Pidan Fermentation. Crit. Rev. Microbiol. 1996, 22, 101–138. [Google Scholar] [CrossRef]
- Kutyna, D.R.; Borneman, A.R. Heterologous Production of Flavour and Aroma Compounds in Saccharomyces cerevisiae. Genes 2018, 9, 326. [Google Scholar] [CrossRef] [Green Version]
- Jeandet, P.; Vasserot, Y.; Chastang, T.; Courot, E. Engineering Microbial Cells for the Biosynthesis of Natural Compounds of Pharmaceutical Significance. BioMed. Res. Int. 2013, 780145. [Google Scholar] [CrossRef]
- Papagianni, M. Metabolic engineering of lactic acid bacteria for the production of industrially important compounds. Comput. Struct. Biotechnol. J. 2012, 3, e201210003. [Google Scholar] [CrossRef] [Green Version]
- Davidson, B.E.; Llanos, R.M.; Cancilla, M.R.; Redman, N.C.; Hillier, A.J. Current research on the genetics of lactic acid production in lactic acid bacteria. Int. Dairy J. 1995, 5, 763–784. [Google Scholar] [CrossRef]
- Escamilla-Hurtado, M.L.; Valdes-Martinez, S.; Soriano-Santos, J.; Tomasini-Campocosio, A. Effect of some nutritional and environmental parameters on the production of diacetyl and on starch consumption by Pediococcus pentosaceus and Lactobacillus acidophilus in submerged cultures. J. Appl. Microbiol. 2000, 88, 142–153. [Google Scholar] [CrossRef]
- Oliveira, A.P.; Nielsen, J.; Forster, J. Modeling Lactococcus lactis using a genome-scale flux model. BMC Microbiol. 2005, 5, 39. [Google Scholar] [CrossRef] [Green Version]
- Lees, G.J.; Jago, G.R. Acetaldehyde: An intermediate in the formation of ethanol from glucose by lactic acid bacteria. J. Dairy Res. 1976, 43, 63–73. [Google Scholar] [CrossRef]
- Lees, G.J.; Jago, G.R. Formation of acetaldehyde from threonine by lactic acid bacteria. J. Dairy Res. 1976, 43, 75–83. [Google Scholar] [CrossRef]
- Bintsis, T. Lactic acid bacteria: Their applications in foods. J. Bacteriol. Mycol. Open Access 2018, 6, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Smid, E.J.; Kleerebezem, M. Production of Aroma Compounds in Lactic Fermentations. Ann. Rev. Food Sci. Technol. 2014, 5, 313–326. [Google Scholar] [CrossRef]
- Swindell, S.M.; Benson, K.H.; Griffin, H.G.; Renauld, P.; Ekrlich, S.D.; Gasson, M.G. Genetic manipulation of the pathway for diacetyl metabolism in Lactococcus lactis. Appl. Environ. Microbiol. 1996, 62, 2641–2643. [Google Scholar]
- Hugenholtz, C.J.; Kleerebezem, M.; Starrenburg, M.; Delcour, J.; de Vos, W.M.; Hols, P. Lactococcus lactis as a cell factory for high-level diacetyl production. Appl. Environ. Microbiol. 2000, 66, 4112–4114. [Google Scholar] [CrossRef] [Green Version]
- Guo, T.; Kong, J.; Zhang, L.; Zhang, C.; Hu, S. Fine tuning of the lactate and diacetyl production through promoter engineering in Lactococcus lactis. PLoS ONE 2012, 7, e36296. [Google Scholar] [CrossRef] [Green Version]
- Chaves, A.C.S.D.; Fernandez, M.; Lerayer, A.L.S.; Mierau, I.; Kleerebezem, M.; Hugenholtz, J. Metabolic engineering of acetaldehyde production by Streptococcus thermophiles. Appl. Environ. Microbiol. 2002, 68, 5656–5662. [Google Scholar] [CrossRef] [Green Version]
- Axel, R. The molecular logic of smell. Sci. Am. 1995, 273, 154–159. [Google Scholar] [CrossRef]
- Mazzatenta, A.; Cellerino, A.; Origlia, N.; Barloscio, D.; Sartucci, F.; Di Giulio, C.; Domenici, L. Olfactory phenotypic expression unveils human aging. Oncotarget 2016, 7, 19193–19200. [Google Scholar] [CrossRef] [Green Version]
- Bushdid, C.; Magnasco, M.O.; Vosshall, L.B.; Keller, A. Humans can discriminate more than 1 trillion olfactory stimuli. Science 2014, 343, 1370–1372. [Google Scholar] [CrossRef] [Green Version]
- Murray, N.; Lee, B.; Qiao, Y.; Muntean, G.M. Olfaction-Enhanced Multimedia. ACM Comput. Surv. 2016, 48, 1–34. [Google Scholar] [CrossRef]
- Greer, P.L.; Bear, D.M.; Lassance, J.M.; Bloom, M.L.; Tsukahara, T.; Pashkovski, S.L. Family of non-GPCR Chemosensors Defines an Alternative Logic for Mammalian Olfaction. Cell 2016, 165, 1734–1748. [Google Scholar] [CrossRef] [Green Version]
- Rivière, S.; Challet, L.; Fluegge, D.; Spehr, M.; Rodriguez, I. Formyl peptide receptor-like proteins are a novel family of vomeronasal chemosensors. Nature 2009, 459, 574–577. [Google Scholar] [CrossRef]
- Villar, P.S.; Delgado, R.; Vergara, C.; Reyes, J.C.; Bacigalupo, J. Energy Requirements of odor transduction in the chemosensory cilia of olfactory sensory neurons rely on oxidative phosphorylation and glycolytic processing of extracellular glucose. J. Neurosci. 2017, 37, 5736–5743. [Google Scholar] [CrossRef] [Green Version]
- Mori, K.; Sakano, H. How Is the Olfactory Map Formed and Interpreted in the Mammalian Brain? Ann. Rev. Neurosci. 2011, 34, 467–499. [Google Scholar] [CrossRef]
- Touhara, K.; Niimura, Y.; Ihara, S. Vertebrate Odorant Receptors (chapter 3). In Chemosensory Transduction; Frank, Z., Steven, D., Munger, Eds.; Academic Press: New York, NY, USA, 2016; pp. 49–66. [Google Scholar] [CrossRef]
- Gu, Y.; Lucas, P.; Rospars, J.-P. Computational Model of the Insect Pheromone Transduction Cascade. PLoS Comput. Biol. 2009, 5, e1000321. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.; Rospars, J.-P. Dynamical Modelling of the Moth Pheromone-Sensitive Olfactory Receptor Neuron within Its Sensillar Environment. PLoS ONE 2011, 6, e17422. [Google Scholar] [CrossRef] [Green Version]
- Münch, D.; Schmeichel, B.; Silbering, A.F.; Galizia, C.G. Weaker ligands can dominate an odor blend due to syntopic interactions. Chem. Sens. 2012, 38, 293–304. [Google Scholar] [CrossRef] [Green Version]
- Rospars, J.-P. Interactions of Odorants with Olfactory Receptors and Other Pre-processing Mechanisms: How Complex and Difficult to Predict? Chem. Senses 2013, 38, 283–287. [Google Scholar] [CrossRef] [Green Version]
- Jiang, P.; Cui, M.; Ji, Q.; Snyder, L.; Liu, Z.; Benard, L.; Margolskee, R.F.; Osman, R.; Max, M. Molecular mechanisms of sweet receptor function. Chem. Senses 2005, 30 (Suppl. 1), i17–i18. [Google Scholar] [CrossRef]
- Civille, G.V.; Oftedal, K.N. Sensory evaluation techniques–Make “good for you” taste “good”. Physiol. Behav. 2012, 107, 598–605. [Google Scholar] [CrossRef]
- Meilgaard, M.C.; Carr, T.; Civille, G.V. Sensory Evaluation Techniques, 5th ed.; Taylor Francis Group: Boca Raton, FL, USA, 2015. [Google Scholar]
- Murray, J.M.; Delahunty, C.M.; Baxter, I.A. Descriptive sensory analysis: Past, present and future. Food Res. Int. 2001, 34, 461–471. [Google Scholar] [CrossRef]
- Noble, A.C.; Arnold, R.A.; Masuda, B.M.; Pecore, S.D.; Schmidt, J.O.; Stern, P.M. Progress Towards a Standardized System of Wine Aroma Terminology. Am. J. Enol. Vitic. 1984, 35, 107–109. [Google Scholar]
- Meilgaard, M.C.; Dalgliesh, C.E.; Clapperton, J.F. Beer Flavor Terminology. J. Inst. Brew. 1979, 85, 38–42. [Google Scholar] [CrossRef]
- Lee, K.M.; Paterson, A.; Piggott, J.R.; Richardson, G.D. Origins of Flavor in Whiskies and a Revised Flavor Wheel: A Review. J. Inst. Brew. 2001, 107, 287–313. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, B.; Vilela, A.; Correia, E. Sensory profile of Pink Port Wines: Development of a flavour lexicon. Flav. Frag. J. 2014, 29, 50–58. [Google Scholar] [CrossRef]
- Venturi, F.; Andrich, G.; Sanmartin, C.; Scalabrelli, G.; Ferroni, G.; Zinnai, A. The expression of a full-bodied red wine as a function of the characteristics of the glass utilized for the tasting. CyTA-J. Food 2014, 12, 291–297. [Google Scholar] [CrossRef] [Green Version]
- Jaeger, S. Non-sensory factors in sensory science research. Food Qual. Pref. 2006, 17, 132–144. [Google Scholar] [CrossRef]
- Köster, E.P. Diversity in the determinants of food choice: A psychological perspective. Food Qual. Pref. 2009, 20, 70–82. [Google Scholar] [CrossRef]
- Prescott, J.; Bell, G. Cross-cultural determinants of food acceptability: Recent research on sensory perceptions and preferences. Trends Food Sci. Technol. 1995, 6, 201–205. [Google Scholar] [CrossRef]
- Raudenbush, B.; Frank, R.A. Assessing food neophobia: The role of stimulus familiarity. Appetite 1999, 32, 261–271. [Google Scholar] [CrossRef]
- Ares, G. Methodological issues in cross-cultural sensory and consumer research. Food Qual. Pref. 2018, 64, 253–263. [Google Scholar] [CrossRef]
- Hu, X.; Lee, J. Emotions elicited while drinking coffee: A cross-cultural comparison between Korean and Chinese consumers. Food Qual. Pref. 2019, 76, 160–168. [Google Scholar] [CrossRef]
- Meiselman, H.L. A review of the current state of emotion research in product development. Food Res. Int. 2015, 76, 192–199. [Google Scholar] [CrossRef]
- Stone, H.; Sidel, J.; Oliver, S.; Woolsey, A.; Singleton, R.C. Sensory evaluation by quantitative descriptive analysis. Food Technol. 1974, 28, 24–34. [Google Scholar]
- Puri, R.; Khamrui, K.; Khetra, Y.; Malhotra, R.; Devraja, H.C. Quantitative descriptive analysis and principal component analysis for sensory characterization of Indian milk product cham-cham. J. Food Sci. Technol. 2016, 53, 1238–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Punter, P.H. Free Choice Profiling. In Descriptive Analysis in Sensory Evaluation; Kemp, S.E., Hort, J., Hollowood, T., Eds.; John Wiley & Sons Ltd.: New York, NY, USA, 2018; pp. 493–511. [Google Scholar] [CrossRef]
- Jaeger, S.R.; Beresford, M.K.; Paisley, A.G.; Antúnez, L.; Vidal, L.; Cadena, R.S.; Giménez, A.; Ares, G. Check-all-that-apply (CATA) questions for sensory product characterization by consumers: Investigations into the number of terms used in CATA questions. Food Qual. Pref. 2015, 42, 154–164. [Google Scholar] [CrossRef]
- Ares, G.; Antúnez, L.; Saldamando, L.; Giménez, A. Polarized Sensory Positioning. In Descriptive Analysis in Sensory Evaluation; Kemp, S.E., Hort, J., Hollowood, T., Eds.; John Wiley & Sons Ltd.: New York, USA, 2018; pp. 561–577. [Google Scholar] [CrossRef]
- Fleming, E.E.; Ziegler, G.R.; Hayes, J.E. Check-all-that-apply (CATA), sorting, and polarized sensory positioning (PSP) with astringent stimuli. Food Qual. Pref. 2015, 45, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Lawless, H.T.; Glatter, S. Consistency of multidimensional scaling models derived from odor sorting. J. Sens. Stud. 1990, 5, 217–230. [Google Scholar] [CrossRef]
- Valentin, D.; Chollet, S.; Lelièvre, M.; Abdi, H. Quick and dirty but still pretty good: A review of new descriptive methods in food science. Int. J. Food Sci. Technol. 2012, 1–16. [Google Scholar] [CrossRef]
- Risvik, E.; McEwan, J.A.; Colwill, J.S.; Rogers, R.; Lyon, D.H. Projective mapping: A tool for sensory analysis and consumer research. Food Qual. Pref. 1994, 5, 263–269. [Google Scholar] [CrossRef]
- Dehlholm, C.; Brockhoff, P.B.; Meinert, L.; Aaslyng, M.D.; Bredie, W.L.P. Rapid descriptive sensory methods – Comparison of Free Multiple Sorting, Partial Napping, Napping, Flash Profiling, and conventional profiling. Food Qual. Pref. 2012, 26, 267–277. [Google Scholar] [CrossRef]
- Dijksterhuis, G.B.; Piggott, J.R. Dynamic methods of sensory analysis. Trends Food Sci. Technol. 2000, 11, 284–290. [Google Scholar] [CrossRef]
- Cliff, M.; Heymann, H. Development and use of time-intensity methodology for sensory evaluation: A review. Food Res. Int. 1993, 26, 375–385. [Google Scholar] [CrossRef]
- Duizer, L.M.; Bloom, K.; Findlay, C.J. Dual-attribute time-intensity sensory evaluation: A new method for temporal measurement of sensory perceptions. Food Qual. Pref. 1997, 8, 261–269. [Google Scholar] [CrossRef]
- Pineau, N.; Schlich, P.; Cordelle, S.; Schlich, P. Temporal Dominance of Sensation: A new technique to record several sensory attributes simultaneously over time. In Proceedings of the 5th Pangborn Symposium, Boston, MA, USA, 20–24 July 2003; pp. 20–24. [Google Scholar]
- Kuesten, C.; Bi, J.; Feng, Y.-H. Exploring taffy product consumption experiences using a multi-attribute time-intensity (MATI) method. Food Qual. Pref. 2013, 30, 260–273. [Google Scholar] [CrossRef]
- Kuesten, C.; Bi, J. Temporal Drivers of Liking Based on Functional Data Analysis and Non-Additive Models for Multi-Attribute Time-Intensity Data of Fruit Chews. Foods 2018, 7, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lekalake, K.; Rosemary, I.; Taylor, J.R.N.; De Kock, H.L. Application of the dual attribute time-intensity (DATI) sensory method to the temporal measurement of bitterness and astringency in sorghum. Int. J. Food Sci. Technol. 2012, 47, 459–466. [Google Scholar] [CrossRef] [Green Version]
- Pineau, N.; Schilch, P. Temporal dominance of sensations (TDS) as a sensory profiling technique. In Rapid Sensory Profiling Techniques Applications in New Product Development and Consumer Research; Julien Delarue, J., Ben Lawlor, M.R., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition: Cambridge, UK, 2015; pp. 269–306. [Google Scholar] [CrossRef]
- Schlich, P.; Pineau, N. Temporal Dominance of Sensations. In Time-Dependent Measures of Perception in Sensory Evaluation; Hort, J., Kemp, S.E., Hollowood, T., Eds.; John Wiley & Sons Ltd.: New York, NY, USA, 2017; pp. 283–320. [Google Scholar] [CrossRef]
- Kauer, J.S.; White, J. Electronic Nose. In Encyclopedia of Neuroscience; Larry, R.S., Ed.; Academic Press: New York, NY, USA, 2009; pp. 871–877. [Google Scholar] [CrossRef]
- Liu, T.; Chen, Y.B.; Li, D.Q.; Wu, M. An active feature selection strategy for DWT in artificial taste. J. Sens. 2018, 1–11. [Google Scholar] [CrossRef] [Green Version]
- McQueen, R.H. Odour control of medical textiles. In Handbook of Medical Textiles; Bartels, V.T., Ed.; Woodhead Publishing Series in Textiles: New York, NY, USA, 2011; pp. 387–416. [Google Scholar] [CrossRef]
- Vilela, A.; Pinto, T.; Gonçalves, B.; Bacelar, E.; Correia, C.; Jordão, A.M.; Cosme, F. Food analysis: From structure, chemistry and flavour to foodomics. In Science within Food: Up-to-Date Advances on Research and Educational Ideas; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2017; pp. 95–115. [Google Scholar]
- Wilson, A.D.; Baietto, M. Applications and advances in electronic-nose technologies. Sensors 2009, 9, 5099–5148. [Google Scholar] [CrossRef]
- Liu, H.; Li, Q.; Yan, B.; Zhang, L.; Gu, Y. Bionic Electronic Nose Based on MOS Sensors Array and Machine Learning Algorithms Used for Wine Properties Detection. Sensors 2018, 19, 45. [Google Scholar] [CrossRef] [Green Version]
- Meixner, H.; Lampe, U. Metal oxide sensors. Sens. Actuators B Chem. 1996, 33, 198. [Google Scholar] [CrossRef]
- Wei, C.; Dai, L.; Roy, A.; Tolle, T.B. Multifunctional chemical vapro sensors of aligned carbon nanotube and polymer composites. J. Am. Chem. Soc. 2006, 128, 1412. [Google Scholar] [CrossRef] [PubMed]
- Gabl, R.; Feucht, H.D.; Zeininger, H.; Eckstein, G.; Schreiter, M.; Primig, R.; Pitzer, D.; Wersing, W. First results on label-free detection of DNA and protein molecules using a novel integrated sensor technology based on gravimetric detection principles. Biosens. Bioelectron. 2004, 19, 615. [Google Scholar] [CrossRef]
- Gorris, H.H.; Blicharz, T.M.; Walt, D.R. Optical-fiber bundles. FEBS J. 2007, 274, 5462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epstein, J.R.; Walt, D.R. Fluorescence-based fibre optic arrays: A universal platform for sensing. Chem. Soc. Rev. 2003, 32, 203. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.-Y.; Deng, S.-P.; Chen, Z.-X. Multifrequency large amplitude pulse voltammetry: A novel electrochemical method for electronic tongue. Sens. Actuators B Chem. 2007, 123, 1049. [Google Scholar] [CrossRef]
- Gallardo, J.; Alegert, S.; Del Valle, M. A flow-injection electronic tongue based on potentiometric sensors for the determination of nitrate in the presence of chloride. Sens. Actuators B Chem. 2004, 101, 72. [Google Scholar] [CrossRef]
- Ionescu, R.; Broza, Y.; Shaltieli, H.; Sadeh, D.; Zilberman, Y.; Feng, X.; Glass-Marmor, L.; Lejbkowicz, I.; Müllen, K.; Miller, A.; et al. Detection of multiple sclerosis from exhaled breath using bilayers of polycyclic aromatic hydrocarbons and single-wall carbon nanotubes. ACS Chem. Neurosci. 2011, 2, 687. [Google Scholar] [CrossRef] [Green Version]
- Zampetti, E.; Pantalei, S.; Macagnano, A.; Proietti, E.; Di Natale, C.; D’Amico, A. Use of a multiplexed oscillator in a miniaturized electronic nose based on a multichannel quartz crystal microbalance. Sens. Actuators B Chem. 2008, 131, 159–166. [Google Scholar] [CrossRef]
- Fitzgerald, J.E.; Shen, J.; Fenniri, H. A Barcoded Polymer-Based Cross-Reactive Spectroscopic Sensor Array for Organic Volatiles. Sensors 2019, 19, 3683. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Li, D.; Chen, J.; Chen, Y.; Yang, T.; Cao, J. Active Learning on Dynamic Clustering for Drift Compensation in an Electronic Nose System. Sensors 2019, 19, 3601. [Google Scholar] [CrossRef] [Green Version]
- Ziyatdinov, A.; Chaudry, A.; Persaud, K. Common principal component analysis for drift compensation of gas sensor array data. AIP Conf. Proc. 2009, 1137, 566–569. [Google Scholar] [CrossRef] [Green Version]
- Artursson, T.; Eklöv, T.; Lundström, I. Drift correction for gas sensors using multivariate methods. J. Chemom. 2000, 14, 711–723. [Google Scholar] [CrossRef]
- Yin, Y.; Wu, W.K.; Ye, M. Drift elimination method of electronic nose signals based on independent component analysis coupled with wavelet energy threshold value. Trans. Chin. Soc. Agric. Eng. 2014, 24, 325–331. [Google Scholar]
- Lavigne, J.J.; Savoy, S.; Clevenger, M.B.; Ritchie, J.E.; McDoniel, B.; Yoo, S.-J.; Anslyn, E.V.; McDevitt, J.T.; Shear, J.B.; Neikirk, D. Solution-based analysis of multiple analytes by a sensor array: Toward the development of an “electronic tongue”. J. Am. Chem. Soc. 1998, 120, 6429–6430. [Google Scholar] [CrossRef]
- Toko, K. Taste sensor with global selectivity. Mat. Sci. Eng. C 1996, 4, 69–82. [Google Scholar] [CrossRef]
- Tahara, Y.; Toko, K. Electronic tongues–A review. IEEE Sens. J. 2013, 13, 3001–3011. [Google Scholar] [CrossRef]
- Sliwinska, M.; Wisniewska, P.; Dymerski, T.; Namiesnik, J.; Wardencki, W. Food analysis using artifcial senses´. J. Agric. Food Chem. 2014, 62, 1423–1448. [Google Scholar] [CrossRef]
- Podrazka, M.; Baczýnska, E.; Kundys, M.; Jelén, P.S.; Nery, E.W. Electronic Tongue—A Tool for All Tastes? Biosensors 2018, 8, 3. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Méndez, M.L.; De Saja, J.A.; González-Antón, R.; García-Hernández, C.; Medina-Plaza, C.; García-Cabezón, C.; Martín-Pedrosa, F. Electronic Noses and Tongues in Wine Industry. Front. Bioeng. Bio. 2016, 4, 81. [Google Scholar] [CrossRef] [Green Version]
- Scampicchio, M.; Benedetti, S.; Brunetti, B.; Mannino, S. Amperometric Electronic Tongue for the Evaluation of the Tea Astringency. Electroanalysis 2006, 18, 1643–1648. [Google Scholar] [CrossRef]
- Winquist, F. Voltammetric electronic tongues—Basic principles and applications. Microchim. Acta 2008, 163, 3. [Google Scholar] [CrossRef] [Green Version]
- Mimendia, A.; Gutiérrez, J.M.; Leija, L.; Hernández, P.R.; Favari, L.; Muñoz, R.; del Valle, M. A review of the use of the potentiometric electronic tongue in the monitoring of environmental systems. Environ. Model. Soft. 2010, 25, 1023–1030. [Google Scholar] [CrossRef]
- Elamine, Y.; Inácio, P.M.C.; Lyoussi, B.; Anjos, O.; Estevinho, L.M.; Miguel, M.G.; Gomes, H.L. Insight into the sensing mechanism of an impedance based electronic tongue for honey botanic origin discrimination. Sens. Actuators B Chem. 2019, 285, 24–33. [Google Scholar] [CrossRef] [Green Version]
- Volpati, D.; Aoki, P.H.B.; Dantas, C.A.R.; Paulovich, F.V.; de Oliveira, M.C.F.; Oliveira, O.N. Toward the optimization of an e-tongue system using information visualization: A case study with perylene tetracarboxylic derivative films in the sensing units. Langmuir 2012, 28, 1029–1040. [Google Scholar] [CrossRef]
- Lvova, L.; Pudi, R.; Galloni, P.; Lippolis, V.; Di Natale, C.; Lundström, I.; Paolesse, R. Multi-transduction sensing films for Electronic Tongue applications. Sens. Actuators B Chem. 2015, 207, 1076–1086. [Google Scholar] [CrossRef]
- Gutiérrez, M.; Llobera, A.; Vila-Planas, J.; Capdevila, F.; Demming, S.; Büttgenbach, S.; Mínguez, S.; Jiménez-Jorquera, C. Hybrid electronic tongue based on optical and electrochemical microsensors for quality control of wine. Analyst 2010, 135, 1718–1725. [Google Scholar] [CrossRef]
- Toko, K. Biochemical Sensors: Mimicking Gustatory and Olfactory Senses; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Lu, L.; Deng, S.; Zhu, Z.; Tian, S. Classification of Rice by Combining Electronic Tongue and Nose. Food Anal. Methods 2015, 8, 1893–1902. [Google Scholar] [CrossRef]
- Cavallari, M.R.; Braga, G.S.; Da Silva, M.F.P.; Izquierdo, J.E.E.; Paterno, L.G.; Dirani, E.A.T.; Kymissis, I.; Fonseca, F.J. A Hybrid Electronic Nose and Tongue for the Detection of Ketones: Improved Sensor Orthogonality Using Graphene Oxide-Based Detectors. IEEE Sens. J. 2017, 17, 1971–1980. [Google Scholar] [CrossRef]
- Di Rosa, A.R.; Leone, F.; Cheli, F.; Chiofalo, V. Fusion of electronic nose, electronic tongue and computer vision for animal source food authentication and quality assessment—A review. J. Food Eng. 2017, 210, 62–75. [Google Scholar] [CrossRef]
- Cole, M.; Covington, J.A.; Gardner, J.W. Combined electronic nose and tongue for a flavour sensing system. Sens. Actuator B Chem. 2011, 156, 832–839. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.K.; Lee, Y.-J.; Kwak, H.S.; Kang, M. Identification of Sensory Attributes That Drive Consumer Liking of Commercial Orange Juice Products in Korea. J. Food Sci. 2013, 78, S1451–S1458. [Google Scholar] [CrossRef]
- Paula, A.M.; Conti-Silva, A.C. Texture profile and correlation between sensory and instrumental analyses on extruded snacks. J. Food Eng. 2014, 121, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Jimenez-Garcia, S.N.; Vázquez-Cruz, M.A.; Miranda-Lopez, R.; Garcia-Mier, L.; Guevara-González, R.G.; Feregrino-Perez, A.A. Effect of Elicitors as Stimulating Substances on Sensory Quality Traits in Color Sweet Bell Pepper (Capsicum annuum L. cv. Fascinato and Orangela) Grown under Greenhouse Conditions. Pol. J. Food Nutr. Sci. 2018, 68, 359–365. [Google Scholar] [CrossRef]
- Esti, M.; Airola, R.L.G.; Moneta, E.; Paperaio, M.; Sinesio, F. Qualitative data analysis for an exploratory sensory study of Grechetto wine. Anal. Chim. Acta 2010, 660, 63–67. [Google Scholar] [CrossRef]
- Murray, J.; Delahunty, C. Mapping consumer preference for the sensory and packaging attributes of Cheddar cheese. Food Qual. Pref. 2000, 11, 419–435. [Google Scholar] [CrossRef]
- Rebollar, R.; Lidón, I.; Serrano, A.; Martín, J.; Fernández, M.J. Influence of chewing gum packaging design on consumer expectation and willingness to buy. An analysis of functional, sensory and experience attributes. Food Qual. Pref. 2012, 24, 162–170. [Google Scholar] [CrossRef]
- Aleixandre-Tudó, J.L.; Alvarez, I.; García, M.J.; Lizama, V.; Aleixandre, J.L. Application of multivariate regression methods to predict sensory quality of red wines. Czech J. Food Sci. 2015, 33, 217–227. [Google Scholar] [CrossRef] [Green Version]
- Poirson, E.; Petiot, J.F.; Richard, F. A method for perceptual evaluation of products by naive subjects: Application to car engine sounds. Int. J. Ind. Ergon. 2010, 40, s504–s516. [Google Scholar] [CrossRef] [Green Version]
- Meulman, J.J.; Van Der Kooij, A.; Heiser, W. Principal Component Analysis with Nonlinear Optimal Scaling Transformations for Ordinal and Nominal Data. In The SAGE Handbook of Quantitative Methodology for the Social Sciences; Kaplan, D., Ed.; Sage: London, UK, 2004. [Google Scholar] [CrossRef]
- Vilela, A.; Monteiro, B.; Correia, E. Sensory profile of port wines: Categorical principal component analysis, an approach for sensory data treatment. Cienc. Tec. Vitivinic. 2015, 30, 1–8. [Google Scholar] [CrossRef]
- Vilela, A.; Marques, C.; Correia, E. Structural Equation Modelling (SEM) applied to sensory profile of Vinho Verde monovarietal wines. Food Res. Int. 2018, 111, 650–660. [Google Scholar] [CrossRef]
- Marôco, J. Análise de Equações Estruturais: Fundamentos teóricos, software & Aplicações; Report Number Lda: Pêro Pinheiro, Portugal, 2014. [Google Scholar]
- Tu, Y.-K.; Wu, Y.-C. Using structural equation modelling for network meta-analysis. BMC Med. Res. Methodol. 2017, 104, 1471–2288. [Google Scholar] [CrossRef] [Green Version]
- Spearman, C.E. General intelligence objectively determined and measured. Am. J. Psychol. 1904, 15, 201–292. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilela, A.; Bacelar, E.; Pinto, T.; Anjos, R.; Correia, E.; Gonçalves, B.; Cosme, F. Beverage and Food Fragrance Biotechnology, Novel Applications, Sensory and Sensor Techniques: An Overview. Foods 2019, 8, 643. https://doi.org/10.3390/foods8120643
Vilela A, Bacelar E, Pinto T, Anjos R, Correia E, Gonçalves B, Cosme F. Beverage and Food Fragrance Biotechnology, Novel Applications, Sensory and Sensor Techniques: An Overview. Foods. 2019; 8(12):643. https://doi.org/10.3390/foods8120643
Chicago/Turabian StyleVilela, Alice, Eunice Bacelar, Teresa Pinto, Rosário Anjos, Elisete Correia, Berta Gonçalves, and Fernanda Cosme. 2019. "Beverage and Food Fragrance Biotechnology, Novel Applications, Sensory and Sensor Techniques: An Overview" Foods 8, no. 12: 643. https://doi.org/10.3390/foods8120643