Impact of Punicalagin on the Physicochemical and Structural Properties of Wheat Flour Dough
Abstract
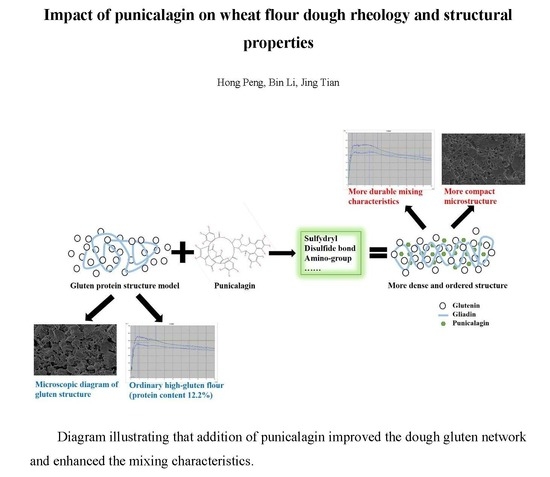
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Sample Preparation
2.3. Dough Mixing Properties
2.4. Dough Stretching Properties
2.5. Rheological Properties Characterization
2.6. Observation of Dough Microstructure
2.7. Measurement of Total and Exposed Free Sulfhydryl (SH) Content
2.8. Determination of the Content of Free Amino Groups
2.9. Fourier Transform Infrared Spectroscopy (FT-IR) Characterization
2.10. Statistical Analyses
3. Results and Discussion
3.1. Impacts of PGN on the Mixing Properties of Dough
3.2. Impacts of PGN on the Stretching Properties of Dough
3.3. Impacts of PGN on Dough Viscoelasticity and Creep-Recovery Profile
3.4. Impacts of PGN on Dough Microstructure
3.5. Analyses of Total and Exposed Free Sulfhydryl (SH) and Free Amino Groups Contents
3.6. Impacts of PGN on Protein Secondary Structure
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shewry, P.R.; Halford, N.G.; Belton, P.S.; Tatham, A.S. The structure and properties of gluten: An elastic protein from wheat grain. Philos. Trans. R. Soc. Lond. Ser. B-Biol. Sci. 2002, 357, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Domenek, S.; Morel, M.H.; Redl, A.; Guilbert, S. Rheological investigation of swollen gluten polymer networks: Effects of process parameters on cross-link density. Macromol. Symp. 2003, 200, 137–145. [Google Scholar] [CrossRef]
- Sapirstein, H.D.; Fu, B.X. Intercultivar variation in the quantity of monomeric proteins, soluble and insoluble glutenin, and residue protein in wheat flour and relationships to breadmaking quality. Cereal Chem. 1998, 75, 500–507. [Google Scholar] [CrossRef]
- Southan, M.; MacRitchie, F. Molecular weight distribution of wheat proteins. Cereal Chem. 1999, 76, 827–836. [Google Scholar] [CrossRef]
- Xu, J.; Wang, W.; Li, Y. Dough properties, bread quality, and associated interactions with added phenolic compounds: A review. J. Funct. Foods 2019, 52, 629–639. [Google Scholar] [CrossRef]
- Jazaeri, S.; Bock, J.E.; Bagagli, M.P.; Iametti, S.; Bonomi, F.; Seetharaman, K. Structural Modifications of Gluten Proteins in Strong and Weak Wheat Dough During Mixing. Cereal Chem. 2015, 92, 105–113. [Google Scholar] [CrossRef]
- Dangi, P.; Chaudhary, N.; Khatkar, B.S. Rheological and microstructural characteristics of low molecular weight glutenin subunits of commercial wheats. Food Chem. 2019, 297, 6. [Google Scholar] [CrossRef]
- Khatkar, B.S.; Bell, A.E.; Schofield, J.D. The dynamic rheological properties of glutens and gluten subfractions from wheats of good and poor bread-making quality. J. Cereal Sci. 1995, 22, 29–44. [Google Scholar] [CrossRef]
- Kohajdova, Z.; Karovicova, J. Impact of potassium iodate on the quality of wheat-spelt baked goods. Acta Sci. Pol.-Technol. Aliment. 2010, 9, 443–450. [Google Scholar]
- Pecivova, P.; Pavlinek, V.; Hrabe, J. The effect of the combination of reducing and oxidising agents on the viscoelastic properties of dough and sensory characteristics of buns. J. Sci. Food Agric. 2010, 90, 1681–1687. [Google Scholar] [CrossRef]
- Decamps, K.; Joye, I.J.; De Vos, D.E.; Courtin, C.M.; Delcour, J.A. Molecular Oxygen and Reactive Oxygen Species in Bread-making Processes: Scarce, but Nevertheless Important. Crit. Rev. Food Sci. Nutr. 2016, 56, 722–736. [Google Scholar] [CrossRef] [PubMed]
- Baiano, A.; Terracone, C. Dough rheology and bread quality of supplemented flours. CyTA-J. Food 2011, 9, 180–186. [Google Scholar] [CrossRef]
- Tang, L.L.; Yang, R.J.; Hua, X.; Yu, C.H.; Zhang, W.B.; Zhao, W. Preparation of immobilized glucose oxidase and its application in improving breadmaking quality of commercial wheat flour. Food Chem. 2014, 161, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Longoria-Garcia, S.; Cruz-Hernandez, M.A.; Flores-Verastegui, M.I.M.; Contreras-Esquivel, J.C.; Montanez-Saenz, J.C.; Belmares-Cerda, R.E. Potential functional bakery products as delivery systems for prebiotics and probiotics health enhancers. J. Food Sci. Technol.-Mysore 2018, 55, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Paucean, A.; Man, S.M.; Chis, M.S.; Muresan, V.; Pop, C.R.; Socaci, S.A.; Muresan, C.C.; Muste, S. Use of Pseudocereals Preferment Made with Aromatic Yeast Strains for Enhancing Wheat Bread Quality. Foods 2019, 8, 443. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, D.J.M.; Moura, E.F.; Cunha, M.A.d.; Medeiros, A.C.Q.d. Analysis on the occurrence of bromate contamination in French type bread. Rev. Inst. Adolfo Lutz 2014, 73, 233–237. [Google Scholar] [CrossRef]
- Aguilar-Zarate, P.; Wong-Paz, J.E.; Michel, M.; Buenrostro-Figueroa, J.; Diaz, H.R.; Ascacio, J.A.; Contreras-Esquivel, J.C.; Gutierrez-Sanchez, G.; Aguilar, C.N. Characterisation of Pomegranate-Husk Polyphenols and Semi-Preparative Fractionation of Punicalagin. Phytochem. Anal. 2017, 28, 433–438. [Google Scholar] [CrossRef]
- Lu, J.J.; Ding, K.; Yuan, Q.P. One-Step Purification of Punicalagin by Preparative HPLC and Stability Study on Punicalagin. Sep. Sci. Technol. 2011, 46, 147–154. [Google Scholar] [CrossRef]
- Heber, D.; Seeram, N.P.; Wyatt, H.; Henning, S.M.; Zhang, Y.J.; Ogden, L.G.; Dreher, M.; Hill, J.O. Safety and antioxidant activity of a pomegranate ellagitannin-enriched polyphenol dietary supplement in overweight individuals with increased waist size. J. Agric. Food Chem. 2007, 55, 10050–10054. [Google Scholar] [CrossRef]
- Vlachojannis, C.; Zimmermann, B.F.; Chrubasik-Hausmann, S. Efficacy and Safety of Pomegranate Medicinal Products for Cancer. Evid.-Based Complement. Altern. Med. 2015, 2015, 258598. [Google Scholar] [CrossRef]
- Nunez-Sanchez, M.A.; Garcia-Villalba, R.; Monedero-Saiz, T.; Garcia-Talavera, N.V.; Gomez-Sanchez, M.B.; Sanchez-Alvarez, C.; Garcia-Albert, A.M.; Rodriguez-Gil, F.J.; Ruiz-Marin, M.; Pastor-Quirante, F.A.; et al. Targeted metabolic profiling of pomegranate polyphenols and urolithins in plasma, urine and colon tissues from colorectal cancer patients. Mol. Nutr. Food Res. 2014, 58, 1199–1211. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Sarrias, A.; Garcia-Villalba, R.; Romo-Vaquero, M.; Alasalvar, C.; Orem, A.; Zafrilla, P.; Tomas-Barberan, F.A.; Selma, M.V.; Espin, J.C. Clustering according to urolithin metabotype explains the interindividual variability in the improvement of cardiovascular risk biomarkers in overweight-obese individuals consuming pomegranate: A randomized clinical trial. Mol. Nutr. Food Res. 2017, 61, 14. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Sarrias, A.; Romo-Vaquero, M.; Garcia-Villalba, R.; Cortes-Martin, A.; Selma, M.V.; Espin, J.C. The Endotoxemia Marker Lipopolysaccharide-Binding Protein is Reduced in Overweight-Obese Subjects Consuming Pomegranate Extract by Modulating the Gut Microbiota: A Randomized Clinical Trial. Mol. Nutr. Food Res. 2018, 62, 10. [Google Scholar] [CrossRef] [PubMed]
- Rana, J.; Diwakar, G.; Saito, L.; Scholten, J.D.; Mulder, T. Inhibition of melanin content by Punicalagins in the super fruit pomegranate (Punica granatum). J. Cosmet. Sci. 2013, 64, 445–453. [Google Scholar] [PubMed]
- Zhao, S.S.; Ma, D.X.; Zhu, Y.; Zhao, J.H.; Zhang, Y.; Chen, J.Q.; Sheng, Z.L. Antidiarrheal effect of bioactivity-guided fractions and bioactive components of pomegranate (Punica granatum L.) peels. Neurogastroenterol. Motil. 2018, 30, 10. [Google Scholar] [CrossRef]
- Turgut, S.S.; Soyer, A.; Işıkçı, F. Effect of pomegranate peel extract on lipid and protein oxidation in beef meatballs during refrigerated storage. Meat Sci. 2016, 116, 126–132. [Google Scholar] [CrossRef]
- Aslam, M.N.N.; Lansky, E.P.; Varani, J. Pomegranate as a cosmeceutical source: Pomegranate fractions promote proliferation and procollagen synthesis and inhibit matrix metalloproteinase-1 production in human skin cells. J. Ethnopharmacol. 2006, 103, 311–318. [Google Scholar] [CrossRef]
- Baltazar, D.; Marto, J.; Berger, T.; Pinto, P.; Ribeiro, H.M. The antiageing efficacy of donkey milk in combination with pomegranate extract and UV protection A traditional ingredient in a modern formulation. Agro Food Ind. Hi-Tech. 2017, 28, 25–27. [Google Scholar]
- Emam-Djomeh, Z.; Moghaddam, A.; Ardakani, S.A.Y. Antimicrobial Activity of Pomegranate (Punica granatum L.) Peel Extract, Physical, Mechanical, Barrier and Antimicrobial Properties of Pomegranate Peel Extract-incorporated Sodium Caseinate Film and Application in Packaging for Ground Beef. Packag. Technol. Sci. 2015, 28, 869–881. [Google Scholar] [CrossRef]
- Uzunlu, S.; Niranjan, K. Laboratory antimicrobial activity of cinnamaldehyde and pomegranate-based polycaprolactone films. J. Appl. Polym. Sci. 2017, 134, 9. [Google Scholar] [CrossRef]
- Arabestani, A.; Kadivar, M.; Shahedi, M.; Goli, S.A.H.; Porta, R. Characterization and antioxidant activity of bitter vetch protein-based films containing pomegranate juice. Lwt-Food Sci. Technol. 2016, 74, 77–83. [Google Scholar] [CrossRef]
- Ozdal, T.; Capanoglu, E.; Altay, F. A review on protein–phenolic interactions and associated changes. Food Res. Int. 2013, 51, 954–970. [Google Scholar] [CrossRef]
- Sivam, A.S.; Sun-Waterhouse, D.; Quek, S.; Perera, C.O. Properties of bread dough with added fiber polysaccharides and phenolic antioxidants: A review. J. Food Sci. 2010, 75, 163–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef]
- Perez-Gregorio, M.R.; Simal-Gandara, J. A Critical Review of the Characterization of Polyphenol-Protein Interactions and of Their Potential Use for Improving Food Quality. Curr. Pharm. Des. 2017, 23, 2742–2753. [Google Scholar] [CrossRef]
- Qi-feng, Y.; Zheng-qi, W.; Xiao-qiang, C.; Yin, Z.; Huang, H. Research progress on tea polyphenol-protein interaction. Sci. Technol. Food Ind. 2019, 40, 337–342. [Google Scholar] [CrossRef]
- Zhu, F.; Sakulnak, R.; Wang, S. Effect of black tea on antioxidant, textural, and sensory properties of Chinese steamed bread. Food Chem. 2016, 194, 1217–1223. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Chen, J.; Chuah, C.; Wibisono, R.; Melton, L.D.; Laing, W.; Ferguson, L.R.; Skinner, M.A. Kiwifruit-based polyphenols and related antioxidants for functional foods: Kiwifruit extract-enhanced gluten-free bread. Int. J. Food Sci. Nutr. 2009, 60, 251–264. [Google Scholar] [CrossRef]
- Das, L.; Raychaudhuri, U.; Chakraborty, R. Supplementation of common white bread by coriander leaf powder. Food Sci. Biotechnol. 2012, 21, 425–433. [Google Scholar] [CrossRef]
- Hye Min, H.; Bong-Kyung, K. Effect of phenolic acids on the rheological properties and proteins of hard wheat flour dough and bread. J. Sci. Food Agric. 2011, 91, 2495–2499. [Google Scholar] [CrossRef]
- Frazier, R.A.; Deaville, E.R.; Green, R.J.; Stringano, E.; Willoughby, I.; Plant, J.; Mueller-Harvey, I. Interactions of tea tannins and condensed tannins with proteins. J. Pharm. Biomed. Anal. 2010, 51, 490–495. [Google Scholar] [CrossRef]
- Girard, A.L.; Castell-Perez, M.E.; Bean, S.R.; Adrianos, S.L.; Awika, J.M. Effect of Condensed Tannin Profile on Wheat Flour Dough Rheology. J. Agric. Food Chem. 2016, 64, 7348–7356. [Google Scholar] [CrossRef] [PubMed]
- Stampfli, L.; Nersten, B.; Molteberg, E.L. Effects of emulsifiers on farinograph and extensograph measurements. Food Chem. 1996, 57, 523–530. [Google Scholar] [CrossRef]
- Gujral, H.S.; Rosell, C.M. Functionality of rice flour modified with a microbial transglutaminase. J. Cereal Sci. 2004, 39, 225–230. [Google Scholar] [CrossRef]
- Bockstaele, F.v.; Leyn, I.d.; Eeckhout, M.; Dewettinck, K. Rheological properties of wheat flour dough and the relationship with bread volume. I. Creep-recovery measurements. Cereal Chem. 2008, 85, 753–761. [Google Scholar] [CrossRef]
- Beveridge, T.; Toma, S.J.; Nakai, S. Determination of SH-groups and SS-groups in some food proteins using ellmans reagent. J. Food Sci. 1974, 39, 49–51. [Google Scholar] [CrossRef]
- Riddles, P.W.; Blakeley, R.L.; Zerner, B. Ellmans reagent-5,5′-dithiobis(2-nitrobenzoic acid)-re-examination. Anal. Biochem. 1979, 94, 75–81. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue Sulfhydryl Groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Wellner, N.; Mills, E.N.C.; Brownsey, G.; Wilson, R.H.; Brown, N.; Freeman, J.; Halford, N.G.; Shewry, P.R.; Belton, P.S. Changes in protein secondary structure during gluten deformation studied by dynamic Fourier transform infrared spectroscopy. Biomacromolecules 2005, 6, 255–261. [Google Scholar] [CrossRef]
- Bock, J.E.; Connelly, R.K.; Damodaran, S. Impact of Bran Addition on Water Properties and Gluten Secondary Structure in Wheat Flour Doughs Studied by Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy. Cereal Chem. 2013, 90, 377–386. [Google Scholar] [CrossRef]
- Bock, J.E.; Damodaran, S. Bran-induced changes in water structure and gluten conformation in model gluten dough studied by Fourier transform infrared spectroscopy. Food Hydrocoll. 2013, 31, 146–155. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, L.B.; Jiang, L.J.; Wang, Y.S.; Yang, G.X.; He, G.Y. Effects of tannic acid on gluten protein structure, dough properties and bread quality of Chinese wheat. J. Sci. Food Agric. 2010, 90, 2462–2468. [Google Scholar] [CrossRef]
- Faria, A.; Calhau, C. The Bioactivity of Pomegranate: Impact on Health and Disease. Crit. Rev. Food Sci. Nutr. 2011, 51, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Ma, Z.; Wang, X.; Li, X.; Liu, L.; Hu, X. Effect of kansui addition on dough rheology and quality characteristics of chickpea-wheat composite flour-based noodles and the underlying mechanism. Food Chem. 2019, 298, 125081. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Luo, Z.; Yang, Q.; Xiao, Z.; Lu, X. Effect of quinoa flour on baking performance, antioxidant properties and digestibility of wheat bread. Food Chem. 2019, 294, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Selinheimo, E.; Autio, K.; Krijus, K.; Buchert, J. Elucidating the mechanism of laccase and tyrosinase in wheat bread making. J. Agric. Food Chem. 2007, 55, 6357–6365. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, E.G.; Bosch, A.; Yantorno, O.; Baran, E.J. A spectroscopy approach for the study of the interactions of bioactive vanadium species with bovine serum albumin. Bioorganic Med. Chem. 2008, 16, 3878–3886. [Google Scholar] [CrossRef]
- Han, C.-W.; Ma, M.; Zhang, H.-H.; Li, M.; Sun, Q.-J. Progressive study of the effect of superfine green tea, soluble tea, and tea polyphenols on the physico-chemical and structural properties of wheat gluten in noodle system. Food Chem. 2019, 308, 125676. [Google Scholar] [CrossRef]



| Flour | Add Amount (mg/g) | Water Absorption Rate (%) | Formation Time (min) | Stability (min) | Weakening Degree (FU) |
|---|---|---|---|---|---|
| High-gluten | 0 | 59.2 ± 0.10 a | 1.50 ± 0.01 a | 3.93 ± 0.15 a | 112.0 ± 4.58 a |
| 0.13 | 59.3 ± 0.25 a | 1.83 ± 0.11 a | 4.57 ± 0.72 a | 91.7 ± 14.6 bc | |
| 0.26 | 59.3 ± 0.10 a | 4.73 ± 1.05 b | 5.57 ± 0.15 b | 92.7 ± 7.37 b | |
| 0.39 | 59.3 ± 0.06 a | 4.60 ± 0.44 b | 5.80 ± 0.20 b | 76.7 ± 4.93 bc | |
| 0.52 | 59.3 ± 0.06 a | 4.73 ± 0.42 b | 5.80 ± 0.36 b | 76.0 ± 7.21 c | |
| Low-gluten | 0 | 58.3 ± 0.20 a | 1.47 ± 0.06 a | 3.17 ± 0.32 a | 120.3 ± 11 a |
| 0.13 | 58.3 ± 0.06 a | 1.63 ± 0.15 ab | 3.30 ± 0.10 b | 103.7 ± 17 a | |
| 0.26 | 58.2 ± 0.06 a | 1.70 ± 0.20 ab | 3.37 ± 0.21 bc | 118.3 ± 4.5 a | |
| 0.39 | 58.3 ± 0.06 a | 1.83 ± 0.35 c | 3.83 ± 0.25 cd | 103.3 ± 13 a | |
| 0.52 | 58.2 ± 0.10 a | 1.57 ± 0.15 a | 4.13 ± 0.38 d | 78.67 ± 5.5 b |
| Flour | Add Amount (mg/g) | Free SH (μM/g dough) | Total SH (μM/g dough) | Free Amino Groups Absorbance |
|---|---|---|---|---|
| High-gluten | 0 | 0.38 ± 0.005 a | 0.57 ± 0.012 a | 0.051 ± 0.008 b |
| 0.13 | 0.39 ± 0.005 a | 0.62 ± 0.014 b | 0.072 ± 0.001 c | |
| 0.26 | 0.41 ± 0.003 bc | 0.64 ± 0.004 bc | 0.070 ± 0.012 c | |
| 0.39 | 0.43 ± 0.013 c | 0.66 ± 0.005 c | 0.069 ± 0.007 c | |
| 0.52 | 0.40 ± 0.002 ab | 0.77 ± 0.018 d | 0.035 ± 0.008 a | |
| Low-gluten | 0 | 0.38 ± 0.007 a | 0.56 ± 0.006 a | 0.047 ± 0.005 a |
| 0.13 | 0.38 ± 0.004 ab | 0.60 ± 0.002 a | 0.071 ± 0.006 b | |
| 0.26 | 0.39 ± 0.001 bc | 0.60 ± 0.005 a | 0.072 ± 0.017 b | |
| 0.39 | 0.39 ± 0.002 bc | 0.71 ± 0.035 b | 0.050 ± 0.011 a | |
| 0.52 | 0.40 ± 0.009 c | 0.71 ± 0.024 b | 0.047 ± 0.015 a |
| Flour | Add Amount (mg/g) | α-helix (%) | β-sheet (%) | β-turn (%) | Random Coil (%) |
|---|---|---|---|---|---|
| High-gluten | 0 | 14.98 ± 0.05 a | 29.90 ± 0.04 a | 40.70 ± 0.07 a | 14.43 ± 0.01 a |
| 0.13 | 15.83 ± 0.01 d | 31.84 ± 0.05 b | 37.78 ± 0.06 b | 14.56 ± 0.05 b | |
| 0.26 | 15.52 ± 0.14 bc | 32.35 ± 0.12 d | 37.11 ± 0.16 d | 15.03 ± 0.12 d | |
| 0.39 | 15.64 ± 0.04 c | 32.04 ± 0.08 c | 37.58 ± 0.08 c | 14.74 ± 0.02 c | |
| 0.52 | 15.41 ± 0.02 b | 32.27 ± 0.09 d | 37.43 ± 0.08 c | 14.88 ± 0.05 d | |
| Low-gluten | 0 | 14.81 ± 0.16 a | 29.21 ± 0.17 a | 41.89 ± 0.15 a | 14.10 ± 0.13 a |
| 0.13 | 15.56 ± 0.07 c | 32.73 ± 0.05 e | 36.90 ± 0.09 d | 14.81 ± 0.04 b | |
| 0.26 | 15.38 ± 0.06 b | 32.26 ± 0.05 d | 37.33 ± 0.03 c | 15.04 ± 0.03 c | |
| 0.39 | 15.56 ± 0.07 c | 31.91 ± 0.05 c | 37.75 ± 0.02 b | 14.78 ± 0.06 b | |
| 0.52 | 15.69 ± 0.05 d | 31.37 ± 0.08 b | 37.76 ± 0.03 b | 15.18 ± 0.04 d |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, H.; Li, B.; Tian, J. Impact of Punicalagin on the Physicochemical and Structural Properties of Wheat Flour Dough. Foods 2019, 8, 606. https://doi.org/10.3390/foods8120606
Peng H, Li B, Tian J. Impact of Punicalagin on the Physicochemical and Structural Properties of Wheat Flour Dough. Foods. 2019; 8(12):606. https://doi.org/10.3390/foods8120606
Chicago/Turabian StylePeng, Hong, Bin Li, and Jing Tian. 2019. "Impact of Punicalagin on the Physicochemical and Structural Properties of Wheat Flour Dough" Foods 8, no. 12: 606. https://doi.org/10.3390/foods8120606