Recent Advances in Techniques for Starch Esters and the Applications: A Review
Abstract
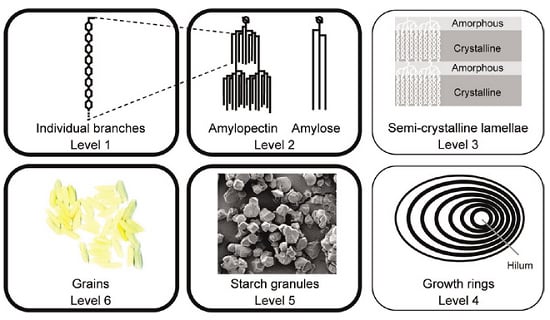
:1. Introduction
2. Determination Methods of DS
3. Comparison of Conventional and Dual Modification Methods
3.1. Conventional Modification
3.2. Dual Modification Combined with Physical Methods
3.2.1. Catalyst
3.2.2. Ball Milling Activation
3.2.3. High Temperature/Pressure
3.2.4. Microwave Technology
3.2.5. Pulsed Electric Fields
3.3. Dual Modification Combined with Chemical Methods
3.3.1. Cross-Linking
3.3.2. Oxidation
4. Emerging Processing Trends in the Future
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ashogbon, A.O.; Akintayo, E.T. Recent trend in the physical and chemical modification of starches from different botanical sources: A review. Starch Starke 2014, 66, 41–57. [Google Scholar] [CrossRef]
- Tran, T.T.B.; Shelat, K.J.; Tang, D.; Li, E.P.; Gilbert, R.G.; Hasjim, J. The degradation on three structural levels of starch in rice flour can be independently controlled during grinding. J. Agric. Food Chem. 2011, 59, 3964–3973. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Zeng, X.A.; Zhang, B.S.; Yu, S.J. Effects of pulsed electric fields (PEF) treatment on the properties of corn starch. J. Food Eng. 2009, 93, 318–323. [Google Scholar] [CrossRef]
- Han, Z.; Zeng, X.A.; Yu, S.J.; Zhang, B.S.; Chen, X.D. Effects of pulsed electric fields (PEF) treatment on physicochemical properties of potato starch. Innov. Food Sci. Emerg. 2009, 10, 481–485. [Google Scholar] [CrossRef]
- Zeng, X.A.; Han, Z.; Zi, Z.H. Effects of pulsed electric field treatments on quality of peanut oil. Food Control 2010, 21, 611–614. [Google Scholar] [CrossRef]
- Lin, Z.R.; Zeng, X.A.; Yu, S.J.; Sun, D.W. Enhancement of ethanol–acetic acid esterification under room temperature and non-catalytic condition via pulsed electric field application. Food Bioprocess Technol. 2012, 5, 2637–2645. [Google Scholar] [CrossRef]
- Sun, W.W.; Yu, S.J.; Zeng, X.A.; Yang, X.Q.; Jia, X. Properties of whey protein isolate–dextran conjugate prepared using pulsed electric field. Food Res. Int. 2011, 44, 1052–1058. [Google Scholar] [CrossRef]
- Kavlani Neelam, S.V.; Singh, L. Various techniques for the modification of starch and the applications of its derivatives. Int. Res. J. Pharm. 2012, 3, 25–31. [Google Scholar]
- Zhang, B.; Zeng, X.A.; Sun, D.W.; Yu, S.J.; Yang, M.F.; Ma, S. Effect of electric field treatments on brandy aging in oak barrels. Food Bioprocess Technol. 2013, 6, 1635–1643. [Google Scholar] [CrossRef]
- Sodhi, N.S.; Singh, N. Characteristics of acetylated starches prepared using starches separated from different rice cultivars. J. Food Eng. 2005, 70, 117–127. [Google Scholar] [CrossRef]
- Wang, Y.J.; Wang, L.F. Characterization of acetylated waxy maize starches prepared under catalysis by different alkali and alkaline-earth hydroxides. Starch Stärke 2002, 54, 25–30. [Google Scholar] [CrossRef]
- Singh, J.; Singh, N.; Saxena, S. Effect of fatty acids on the rheological properties of corn and potato starch. J. Food Eng. 2002, 52, 9–16. [Google Scholar] [CrossRef]
- Singh, N.; Chawla, D.; Singh, J. Influence of acetic anhydride on physicochemical, morphological and thermal properties of corn and potato starch. Food Chem. 2004, 86, 601–608. [Google Scholar] [CrossRef]
- Shogren, R.L. Rapid preparation of starch esters by high temperature/pressure reaction. Carbohydr. Polym. 2003, 52, 319–326. [Google Scholar] [CrossRef]
- Rajan, A.; Prasad, V.S.; Abraham, T.E. Enzymatic esterification of starch using recovered coconut oil. Int. J. Biol. Macromol. 2006, 39, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; Xiong, S.; Jiang, B.; Jiang, H.; Cui, S.W.; Zhang, T. Dual-enzymatic modification of maize starch for increasing slow digestion property. Food Hydrocoll. 2014, 38, 180–185. [Google Scholar] [CrossRef]
- Singh, V.; Tiwari, A. Microwave-accelerated methylation of starch. Carbohydr. Res. 2008, 343, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Lidström, P.; Tierney, J.; Wathey, B.; Westman, J. Microwave assisted organic synthesis—A review. Tetrahedron 2001, 57, 9225–9283. [Google Scholar] [CrossRef]
- Aadil, R.M.; Zeng, X.A.; Sun, D.W.; Wang, M.S.; Liu, Z.W.; Zhang, Z.H. Combined effects of sonication and pulsed electric field on selected quality parameters of grapefruit juice. LWT Food Sci. Technol. 2015, 62, 890–893. [Google Scholar] [CrossRef]
- Lukasiewicz, M.; Kowalski, S. Low power microwave-assisted enzymatic esterification of starch. Starch Starke 2012, 64, 188–197. [Google Scholar] [CrossRef]
- Zięba, T.; Kapelko, M.; Szumny, A. Effect of preparation method on the properties of potato starch acetates with an equal degree of substitution. Carbohydr. Polym. 2013, 94, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, K.; Nishinari, K. Effects of concentration dependence of retrogradation behaviour of dispersions for native and chemically modified potato starch. Food Hydrocoll. 2000, 14, 395–401. [Google Scholar] [CrossRef]
- Zeng, X.A.; Yu, S.J.; Zhang, L.; Chen, X.D. The effects of AC electric field on wine maturation. Innov. Food Sci. Emerg. 2008, 9, 463–468. [Google Scholar] [CrossRef]
- Zukowska, E.A.; Rudnik, E. Commercial use of starch esters. Przem. Chem. 2006, 85, 406–409. [Google Scholar]
- Abbas, K.A.; Khalil, S.K.; Hussin, A.S.M. Modified starches and their usages in selected food products: A review study. J. Agric. Sci. 2010, 2, P90. [Google Scholar] [CrossRef]
- Alissandratos, A.; Halling, P.J. Enzymatic acylation of starch. Bioresour. Technol. 2012, 115, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Gu, J.; Yang, L.; Qiao, Z.; Tan, H.; Zhang, Y. Synthesis and characterization of maleic anhydride esterified corn starch by the dry method. Int. J. Biol. Macromol. 2013, 62, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Golachowski, A.; Zięba, T.; Kapelko-Żeberska, M.; Drożdż, W.; Gryszkin, A.; Grzechac, M. Current research addressing starch acetylation. Food Chem. 2015, 176, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Sweedman, M.C.; Tizzotti, M.J.; Schafer, C.; Gilbert, R.G. Structure and physicochemical properties of octenyl succinic anhydride modified starches: A review. Carbohydr. Polym. 2013, 92, 905–920. [Google Scholar] [CrossRef] [PubMed]
- Ayucitra, A. Preparation and characterization of acetylated corn starches. Int. J. Chem. Eng. Appl. 2012, 3, 156–159. [Google Scholar]
- Bhosale, R.; Singhal, R. Process optimization for the synthesis of octenyl succinyl derivative of waxy corn and amaranth starches. Carbohydr. Polym. 2006, 66, 521–527. [Google Scholar] [CrossRef]
- Chi, H.; Xu, K.; Wu, X.; Chen, Q.; Xue, D.; Song, C.; Zhang, W.; Wang, P. Effect of acetylation on the properties of corn starch. Food Chem. 2008, 106, 923–928. [Google Scholar] [CrossRef]
- Bao, J.S.; Xing, J.; Phillips, D.L.; Corke, H. Physical properties of octenyl succinic anhydride modified rice, wheat, and potato starches. J. Agric. Food Chem. 2003, 51, 2283–2287. [Google Scholar] [CrossRef] [PubMed]
- Ruan, H.; Chen, Q.H.; Fu, M.L.; Xu, Q.; He, G.Q. Preparation and properties of octenyl succinic anhydride modified potato starch. Food Chem. 2009, 114, 81–86. [Google Scholar]
- Hong, J.; Chen, R.; Zeng, X.A.; Han, Z. Effect of pulsed electric fields assisted acetylation on morphological, structural and functional characteristics of potato starch. Food Chem. 2016, 192, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Jyothi, A.N.; Rajasekharan, K.N.; Moorthy, S.N.; Sreekumar, J. Microwave-assisted synthesis and characterization of succinate derivatives of cassava (Manihot esculenta Crantz) starch. Starch Starke 2005, 57, 556–563. [Google Scholar] [CrossRef]
- Rajan, A.; Sudha, J.D.; Abraham, T.E. Enzymatic modification of cassava starch by fungal lipase. Ind. Crop Prod. 2008, 27, 50–59. [Google Scholar] [CrossRef]
- Halal, S.L.M.E.; Colussi, R.; Pinto, V.Z.; Bartz, J.; Radunz, M.; Carreno, N.L.V.; Dias, A.R.G.; Zavareze, E.D.R. Structure, morphology and functionality of acetylated and oxidised barley starches. Food Chem. 2015, 168, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; Li, R.; Jiang, B.; Cui, S.W.; Zhang, T.; Jin, Z. Structure and physicochemical properties of octenyl succinic esters of sugary maize soluble starch and waxy maize starch. Food Chem. 2014, 151, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Luo, Z.; Fu, X.; Xiao, Z. Two-step method of enzymatic synthesis of starch laurate in ionic liquids. J. Agric. Food Chem. 2013, 61, 9882–9891. [Google Scholar] [CrossRef] [PubMed]
- Shogren, R.L.; Biswas, A. Preparation of water-soluble and water-swellable starch acetates using microwave heating. Carbohydr. Polym. 2006, 64, 16–21. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, Q.; Luo, F.X.; Fu, X.; Jiang, H.; Jane, J. Effects of octenylsuccinylation on the structure and properties of high-amylose maize starch. Carbohydr. Polym. 2011, 84, 1276–1281. [Google Scholar] [CrossRef]
- Biswas, A.; Shogren, R.L.; Selling, G.; Salch, J.; Willett, J.L.; Buchanan, C.M. Rapid and environmentally friendly preparation of starch esters. Carbohydr. Polym. 2008, 74, 137–141. [Google Scholar] [CrossRef]
- Tizzotti, M.J.; Sweedman, M.C.; Tang, D.; Schaefer, C.; Gilbert, R.G. New 1H NMR procedure for the characterization of native and modified food-grade starches. J. Agric. Food Chem. 2011, 59, 6913–6919. [Google Scholar] [CrossRef] [PubMed]
- Konowal, E.; Lewandowicz, G.; Le Thanh-Blicharz, J.; Prochaska, K. Physicochemical characterisation of enzymatically hydrolysed derivatives of acetylated starch. Carbohydr. Polym. 2012, 87, 1333–1341. [Google Scholar] [CrossRef]
- Zhong, J.F.; Chai, X.S.; Hu, H.C.; Fu, S.Y. Determination of degree of substitution in succinic anhydride modified cellulose by headspace gas chromatography. J. Chromatogr. A 2012, 1229, 302–304. [Google Scholar] [CrossRef] [PubMed]
- Carlos-Amaya, F.; Osorio-Diaz, P.; Agama-Acevedo, E.; Yee-Madeira, H.; Bello-Perez, L.A. Physicochemical and digestibility properties of double-modified banana (musa paradisiaca L.) starches. J. Agric. Food Chem. 2011, 59, 1376–1382. [Google Scholar] [CrossRef] [PubMed]
- Lawal, O.S. Succinyl and acetyl starch derivatives of a hybrid maize: Physicochemical characteristics and retrogradation properties monitored by differential scanning calorimetry. Carbohydr. Res. 2004, 339, 2673–2682. [Google Scholar] [CrossRef] [PubMed]
- Mccomb, E.A.; Mccready, R.M.; Chem, A. Determination of acetyl in pectin and in acetylated carbohydrate polymers. Anal. Chem. 2002, 29, 819–821. [Google Scholar] [CrossRef]
- Elomaa, M.; Asplund, T.; Soininen, P.; Laatikainen, R.; Peltonen, S.; Hyvarinen, S.; Urtti, A. Determination of the degree of substitution of acetylated starch by hydrolysis, 1H NMR and TGA/IR. Carbohydr. Polym. 2004, 57, 261–267. [Google Scholar] [CrossRef]
- Biswas, A.; Shogren, R.L.; Kim, S.; Willett, J.L. Rapid preparation of starch maleate half–esters. Carbohydr. Polym. 2006, 64, 484–487. [Google Scholar] [CrossRef]
- Zięba, T.; Szumny, A.; Kapelko, M. Properties of retrograded and acetylated starch preparations: Part 1. Structure, susceptibility to amylase, and pasting characteristics. LWT Food Sci. Technol. 2011, 44, 1314–1320. [Google Scholar] [CrossRef]
- Fringant, C.; Desbrieres, J.; Rinaudo, M. Physical properties of acetylated starch-based materials: Relation with their molecular characteristics. Polymer 1996, 37, 2663–2673. [Google Scholar] [CrossRef]
- Liu, H.; Ramsden, L.; Corke, H. Physical poperties of cross-linked and acetylated normal and waxy rice starch. Starch Stärke 1999, 51, 249–252. [Google Scholar] [CrossRef]
- Agboola, S.O.; Akingbala, J.O.; Oguntimein, G.B. Physicochemical and functional properties of low DS cassava starch acetates and citrates. Starch Stärke 1991, 43, 62–66. [Google Scholar] [CrossRef]
- Katerinopoulou, K.; Giannakas, A.; Grigoriadi, K.; Barkoula, N.M.; Ladavos, A. Preparation and characterization of acetylated corn starch–(PVOH)/clay nanocomposite films. Carbohydr. Polym. 2014, 102, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, D.S. The glycemic index: Physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA 2002, 287, 2414–2423. [Google Scholar] [CrossRef] [PubMed]
- Han, J.A.; BeMiller, J.N. Preparation and physical characteristics of slowly digesting modified food starches. Carbohydr. Polym. 2007, 67, 366–374. [Google Scholar] [CrossRef]
- Boutboul, A.; Giampaoli, P.; Feigenbaum, A.; Ducruet, V. Influence of the nature and treatment of starch on aroma retention. Carbohydr. Polym. 2002, 47, 73–82. [Google Scholar] [CrossRef]
- Fang, J.M.; Fowler, P.A.; Tomkinson, J.; Hill, C.A.S. The preparation and characterisation of a series of chemically modified potato starches. Carbohydr. Polym. 2002, 47, 245–252. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Chen, L.; Xie, F.; Yu, L.; Li, B. Preparation and characterisation of octenyl succinate starch as a delivery carrier for bioactive food components. Food Chem. 2011, 126, 1218–1225. [Google Scholar] [CrossRef]
- Fei, H.; Gao, C.; Liu, M.; Feng, H.; Bing, Z. Synthesis, optimization and characterization of acetylated corn starch with the high degree of substitution. Int. J. Biol. Macromol. 2013, 59, 372–376. [Google Scholar]
- Huang, Q.; Fu, X.; He, X.W.; Luo, F.X.; Yu, S.J.; Li, L. The effect of enzymatic pretreatments on subsequent octenyl succinic anhydride modifications of cornstarch. Food Hydrocoll. 2010, 24, 60–65. [Google Scholar] [CrossRef]
- Moraes, J.; Alves, F.S.; Franco, C.M.L. Effect of ball milling on structural and physicochemical characteristics of cassava and Peruvian carrot starches. Starch Starke 2013, 65, 200–209. [Google Scholar] [CrossRef]
- Tian, B.H.; Zhao, Y.L.; Huang, Z.Q.; Tong, Z.F. Synthetic technics of starch phosphate with mechanically activated cassava starch. J. Chem. Eng. Chin. Univ. 2009, 3, 491–495. [Google Scholar]
- Zhang, Z.; Zhao, S.; Xiong, S. Synthesis of octenyl succinic derivative of mechanically activated indica rice starch. Starch Stärke 2010, 62, 78–85. [Google Scholar] [CrossRef]
- Shogren, R.L. Preparation, thermal properties, and extrusion of high-amylose starch acetates. Carbohydr. Polym. 1996, 29, 57–62. [Google Scholar] [CrossRef]
- Kim, H.N.; Sandhu, K.S.; Lee, J.H.; Lim, H.S.; Lim, S.T. Characterisation of 2-octen-1-ylsuccinylated waxy rice amylodextrins prepared by dry-heating. Food Chem. 2010, 119, 1189–1194. [Google Scholar] [CrossRef]
- Vaca-Garcia, C.; Borredon, M.E. A Method for making a cellulose or starch ester by esterification or trans-esterification. Patent 2000. [Google Scholar]
- Rivero, I.E.; Balsamo, V.; Muller, A.J. Microwave-assisted modification of starch for compatibilizing LLDPE/starch blends. Carbohydr. Polym. 2009, 75, 343–350. [Google Scholar] [CrossRef]
- Zeng, X.A.; Zhang, B.S.; Geng, Y.H. FTIR analysis of the alcohol solution treated by high voltage electric field. Guang Pu Xue Yu Guang Pu Fen Xi 2002, 22, 29–32. [Google Scholar] [PubMed]
- Makuuchi, K. Critical review of radiation processing of hydrogel and polysaccharide. Radiat. Phys. Chem. 2010, 79, 267–271. [Google Scholar] [CrossRef]
- Hou, C.; Chen, Y.; Chen, W.; Li, W. Microwave-assisted methylation of cassava starch with dimethyl carbonate. Carbohydr. Res. 2011, 346, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Zeng, X.A.; Buckow, R.; Han, Z.; Wang, M.S. Nanostructure, morphology and functionality of cassava starch after pulsed electric fields assisted acetylation. Food Hydrocoll. 2016, 54, 139–150. [Google Scholar] [CrossRef]
- Ratnayake, W.S.; Jackson, D.S. Phase transition of cross-linked and hydroxypropylated corn (Zea mays L.) starches. LWT Food Sci. Technol. 2008, 41, 346–358. [Google Scholar] [CrossRef]
- Das, A.B.; Singh, G.; Singh, S.; Riar, C.S. Effect of acetylation and dual modification on physico-chemical, rheological and morphological characteristics of sweet potato (Ipomoea batatas) starch. Carbohydr. Polym. 2010, 80, 725–732. [Google Scholar] [CrossRef]
- Lopez, O.V.; Zaritzky, N.E.; Garcia, M.A. Physicochemical characterization of chemically modified corn starches related to rheological behavior, retrogradation and film forming capacity. J. Food Eng. 2010, 100, 160–168. [Google Scholar] [CrossRef]
- Kuakpetoon, D.; Wang, Y.J. Characterization of different starches oxidized by hypochlorite. Starch Starke 2001, 53, 211–218. [Google Scholar] [CrossRef]
- Aini, N.; Purwiyatno, H. Gelatinization properties of white maize starch from three varieties of corn subject to oxidized and acetylated-oxidized modification. Food Res. Int. J. 2010, 17, 961–968. [Google Scholar]
- Kweon, D.K.; Choi, J.K.; Kim, E.K.; Lim, S.T. Adsorption of divalent metal ions by succinylated and oxidized corn starches. Carbohydr. Polym. 2001, 46, 171–177. [Google Scholar] [CrossRef]


| Starch Type | Method | Starch Concentration (w/w) | Anhydride Content (%) | pH | NaOH (w/w) | Reaction Time (min) | Acetyl (%) | DS (%) | References |
|---|---|---|---|---|---|---|---|---|---|
| Corn | Conventional | 29 | 8 | 7.8–8.4 | 4.0 | 60–180 | 2.2–5.3 | 0.080–0.210 | Ayucitra [30] |
| Conventional | 25 | 1–3 | 8.0 | 3.0 | 360–1440 | - | 0.015–0.023 | Bhosale, et al. [31] | |
| Inorganic reagents Catalyzed | 30 | 2–12 | 8.0–8.4 | 3.0 | ~100 | 3.4–4.7 | 0.133–0.154 | Singh, et al. [13] | |
| High temperature | 32 | 32 | 8.0–8.5 | 3.0 | 200 | - | 0.810–2.890 | Chi, et al. [32] | |
| Rice | Conventional | 31 | 6 | 8.0–8.4 | 3.0 | ~100 | 2.3–3.7 | 0.095–0.144 | Sodhi, et al. [10] |
| Sodium carbonate assisted | 40 | 1–6 mL | 8.0–9.0 | - | 840 | - | 0.018–0.045 | Bao, et al. [33] | |
| Potato | Conventional | 20–40 | 3 | 8.0, 8.5 | 3.0 | 120–360 | - | 0.012–0.015 | Ruan, et al. [34] |
| Sodium carbonate assisted | 40 | 1–6 mL | 8.0–9.0 | - | 840 | - | 0.017–0.049 | Bao, et al. [33] | |
| Inorganic reagents catalyzed | 30 | 2–12 | 8.0–8.4 | 3.0 | ~100 | 4.7–6.0 | 0.180–0.238 | Singh, et al. [13] | |
| PEF–assisted | 30–40 | 6 | 8.0–8.5 | 3.0 | 60 | - | 0.054–0.130 | Hong, et al. [35] | |
| Cassava | Microwave-assisted & High temperature | 87–93 | 3–5 | - | No use | 3–7 | 2 | 0.007–0.051 | Jyothi, et al. [36] |
| Enzyme catalyzed & Microwave-assisted | 50 | - | - | No use | 2 | - | 0.330–1.10 | Rajan, et al. [37] | |
| Amaranth | Conventional | 25 | 1–3 | 8.0 | 3.0 | 360–1440 | - | 0.016–0.027 | Bhosale, et al. [31] |
| Determination Method | Types of Esters | Merits | Shortcomings | References |
|---|---|---|---|---|
| Titration | OSA and ACS | Widely used; Better acceptance of its reaction mechanism; Determination of native starch | Complicated process for pretreatment; Increased sample consumption; Time-consuming; Special reaction environment; Color reversion after end point; CO2 disturbance; Pyridine used in sometimes | [16,32,38,47] |
| Back-titration | OSA and ACS | Widely used; Better acceptance of its reaction mechanism; Avoids interference of atmospheric CO2 atmosphere; Determination of native starch | Excess alkali; Increased sample consumption; Color reversion after end point; | [10,13,35,48] |
| Spectrophotometric | ACS | Less sample weight | double color determination; Repeated procedures; | [11,45,49] |
| FT-IR | ACS | Less sample weight | Determination of series of standard with diverse DS; High cost; Use of titration method; Complicated and fussy procedures | [14] |
| NMR (involving 1H-NMR, 13C-NMR) | OSA and ACS | Less sample weight; Simple procedure; Consistent results | the use of internal standards; High cost; Complicated and fussy procedure; Uses of DMSO and chloroform; Determination of DS and DB | [14,41,43,50,51,52] |
| TGA/IR | ACS | Combination of two instruments | High energy consumption; High temperature; Diverse standard samples | [45] |
| HS-GC | Cellulose | Less sample weight; Avoids interference of atmospheric CO2 | - | [46] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, J.; Zeng, X.-A.; Brennan, C.S.; Brennan, M.; Han, Z. Recent Advances in Techniques for Starch Esters and the Applications: A Review. Foods 2016, 5, 50. https://doi.org/10.3390/foods5030050
Hong J, Zeng X-A, Brennan CS, Brennan M, Han Z. Recent Advances in Techniques for Starch Esters and the Applications: A Review. Foods. 2016; 5(3):50. https://doi.org/10.3390/foods5030050
Chicago/Turabian StyleHong, Jing, Xin-An Zeng, Charles S. Brennan, Margaret Brennan, and Zhong Han. 2016. "Recent Advances in Techniques for Starch Esters and the Applications: A Review" Foods 5, no. 3: 50. https://doi.org/10.3390/foods5030050