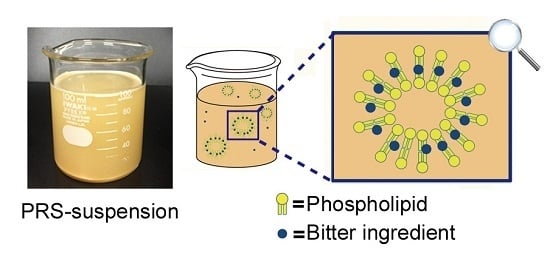
Evaluation of the Bitterness-Masking Effect of Powdered Roasted Soybeans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Bitter Taste Sensing
2.3. Dynamic Light Scattering
2.4. Nuclear Magnetic Resonance
2.5. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Conflicts of Interest
Abbreviations
| PRS | Powdered roasted soybeans |
| T2Rs | Bitter taste receptors |
| QH | Quinine hydrochloride |
| DB | Denatonium benzoate |
| DLS | Dynamic light scattering |
| NMR | nuclear magnetic resonance |
| Vr | Reference potential |
| Vs | Membrane potential |
References
- Drewnowski, A.; Gomez-Carneros, C. Bitter taste, phytonutrients, and the consumer: A review. Am. J. Clin. Nutr. 2000, 72, 1424–1435. [Google Scholar] [PubMed]
- Chandrashekar, J.; Hoon, M.A.; Ryba, N.J.P.; Zuker, C.S. The receptors and cells for mammalian taste. Nature 2006, 444, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Tanigake, A.; Miyanaga, Y.; Ogawa, T.; Akiyoshi, T.; Matsuyama, K.; Uchida, T. The effect of various substances on the suppression of the bitterness of quinine–human gustatory sensation, binding, and taste sensor studies. Chem. Pharm. Bull. 2002, 50, 1589–1593. [Google Scholar] [CrossRef] [PubMed]
- Squier, C.A.; Mantz, M.J.; Wertz, P.W. Effect of menthol on the penetration of tobacco carcinogens and nicotine across porcine oral mucosa ex vivo. Nicotine Tob. Res. 2010, 12, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Wilkie, L.M.; Capaldi Phillips, E.D.; Wadhera, D. Sodium chloride suppresses vegetable bitterness only when plain vegetables are perceived as highly bitter. Chemosens. Percept. 2014, 7, 10–22. [Google Scholar] [CrossRef]
- Slack, J.P.; Brockhoff, A.; Batram, C.; Menzel, S.; Sonnabend, C.; Born, S.; Galindo, M.M.; Kohl, S.; Thalmann, S.; Ostopovici-Halip, L.; et al. Modulation of bitter taste perception by a small molecule hTAS2R antagonist. Curr. Biol. 2010, 20, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Brockhoff, A.; Behrens, M.; Roudnitzky, N.; Appendino, G.; Avonto, C.; Meyerhof, W. Receptor agonism and antagonism of dietary bitter compounds. J. Neurosci. 2011, 31, 14775–14782. [Google Scholar] [CrossRef] [PubMed]
- Greene, T.A.; Alarcon, S.; Thomas, A.; Berdougo, E.; Doranz, B.J.; Breslin, P.A.S.; Rucker, J.B. Probenecid inhibits the human bitter taste receptor TAS2R16 and suppresses bitter perception of salicin. PLoS ONE 2011, 6, e20123. [Google Scholar] [CrossRef] [PubMed]
- Roland, W.S.U.; Gouka, R.J.; Gruppen, H.; Driesse, M.; van Buren, L.; Smit, G.; Vincken, J.-P. 6-Methoxyflavanones as bitter taste receptor blockers for hTAS2R39. PLoS ONE 2014, 9, e94451. [Google Scholar] [CrossRef]
- Khan, S.; Kataria, P.; Nakhat, P.; Yeole, P. Taste masking of ondansetron hydrochloride by polymer carrier system and formulation of rapid-disintegrating tablets. AAPS PharmSciTech 2007, 8, E127–E133. [Google Scholar] [CrossRef] [PubMed]
- Bora, D.; Borude, P.; Bhise, K. Taste masking by spray-drying technique. AAPS PharmSciTech 2008, 9, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.-D.; Woo, J.S.; Kang, J.H.; Yong, C.S.; Choi, H.-G. Preparation and evaluation of taste-masked donepezil hydrochloride orally disintegrating tablets. Biol. Pharm. Bull. 2010, 33, 1364–1370. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, S.T.; Patel, P.B.; Patel, C.N. Formulation and evaluation of sublingual tablets containing Sumatriptan succinate. Int. J. Pharm. Invest. 2012, 2, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Petereit, H.-U. Film coatings for taste masking and moisture protection. Int. J. Pharm. 2013, 457, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Yewale, C.P.; Rathi, M.N.; Kore, G.G.; Jadhav, G.V.; Wagh, M.P. Formulation and development of taste masked fast-disintegrating tablets (FDTs) of Chlorpheniramine maleate using ion-exchange resins. Pharm. Dev. Technol. 2013, 18, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Cantor, S.L.; Khan, M.A.; Gupta, A. Development and optimization of taste-masked orally disintegrating tablets (ODTs) of clindamycin hydrochloride. Drug Dev. Ind. Pharm. 2015, 41, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Khobragade, D.S.; Potbhare, M.S.; Patil, A.T. Evaluation of gum sandarac as a novel release controlling coating polymer for formulation of sustained release pellets. Int. J. Adv. Pharm. 2016, 5, 39–45. [Google Scholar]
- Binello, A.; Cravotto, G.; Nano, G.M.; Spagliardi, P. Synthesis of chitosan–cyclodextrin adducts and evaluation of their bitter-masking properties. Flavour Fragr. J. 2004, 19, 394–400. [Google Scholar] [CrossRef]
- Szejtli, J.; Szente, L. Elimination of bitter, disgusting tastes of drugs and foods by cyclodextrins. Eur. J. Pharm. Biopharm. 2005, 61, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Aytac, Z.; Uyar, T. Antioxidant activity and photostability of α-tocopherol/β-cyclodextrin inclusion complex encapsulated electrospun polycaprolactone nanofibers. Eur. Polym. J. 2016, 79, 140–149. [Google Scholar] [CrossRef]
- Katsuragi, Y. Specific inhibitor for bitter taste. Nature 1993, 365, 213–214. [Google Scholar] [CrossRef] [PubMed]
- Tokuyama, E.; Shibasaki, T.; Kawabe, H.; Mukai, J.; Okada, S.; Uchida, T. Bitterness suppression of BCAA solutions by L-ornithine. Chem. Pharm. Bull. 2006, 54, 1288–1292. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture. USDA National Nutrient Database for Standard Reference 28: Soybeans, Mature Seeds, Roasted, no Salt Added; Basic Report 16410; USDA: Washington, DC, USA, 2016.
- Makita, Y.; Ishida, T.; Kobayashi, N.; Fujio, M.; Fujimoto, K.; Moritomo, R.; Fujiwara, S. Study on masking effect of kinako on bitterness. Jpn. J. Taste Smell Res. 2014, 21, 343–344. [Google Scholar]
- Akitomi, H.; Tahara, Y.; Yasuura, M.; Kobayashi, Y.; Ikezaki, H.; Toko, K. Quantification of tastes of amino acids using taste sensors. Sens. Actuators B 2013, 179, 276–281. [Google Scholar] [CrossRef]
- Tahara, Y.; Toko, K. Electronic tongues–A review. IEEE Sens. J. 2013, 13, 3001–3011. [Google Scholar] [CrossRef]
- Uchida, T. Comprehensive evaluation of palatability for commercial medicine by taste sensing system. Yakugaku Zasshi 2014, 134, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Preis, M.; Eckerta, C.; Häuslerb, H.; Breitkreutza, J. A comparative study on solubilizing and taste-masking capacities of hydroxypropyl-β-cyclodextrin and maltodextrins with high amylose content. Sens. Actuat. B Chem. 2014, 193, 442–450. [Google Scholar] [CrossRef]
- Choi, D.H.; Kim, N.A.; Nam, T.S.; Lee, S.; Jeong, S.H. Evaluation of taste-masking effects of pharmaceutical sweeteners with an electronic tongue system. Drug Dev. Ind. Pharm. 2014, 40, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, T.; Uchida, T.; Hazekawa, M.; Yoshida, M.; Nakashima, M.; Sanda, H.; Hase, T.; Tomoda, Y. Ability of food/drink to reduce the bitterness intensity of topiramate as determined by taste sensor analysis. Chem. Pharm. Bull. 2016, 64, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Onitake, H.; Haraguchi, T.; Tahara, Y.; Yatabe, R.; Yoshida, M.; Uchida, T.; Ikezaki, H.; Toko, K. Quantitative prediction of bitterness masking effect of high-potency sweeteners using taste sensor. Sens. Actuat. B 2016, 235, 11–17. [Google Scholar] [CrossRef]
- Shin, K.; Maeda, H.; Fujiwara, T.; Akutsu, H. Molecular miscibility of phosphatidylcholine and phosphatidylethanolamine in binary mixed bilayers with acidic phospholipids studied by 2H- and 31P-NMR. Biochim. Biophys. Acta 1995, 1238, 42–48. [Google Scholar] [CrossRef]
- Katsuragi, Y.; Sugiura, Y.; Lee, C.; Otsuji, K.; Kurihara, K. Selective inhibition of bitter taste of various drugs by lipoprotein. Pharm. Res. 1995, 12, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Sohi, H.; Sultana, Y.; Khar, R.K. Taste masking technologies in oral pharmaceuticals: Recent developments and approaches. Drug Dev. Ind. Pharm. 2004, 30, 429–448. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Parmar, D.; Patel, U.; Patel, G.; Daslaniya, D.; Bhimani, B. Taste masking: A novel approach for bitter and obnoxious drugs. J. Pharm. Sci. Biosci. Res. 2011, 1, 136–142. [Google Scholar]



© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makita, Y.; Ishida, T.; Kobayashi, N.; Fujio, M.; Fujimoto, K.; Moritomo, R.; Fujita, J.-i.; Fujiwara, S.-i. Evaluation of the Bitterness-Masking Effect of Powdered Roasted Soybeans. Foods 2016, 5, 44. https://doi.org/10.3390/foods5020044
Makita Y, Ishida T, Kobayashi N, Fujio M, Fujimoto K, Moritomo R, Fujita J-i, Fujiwara S-i. Evaluation of the Bitterness-Masking Effect of Powdered Roasted Soybeans. Foods. 2016; 5(2):44. https://doi.org/10.3390/foods5020044
Chicago/Turabian StyleMakita, Yoshimasa, Tomoko Ishida, Noriko Kobayashi, Mai Fujio, Kyoko Fujimoto, Rina Moritomo, Jun-ichi Fujita, and Shin-ichi Fujiwara. 2016. "Evaluation of the Bitterness-Masking Effect of Powdered Roasted Soybeans" Foods 5, no. 2: 44. https://doi.org/10.3390/foods5020044