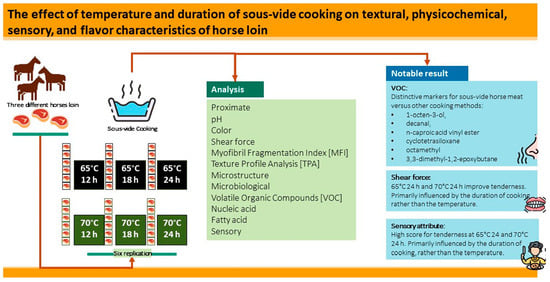
Physicochemical Features and Volatile Organic Compounds of Horse Loin Subjected to Sous-Vide Cooking
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Sample and Experimental Design
2.2. Proximate Analysis and Cooking Loss
2.3. Determination of pH Value
2.4. Instrumental Color Analysis
2.5. Instrumental Texture Analysis
2.6. Measurement of Myofibril Fragmentation Index (MFI)
2.7. Scanning Electron Microscopy (SEM)
2.8. Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.9. Microbiological Analysis
2.10. Measurement of VOC
2.11. Analysis of Nucleotide-Related Compounds
2.12. Analysis of Fatty Acids
2.13. Statistical Analysis
3. Results and Discussion
3.1. Proximate Components and Cooking Losses
3.2. Changes in pH and Instrumental Color
3.3. Changes in Instrumental Texture
3.4. Changes in Microstructure
3.5. Changes in Myofibrillar Protein
3.6. Changes in Microbiology
3.7. Changes in Volatile Compounds
3.8. Change in Nucleotide-Related Compound
3.9. Changes in Fatty Acids
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Belaunzaran, X.; Bessa, R.J.B.; Lavín, P.; Mantecón, A.R.; Kramer, J.K.G.; Aldai, N. Horse-Meat for Human Consumption—Current Research and Future Opportunities. Meat Sci. 2015, 108, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Stanisławczyk, R.; Rudy, M.; Gil, M.; Duma-Kocan, P.; Dziki, D.; Rudy, S. The Effect of Citric Acid, NaCl, and CaCl2 on Qualitative Changes of Horse Meat in Cold Storage. Processes 2020, 8, 1099. [Google Scholar] [CrossRef]
- Kim, D.-S.; Joo, N. Texture Characteristics of Horse Meat for the Elderly Based on the Enzyme Treatment. Food Sci. Anim. Resour. 2020, 40, 74–86. [Google Scholar] [CrossRef]
- Leadon, D.P. Unwanted and Slaughter Horses:A European and Irish Perspective. Anim. Front. 2012, 2, 72–75. [Google Scholar] [CrossRef]
- Thathsarani, A.P.K.; Alahakoon, A.U.; Liyanage, R. Current Status and Future Trends of Sous Vide Processing in Meat Industry; A Review. Trends Food Sci. Technol. 2022, 129, 353–363. [Google Scholar] [CrossRef]
- Latoch, A.; Głuchowski, A.; Czarniecka-Skubina, E. Sous-Vide as an Alternative Method of Cooking to Improve the Quality of Meat: A Review. Foods 2023, 12, 3110. [Google Scholar] [CrossRef]
- Del Pulgar, J.S.; Roldan, M.; Ruiz-Carrascal, J. Volatile Compounds Profile of Sous-Vide Cooked Pork Cheeks as Affected by Cooking Conditions (Vacuum Packaging, Temperature and Time). Molecules 2013, 18, 12538–12547. [Google Scholar] [CrossRef] [PubMed]
- Roldán, M.; Antequera, T.; Martín, A.; Mayoral, A.I.; Ruiz, J. Effect of Different Temperature–Time Combinations on Physicochemical, Microbiological, Textural and Structural Features of Sous-Vide Cooked Lamb Loins. Meat Sci. 2013, 93, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Pereira, J.; Zhou, L.; Lorenzo, J.M.; Tian, X.; Zhang, W. Insight into the Effects of Sous Vide on Cathepsin B and L Activities, Protein Degradation and the Ultrastructure of Beef. Foods 2020, 9, 1441. [Google Scholar] [CrossRef]
- Naqvi, Z.B.; Thomson, P.C.; Ha, M.; Campbell, M.A.; McGill, D.M.; Friend, M.A.; Warner, R.D. Effect of Sous Vide Cooking and Ageing on Tenderness and Water-Holding Capacity of Low-Value Beef Muscles from Young and Older Animals. Meat Sci. 2021, 175, 108435. [Google Scholar] [CrossRef] [PubMed]
- Christensen, L.; Ertbjerg, P.; Løje, H.; Risbo, J.; van den Berg, F.W.J.; Christensen, M. Relationship between Meat Toughness and Properties of Connective Tissue from Cows and Young Bulls Heat Treated at Low Temperatures for Prolonged Times. Meat Sci. 2013, 93, 787–795. [Google Scholar] [CrossRef]
- Baldwin, D.E. Sous Vide Cooking: A Review. Int. J. Gastron. Food Sci. 2012, 1, 15–30. [Google Scholar] [CrossRef]
- Suriaatmaja, D.; Lanier, T. Mechanism of Meat Tenderization by Sous Vide Cooking. Meat Sci. 2014, 96, 457. [Google Scholar] [CrossRef]
- Singh, P.; Sultan, Z.; Pandey, V.K.; Singh, R. Sous Vide Processing for Food Quality Enhancement: A Review. Food Humanit. 2023, 1, 543–552. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, Q.; Zhang, Y.; Yang, X.; Mao, Y.; Luo, X.; Hopkins, D.L.; Niu, L.; Liang, R. Sous Vide Cooking Improved the Physicochemical Parameters of Hot-Boned Bovine Semimembranosus Muscles. Meat Sci. 2023, 206, 109326. [Google Scholar] [CrossRef]
- Hong, G.-E.; Kim, J.-H.; Ahn, S.-J.; Lee, C.-H. Changes in Meat Quality Characteristics of the Sous-Vide Cooked Chicken Breast during Refrigerated Storage. Korean J. Food Sci. Anim. Resour. 2015, 35, 757–764. [Google Scholar] [CrossRef]
- Jiang, S.; Xue, D.; Zhang, Z.; Shan, K.; Ke, W.; Zhang, M.; Zhao, D.; Nian, Y.; Xu, X.; Zhou, G.; et al. Effect of Sous-Vide Cooking on the Quality and Digestion Characteristics of Braised Pork. Food Chem. 2022, 375, 131683. [Google Scholar] [CrossRef]
- Ismail, I.; Hwang, Y.H.; Joo, S.T. Interventions of Two-Stage Thermal Sous-Vide Cooking on the Toughness of Beef Semitendinosus. Meat Sci. 2019, 157, 107882. [Google Scholar] [CrossRef]
- Kaur, L.; Hui, S.X.; Boland, M. Changes in Cathepsin Activity during Low-Temperature Storage and Sous Vide Processing of Beef Brisket. Food Sci. Anim. Resour. 2020, 40, 415–425. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 6th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar]
- CIE. Colorimetry—Part 4: CIE 1976L*a*a* Colour Space (11664–4; p. 8). International Commission on Illumination. 2019. Available online: https://cie.co.at/publications/colorimetry-part-4-cie-1976-lab-colour-space-1 (accessed on 17 December 2023).
- Rosenthal, A.J. Texture Profile Analysis—How Important Are the Parameters? J. Texture Stud. 2010, 41, 672–684. [Google Scholar] [CrossRef]
- Mittal, G.S.; Nadulski, R.; Barbut, S.; Negi, S.C. Textural Profile Analysis Test Conditions for Meat Products. Food Res. Int. 1992, 25, 411–417. [Google Scholar] [CrossRef]
- Culler, R.D.; Jr, F.C.P.; Smith, G.C.; Cross, H.R. Relationship of Myofibril Fragmentation Index to Certain Chemical, Physical and Sensory Characteristics of Bovine Longissimus Muscle. J. Food Sci. 1978, 43, 1177–1180. [Google Scholar] [CrossRef]
- Olson, D.G.; Parrish, F.C., Jr.; Stromer, M.H. Myofibril Fragmentation and Shear Resistance of Three Bovine Muscles during Postmortem Storage. J. Food Sci. 1976, 41, 1036–1041. [Google Scholar] [CrossRef]
- Garcia-Esteban, M.; Ansorena, D.; Astiasaran, I.; Martin, D.; Ruiz, J. Comparison of Simultaneous Distillation Extraction (SDE) and Solid-phase Microextraction (SPME) for the Analysis of Volatile Compounds in Dry-cured Ham. J. Sci. Food Agric. 2004, 84, 1364–1370. [Google Scholar] [CrossRef]
- NIST US National Institute of Standards and Technology (NIST); Gaithersburg. 2021. Available online: https://www.nist.gov/oles/forensic-database-chemistry-toxicology-table (accessed on 12 January 2024).
- Garg, N.; Sethupathy, A.; Tuwani, R.; NK, R.; Dokania, S.; Iyer, A.; Gupta, A.; Agrawal, S.; Singh, N.; Shukla, S.; et al. FlavorDB: A Database of Flavor Molecules. Nucleic Acids Res. 2018, 46, 1210–1216. [Google Scholar] [CrossRef]
- Jayasena, D.D.; Jung, S.; Kim, H.J.; Bae, Y.S.; Yong, H.I.; Lee, J.H.; Kim, J.G.; Jo, C. Comparison of Quality Traits of Meat from Korean Native Chickens and Broilers Used in Two Different Traditional Korean Cuisines. Asian Australas. J. Anim. Sci. 2013, 26, 1038–1046. [Google Scholar] [CrossRef]
- Man, L.; Ren, W.; Sun, M.; Du, Y.; Chen, H.; Qin, H.; Chai, W.; Zhu, M.; Liu, G.; Wang, C.; et al. Characterization of Donkey-Meat Flavor Profiles by GC–IMS and Multivariate Analysis. Front. Nutr. 2023, 10, 1079799. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Sarriés, M.V.; Tateo, A.; Polidori, P.; Franco, D.; Lanza, M. Carcass Characteristics, Meat Quality and Nutritional Value of Horse Meat: A Review. Meat Sci. 2014, 96, 1478–1488. [Google Scholar] [CrossRef]
- USDA. FoodData Central Search Results on Game Meat, Horse, Cooked, Roasted. 2019. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/175087/nutrients (accessed on 29 October 2023).
- Vaudagna, S.R.; Sá Nchez, G.; Neira, M.S.; Insani, E.M.; Picallo, A.B.; Gallinger, M.M.; Lasta, J.A. Sous Vide Cooked Beef Muscles: Effects of Low Temperature-Long Time (LT-LT) Treatments on Their Quality Characteristics and Storage Stability. Int. J. Food Sci. Technol. 2002, 37, 425–441. [Google Scholar] [CrossRef]
- Sánchez del Pulgar, J.; Gázquez, A.; Ruiz-Carrascal, J. Physico-Chemical, Textural and Structural Characteristics of Sous-Vide Cooked Pork Cheeks as Affected by Vacuum, Cooking Temperature, and Cooking Time. Meat Sci. 2012, 90, 828–835. [Google Scholar] [CrossRef]
- García-Segovia, P.; Andrés-Bello, A.; Martínez-Monzó, J. Effect of Cooking Method on Mechanical Properties, Color and Structure of Beef Muscle (M. Pectoralis). J. Food Eng. 2007, 80, 813–821. [Google Scholar] [CrossRef]
- Suman, S.P.; Nair, M.N.; Joseph, P.; Hunt, M.C. Factors Influencing Internal Color of Cooked Meats. Meat Sci. 2016, 120, 133–144. [Google Scholar] [CrossRef]
- Sen, A.R.; Naveena, B.M.; Muthukumar, M.; Vaithiyanathan, S. Colour, Myoglobin Denaturation and Storage Stability of Raw and Cooked Mutton Chops at Different End Point Cooking Temperature. J. Food Sci. Technol. 2014, 51, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Christensen, L.; Ertbjerg, P.; Aaslyng, M.D.; Christensen, M. Effect of Prolonged Heat Treatment from 48 °C to 63 °C on Toughness, Cooking Loss and Color of Pork. Meat Sci. 2011, 88, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Laakkonen, E.; Wellington, G.H.; Sherbon, J.N. Low-temperature, Long-time Heating of Bovine Muscle 1. Changes in Tenderness, Water-binding Capacity, PH and Amount of Water-soluble Components. J. Food Sci. 1970, 35, 175–177. [Google Scholar] [CrossRef]
- Tornberg, E. Effects of Heat on Meat Proteins—Implications on Structure and Quality of Meat Products. Meat Sci. 2005, 70, 493–508. [Google Scholar] [CrossRef] [PubMed]
- Aroeira, C.N.; Torres Filho, R.A.; Fontes, P.R.; Ramos, A.L.S.; Contreras Castillo, C.J.; Hopkins, D.L.; Ramos, E.M. Comparison of Different Methods for Determining the Extent of Myofibrillar Fragmentation of Chilled and Frozen/Thawed Beef across Postmortem Aging Periods. Meat Sci. 2020, 160, 107955. [Google Scholar] [CrossRef] [PubMed]
- Karki, R.; Bremer, P.; Silcock, P.; Oey, I. Effect of Sous Vide Processing on Quality Parameters of Beef Short Ribs and Optimisation of Sous Vide Time and Temperature Using Third-Order Multiple Regression. Food Bioproc Tech. 2022, 15, 1629–1646. [Google Scholar] [CrossRef]
- Li, C.B.; Zhou, G.H.; Xu, X.L. Dynamical Changes of Beef Intramuscular Connective Tissue and Muscle Fiber during Heating and Their Effects on Beef Shear Force. Food Bioproc Tech. 2010, 3, 521–527. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Morton, J.D.; Zhang, X.; Mason, S.L.; Bekhit, A.E.-D.A. Sous-Vide Cooking Improves the Quality and in-Vitro Digestibility of Semitendinosus from Culled Dairy Cows. Food Res. Int. 2020, 127, 108708. [Google Scholar] [CrossRef]
- Díaz, P.; Nieto, G.; Garrido, M.D.; Bañón, S. Microbial, Physical–Chemical and Sensory Spoilage during the Refrigerated Storage of Cooked Pork Loin Processed by the Sous Vide Method. Meat Sci. 2008, 80, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Kim, H.-J.; Kim, D.; Joo, B.; Jhoo, J.-W.; Jang, A. Physicochemical Properties and Volatile Organic Compounds of Dairy Beef Round Subjected to Various Cooking Methods. Food Sci Anim Resour 2023, 43, 767–791. [Google Scholar] [CrossRef] [PubMed]
- Maggiolino, A.; Lorenzo, J.M.; Marino, R.; della Malva, A.; Centoducati, P.; De Palo, P. Foal Meat Volatile Compounds: Effect of Vacuum Ageing on Semimembranosus Muscle. J. Sci. Food Agric. 2019, 99, 1660–1667. [Google Scholar] [CrossRef] [PubMed]
- Tateo, A.; Maggiolino, A.; Domínguez, R.; Lorenzo, J.M.; Dinardo, F.R.; Ceci, E.; Marino, R.; della Malva, A.; Bragaglio, A.; De Palo, P. Volatile Organic Compounds, Oxidative and Sensory Patterns of Vacuum Aged Foal Meat. Animals 2020, 10, 1495. [Google Scholar] [CrossRef] [PubMed]
- Maggi, F.; Papa, F.; Cristalli, G.; Sagratini, G.; Vittori, S. Characterisation of the Mushroom-like Flavour of Melittis Melissophyllum L. Subsp. Melissophyllum by Headspace Solid-Phase Microextraction (HS-SPME) Coupled with Gas Chromatography (GC–FID) and Gas Chromatography–Mass Spectrometry (GC–MS). Food Chem. 2010, 123, 983–992. [Google Scholar] [CrossRef]
- Sawamura, M.; Thi Minh Tu, N.; Onishi, Y.; Ogawa, E.; Choi, H.-S. Characteristic Odor Components of Citrus Reticulata Blanco (Ponkan) Cold-Pressed Oil. Biosci. Biotechnol. Biochem. 2004, 68, 1690–1697. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information PubChem Compound Summary for CID 5283356, 2-Undecenal. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/5283356 (accessed on 5 October 2023).
- Hwang, Y.H.; Ismail, I.; Joo, S.T. Identification of Umami Taste in Sous-Vide Beef by Chemical Analyses, Equivalent Umami Concentration, and Electronic Tongue System. Foods 2020, 9, 251. [Google Scholar] [CrossRef]
- Kawai, M. Taste Enhancements between Various Amino Acids and IMP. Chem. Senses 2002, 27, 739–745. [Google Scholar] [CrossRef]
- Seong, P.N.; Park, K.M.; Kang, G.H.; Cho, S.H.; Park, B.Y.; Chae, H.S.; Ba, H. Van The Differences in Chemical Composition, Physical Quality Traits and Nutritional Values of Horse Meat as Affected by Various Retail Cut Types. Asian Australas. J. Anim. Sci. 2016, 29, 89–99. [Google Scholar] [CrossRef]
- Stanisławczyk, R.; Rudy, M. Effect of the Slaughter Age of Horses on Changes Occurring in the Horse Fat during the Frozen Storage. Nauka Przyr. Technol. 2015, 9, 10. [Google Scholar] [CrossRef]
- Stanisławczyk, R.; Żurek, J.; Rudy, M.; Gil, M.; Krajewska, A.; Dziki, D. Effect of Time and Temperature in Sous-Vide Heat Treatment on Selected Physicochemical Properties of Horsemeat. Processes 2023, 11, 3126. [Google Scholar] [CrossRef]



| Parameter | C | 65 °C | 70 °C | SEM | P (T) | P (t) | P (T*t) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 12 h | 18 h | 24 h | 12 h | 18 h | 24 h | ||||||
| Moisture NS | 67.25 | 65.21 | 64.46 | 64.31 | 62.64 | 62.68 | 63.54 | 1.125 | 0.0834 | 0.9384 | 0.7428 |
| Crude protein | 26.38 c | 28.14 bc | 29.57 ab | 29.46 ab | 29.92 ab | 30.72 a | 30.41 ab | 0.559 | 0.0099 | 0.1379 | 0.7518 |
| Crude lipid NS | 6.14 | 8.31 | 7.30 | 7.45 | 7.39 | 8.49 | 7.59 | 0.535 | 0.7738 | 0.7709 | 0.2016 |
| Crude ash NS | 1.13 | 1.21 | 1.21 | 1.13 | 1.14 | 1.23 | 1.04 | 0.086 | 0.5182 | 0.3432 | 0.8211 |
| Cooking loss | 22.88 c | 32.09 b | 32.97 ab | 33.68 ab | 37.28 ab | 37.58 a | 35.95 a | 1.229 | 0.0003 | 0.8768 | 0.4477 |
| Parameter | C | 65 °C | 70 °C | SEM | P (T) | P (t) | P (T*t) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12 h | 18 h | 24 h | 12 h | 18 h | 24 h | |||||||
| pH | 6.20 b | 6.34 ab | 6.36 a | 6.36 a | 6.40 a | 6.34 a | 6.37 a | 0.032 | 0.5301 | 0.7991 | 0.4176 | |
| Color | L* | 46.99 ab | 48.23 ab | 48.56 ab | 48.70 a | 46.70 ab | 46.41 b | 48.51 ab | 0.515 | 0.0037 | 0.0479 | 0.1518 |
| a* | 16.95 a | 16.29 a | 15.57 ab | 15.03 ab | 14.58 ab | 13.84 b | 13.60 b | 0.553 | <0.0001 | 0.0194 | 0.9028 | |
| b* | 12.96 c | 13.30 bc | 13.40 bc | 13.91 ab | 13.50 bc | 13.61 abc | 14.31 a | 0.158 | 0.0586 | 0.0004 | 0.8159 | |
| Parameter | C | 65 °C | 70 °C | SEM | P (T) | P (t) | P (T*t) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 12 h | 18 h | 24 h | 12 h | 18 h | 24 h | ||||||
| Shear force (N) | 52.64 a | 50.72 ab | 44.65 ab | 36.36 b | 50.57 ab | 44.45 ab | 35.70 b | 3.426 | 0.9080 | 0.0012 | 0.9969 |
| Hardness (N) | 165.78 | 142.72 | 151.83 | 130.29 | 158.79 | 131.98 | 140.66 | 10.984 | 0.8164 | 0.4217 | 0.2607 |
| Cohesiveness | 0.45 a | 0.37 bc | 0.41 ab | 0.38 bc | 0.41 abc | 0.38 bc | 0.36 c | 0.012 | 0.6640 | 0.0889 | 0.0108 |
| Springiness (cm) | 0.76 b | 0.89 ab | 0.73 b | 0.95 a | 0.87 ab | 0.94 a | 0.93 a | 0.036 | 0.0217 | 0.0074 | 0.0003 |
| Gumminess (N) | 76.64 a | 54.24 abc | 67.77 abc | 49.93 abc | 74.44 ab | 47.91 bc | 46.05 c | 6.417 | 0.8334 | 0.0690 | 0.0212 |
| Chewiness (N) NS | 56.76 | 52.78 | 48.81 | 46.46 | 66.28 | 44.65 | 40.87 | 6.672 | 0.8281 | 0.0711 | 0.3289 |
| MFI | 41.15 b | 50.75 ab | 50.78 ab | 47.59 ab | 55.03 a | 56.71 a | 52.49 a | 2.413 | 0.0145 | 0.2789 | 0.9405 |
| Parameter | R | C | 65 °C | 70 °C | SEM | ||||
|---|---|---|---|---|---|---|---|---|---|
| 12 H | 18 H | 24 H | 12 H | 18 H | 24 H | ||||
| Total aerobic bacteria | 2.84 a | 0.00 b | 0.00 b | 0.00 b | 0.17 b | 0.00 b | 0.00 b | 0.00 b | 0.608 |
| Coliforms | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | - |
| E. coli | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | - |
| Compound | R | C | 65 °C | 70 °C | SEM | ||||
|---|---|---|---|---|---|---|---|---|---|
| 12 h | 18 h | 24 h | 12 h | 18 h | 24 h | ||||
| (S)-(+)-3-Methyl1-pentanol | 0.06 b | 0.18 b | 0.63 a | 0.53 a | 0.10 b | 0.45 a | 0.00 b | 0.07 b | 0.052 |
| 1-Octen-3-ol | 0.66 d | 2.91 cd | 16.66 b | 26.87 a | 4.84 c | 16.97 b | 3.40 cd | 1.62 cd | 0.913 |
| 2-Octen-1-ol, (E)- | 0.00 c | 0.00 c | 0.88 b | 1.30 a | 0.15 c | 0.60 b | 0.00 c | 0.00 c | 0.091 |
| Ethanone, 1-(4,5-dihydro-2-thiazolyl)- | 0.15 a | 0.08 b | 0.00 c | 0.00 c | 0.00 c | 0.00 c | 0.00 c | 0.00 c | 0.013 |
| 2-Decenal, (E)- | 0.00 d | 0.00 d | 0.05 a | 0.00 d | 0.02 b | 0.00 d | 0.01 cd | 0.02 bc | 0.003 |
| 2-Octenal, (E)- | 0.00 c | 0.00 c | 0.02 b | 0.03 a | 0.00 c | 0.01 bc | 0.00 c | 0.00 c | 0.003 |
| 2-Octenal, 2-butyl- | 0.00 c | 0.00 c | 0.06 b | 0.18 a | 0.02 c | 0.07 b | 0.00 c | 0.00 c | 0.006 |
| 2-Undecenal | 0.00 d | 0.00 d | 3.82 a | 4.42 a | 1.29 b | 3.69 a | 1.11 bc | 0.55 cd | 0.160 |
| Cyclododecanol | 0.00 b | 0.00 b | 0.08 a | 0.01 b | 0.00 b | 0.00 b | 0.00 b | 0.00 b | 0.003 |
| Decanal | 0.01 e | 0.02 e | 3.97 b | 8.05 a | 1.76 c | 3.81 b | 1.21 cd | 0.57 de | 0.257 |
| Dodecanal | 0.00 c | 0.00 c | 0.10 a | 0.03 b | 0.00 c | 0.01 bc | 0.01 bc | 0.00 c | 0.004 |
| Hexadecanal | 0.02 c | 0.14 c | 0.88 a | 0.36 b | 0.08 c | 0.14 c | 0.07 c | 0.08 c | 0.042 |
| Octadecanal | 0.00 d | 0.00 d | 0.08 b | 0.12 a | 0.00 d | 0.03 c | 0.00 d | 0.00 d | 0.005 |
| Pentadecanal- | 0.02 c | 0.05 c | 4.06 a | 1.26 b | 1.31 b | 0.86 bc | 0.44 bc | 1.12 bc | 0.240 |
| Tridecanal | 0.01 de | 0.02 cd | 0.05 b | 0.07 a | 0.01 cde | 0.03 c | 0.00 e | 0.01 e | 0.004 |
| Undecanal | 0.00 c | 0.01 c | 0.62 a | 0.41 b | 0.07 c | 0.11 c | 0.05 c | 0.05 c | 0.028 |
| 2,4-Nonadienal, (E,E)- | 0.00 d | 0.00 d | 0.30 b | 0.36 ab | 0.34 ab | 0.18 c | 0.19 c | 0.39 a | 0.017 |
| Heptadecanal | 0.00 c | 0.00 c | 0.03 a | 0.03 a | 0.00 c | 0.01 b | 0.00 c | 0.00 c | 0.002 |
| Hexanoic acid, ethyl ester | 0.36 a | 0.00 b | 0.00 b | 0.00 b | 0.00 b | 0.00 b | 0.00 b | 0.00 b | 0.012 |
| n-Caproic acid vinyl ester | 0.22 e | 1.90 de | 12.36 c | 36.95 a | 6.19 d | 21.37 b | 4.82 de | 1.30 e | 1.013 |
| Cyclotetrasiloxane, octamethyl | 20.27 | 25.70 | 22.38 | 20.11 | 21.84 | 15.11 | 24.94 | 24.82 | 2.860 |
| Dodecane | 0.06 c | 0.16 | 0.64 a | 0.41 ab | 0.25 bc | 0.42 ab | 0.21 bc | 0.33 abc | 0.074 |
| Nonane, 3,7-dimethyl | 0.00 c | 0.20 bc | 0.67 ab | 0.29 abc | 0.84 a | 0.46 abc | 0.38 abc | 0.26 bc | 0.121 |
| Oxirane, hexadecyl | 0.00 b | 0.00 b | 0.07 a | 0.00 b | 0.00 b | 0.00 b | 0.00 b | 0.00 b | 0.004 |
| Pentadecane | 0.07 c | 0.05 c | 0.76 a | 0.42 b | 0.10 c | 0.19 bc | 0.11 c | 0.14 c | 0.051 |
| Tetradecane | 0.05 b | 0.06 b | 0.40 a | 0.34 a | 0.09 b | 0.15 b | 0.06 b | 0.08 b | 0.025 |
| Tridecane | 0.05 b | 0.06 b | 0.53 a | 0.44 a | 0.12 b | 0.22 b | 0.07 b | 0.09 b | 0.043 |
| 2-Nonenal, (E)- | 0.00 d | 0.00 d | 0.04 c | 0.19 a | 0.00 d | 0.08 b | 0.00 d | 0.00 d | 0.007 |
| 3,3-Dimethyl-1, 2-epoxybutane | 0.32 d | 1.55 c | 2.72 b | 2.86 ab | 1.06 cd | 3.89 a | 1.21 cd | 0.51 d | 0.225 |
| 5,9-Undecadien-2-one, 6,10-dimethyl-, (E)- | 0.01 c | 0.01 c | 0.12 a | 0.08 b | 0.00 c | 0.02 c | 0.02 c | 0.01 c | 0.007 |
| D-Limonene | 0.00 d | 0.02 | 0.11 b | 0.11 b | 0.09 bc | 0.21 a | 0.06 bcd | 0.03 cd | 0.015 |
| Compound | C | 65 °C | 70 °C | SEM | P (T) | P (t) | P (T*t) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 12 h | 18 h | 24 h | 12 h | 18 h | 24 h | ||||||
| ATP | 3.03 f | 14.42 e | 16.55 d | 20.58 c | 18.03 d | 22.80 b | 26.01 a | 0.382 | <0.0001 | <0.0001 | 0.0208 |
| ADP | 1.84 a | 0.28 b | 0.35 b | 0.33 b | 0.49 b | 0.47 b | 0.42 b | 0.074 | 0.0052 | 0.7630 | 0.4225 |
| AMP | 26.91 a | 21.84 bc | 21.98 bc | 19.83 c | 25.50 ab | 24.80 ab | 24.72 ab | 0.823 | <0.0001 | 0.2341 | 0.4636 |
| IMP | 42.35 a | 22.58 bc | 25.00 bc | 21.35 c | 29.42 abc | 41.02 a | 37.27 ab | 3.107 | <0.0001 | 0.0761 | 0.2049 |
| Inosine | 45.83 a | 44.79 a | 38.63 ab | 31.86 b | 31.13 b | 31.69 b | 29.61 b | 2.195 | 0.0004 | 0.0096 | 0.0378 |
| GMP | 0.98 ab | 0.79 b | 0.68 b | 0.73 b | 0.89 ab | 1.03 ab | 1.35 a | 0.103 | 0.0019 | 0.1706 | 0.1019 |
| Hypoxanthine | 22.16 c | 27.99 b | 29.30 ab | 32.28 a | 31.03 ab | 31.35 a | 32.03 a | 0.685 | 0.0174 | 0.0092 | 0.1032 |
| Compound NS | C | 65 °C | 70 °C | SEM | P (T) | P (t) | P (T*t) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 12 h | 18 h | 24 h | 12 h | 18 h | 24 h | ||||||
| C14:0 (myristic acid) | 2.95 | 3.65 | 2.86 | 3.30 | 4.65 | 3.63 | 3.06 | 0.493 | 0.2534 | 0.1564 | 0.4709 |
| C16:0 (palmitic acid) | 25.10 | 25.58 | 25.01 | 25.25 | 26.35 | 25.68 | 25.47 | 0.716 | 0.3351 | 0.5932 | 0.9104 |
| C16:1n7 (palmitoleic acid) | 2.46 | 3.12 | 2.20 | 2.96 | 3.26 | 3.10 | 2.22 | 0.464 | 0.8060 | 0.4427 | 0.2930 |
| C18:0 (stearic acid) | 8.79 | 7.01 | 9.38 | 7.69 | 6.67 | 7.15 | 8.60 | 1.064 | 0.5576 | 0.3998 | 0.3980 |
| C18:1n9 (oleic acid) | 19.50 | 21.89 | 19.76 | 21.88 | 19.32 | 24.23 | 19.91 | 2.666 | 0.9908 | 0.8771 | 0.4183 |
| C18:1n7 (vaccenic acid) | 1.76 | 1.59 | 1.53 | 1.86 | 2.09 | 1.62 | 1.41 | 0.374 | 0.8932 | 0.7747 | 0.4956 |
| C18:2n6 (linoleic acid) | 29.00 | 28.75 | 28.50 | 28.02 | 27.89 | 25.54 | 28.57 | 2.184 | 0.5721 | 0.8153 | 0.7508 |
| C18:3n6 (r-linolenic acid) | 0.05 | 0.05 | 0.05 | 0.06 | 0.08 | 0.06 | 0.06 | 0.019 | 0.4411 | 0.8684 | 0.8696 |
| C18:3n3 (a-linolenic acid) | 5.41 | 4.69 | 5.72 | 4.90 | 6.08 | 5.69 | 6.22 | 0.938 | 0.2915 | 0.9498 | 0.7298 |
| C20:1n9 (eicosenoic acid) | 0.33 | 0.27 | 0.35 | 0.32 | 0.30 | 0.37 | 0.33 | 0.073 | 0.7747 | 0.5768 | 0.9954 |
| C20:4n6 (arachidonic acid) | 4.04 | 3.00 | 4.04 | 3.25 | 2.89 | 2.46 | 3.60 | 0.902 | 0.5863 | 0.8840 | 0.5949 |
| C20:5n3 (eicosapentaenoic acid, EPA) | 0.20 | 0.13 | 0.18 | 0.19 | 0.11 | 0.14 | 0.15 | 0.046 | 0.3878 | 0.5867 | 0.9465 |
| C22:4n6 (adrenic acid) | 0.17 | 0.11 | 0.19 | 0.12 | 0.15 | 0.11 | 0.17 | 0.041 | 0.9850 | 0.9100 | 0.3022 |
| C22:6n3 (docosahexaenoic acid, DHA) | 0.24 | 0.15 | 0.20 | 0.19 | 0.15 | 0.22 | 0.22 | 0.058 | 0.7407 | 0.4801 | 0.9666 |
| SFA | 36.83 | 36.23 | 37.26 | 36.24 | 37.67 | 36.45 | 37.13 | 0.907 | 0.5231 | 0.9611 | 0.4872 |
| UFA | 63.17 | 63.77 | 62.74 | 63.76 | 62.33 | 63.55 | 62.87 | 0.907 | 0.5231 | 0.9611 | 0.4872 |
| MUFA | 24.05 | 26.88 | 23.85 | 27.03 | 24.97 | 29.33 | 23.87 | 3.068 | 0.9602 | 0.9413 | 0.3899 |
| PUFA | 39.12 | 36.89 | 38.90 | 36.72 | 37.36 | 34.22 | 39.00 | 2.686 | 0.7886 | 0.9029 | 0.4778 |
| MUFA/SFA | 0.65 | 0.75 | 0.64 | 0.75 | 0.66 | 0.81 | 0.64 | 0.098 | 0.9274 | 0.9587 | 0.3528 |
| PUFA/SFA | 1.06 | 1.02 | 1.04 | 1.01 | 0.99 | 0.94 | 1.06 | 0.071 | 0.6533 | 0.8528 | 0.6184 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sujiwo, J.; Lee, S.; Kim, D.; Lee, H.-J.; Oh, S.; Jung, Y.; Jang, A. Physicochemical Features and Volatile Organic Compounds of Horse Loin Subjected to Sous-Vide Cooking. Foods 2024, 13, 280. https://doi.org/10.3390/foods13020280
Sujiwo J, Lee S, Kim D, Lee H-J, Oh S, Jung Y, Jang A. Physicochemical Features and Volatile Organic Compounds of Horse Loin Subjected to Sous-Vide Cooking. Foods. 2024; 13(2):280. https://doi.org/10.3390/foods13020280
Chicago/Turabian StyleSujiwo, Joko, Sangrok Lee, Dongwook Kim, Hee-Jeong Lee, Soomin Oh, Yousung Jung, and Aera Jang. 2024. "Physicochemical Features and Volatile Organic Compounds of Horse Loin Subjected to Sous-Vide Cooking" Foods 13, no. 2: 280. https://doi.org/10.3390/foods13020280