Protein-Stabilized Emulsion Gels with Improved Emulsifying and Gelling Properties for the Delivery of Bioactive Ingredients: A Review
Abstract
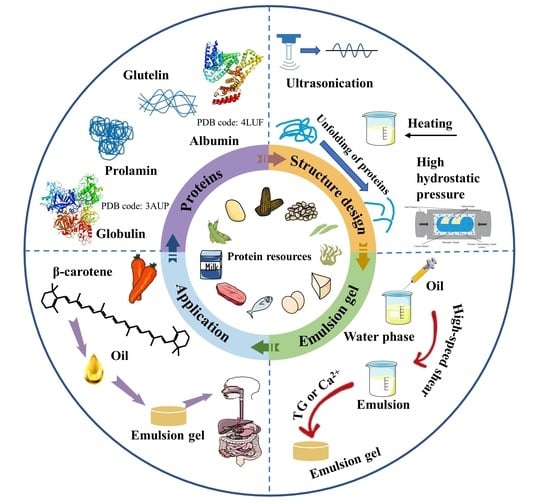
:1. Introduction
2. Proteins Used as Emulsifiers or Gelling Agents
2.1. Animal Proteins
2.2. Plant Proteins
2.3. Insect Proteins
2.4. Algae Proteins
3. Methods for Improving the Emulsifying and Gelling Properties of Proteins
3.1. Heat Treatment
3.2. Microwave Treatment
3.3. Ultrasound Treatment
3.4. High Hydrostatic Pressure Treatment
3.5. Cold Plasma Treatment
3.6. Interactions between Food-Grade-Biopolymers
3.6.1. Protein–Protein Interactions
3.6.2. Protein–Polysaccharide Interactions

4. Formation of Protein-Based Emulsion Gels
4.1. Heat-Set Gels
4.2. Cold-Set Gels
4.3. Homogenization Methods
5. Protein-Based Emulsion Gels as Nutrient Delivery Vehicles
| Protein Type | Modification | Ingredient | Homogenization Technology | Gelation Triggers | Bioactive Components | Main Findings | References |
|---|---|---|---|---|---|---|---|
| Whey protein isolate | High hydrostatic pressure (600 Mpa) to obtain protein gels | Canola oil | Ultra-high-speed homogenization (12,000 rpm for 1 min) | Curcumin |
| [85] | |
| No | Medium chain triglycerides (MCT) | Homogenization (9,000 rpm for 90 s) and Microfluidization (18000 psi, 2 cycles) | Heat treatment | β-carotene |
| [54] | |
| Heated treatment (90 °C for 5 min) | Sunflower oil | Homogenization (10,000 rpm for 1 min) and Microfluidization (50 MPa, 3 passes) | Acidification treatment (Glucono-δ-lactone) | Modulate volatile release (propanol, diacetyl, pentanone, hexanal, and heptanone) |
| [11] | |
| β-lactoglobulin | Heated treatment (85 °C for 45 min) | Sunflower oil | Homogenization (20,000 rpm for 2 min) and Microfluidization (100 MPa for the first-stage, 10 MPa for second-stage) | Addition of ions (Ca2+) | α-tocopherol |
| [9] |
| Egg yolk granule protein | No | Sunflower oil | Homogenization (15,000 rpm for 1 min) | Addition of ions (Ca2+) | β-carotene |
| [174] |
| Soybean protein isolate | Soybean protein isolate (final concentration 7%) with pectin (final concentration 3%) | Soybean oil | Homogenization (20,000 rpm for 5 min) | Ultrasonication (0, 150, 300, 450, and 600 W, for 15 min) and then heat treatment | β-carotene |
| [8] |
| Soybean protein isolate (6.0%, w/w) with sugar beet pectin (2.0%, w/w) and then heated treatment (85 °C for 15 min) | Medium-chain triglycerides | Homogenization (10,000 rpm for 3 min) and Microfluidization (30 MPa, 5 passes) | Acidification treatment (Glucono-δ-lactone) and then added laccase/Enzyme treatment (Transglutaminase) and then added laccase | The hydrophilic phase was loaded with the riboflavin, and the lipophilic phase (MCT) was loaded with β-carotene |
| [26] | |
| Heated treatment (90 °C for 30 min) | Sunflower oil | Homogenization (14,000 rpm for 3 min) and Ultrasonication (20 kHz, 90 W for 3 min) | Addition of ions (Ca2+)/Acidification treatment (Glucono-δ-lactone)/Enzyme treatment (Transglutaminase) | β-carotene |
| [148] | |
| Zein | Zein with heated (150 °C) food-grad glycerol solutions | Soybean oil | Soybean oil was preheated to 95 °C and was added to the heated zein-glycerol mixed solutions and homogenization (10,000 rpm for 3 min) | β-carotene |
| [175] | |

6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chang, C.; Li, J.; Su, Y.; Gu, L.; Yang, Y.; Zhai, J. Protein particle-based vehicles for encapsulation and delivery of nutrients: Fabrication, digestion, and release properties. Food Hydrocoll. 2022, 123, 106963. [Google Scholar] [CrossRef]
- Mao, L.; Lu, Y.; Cui, M.; Miao, S.; Gao, Y. Design of gel structures in water and oil phases for improved delivery of bioactive food ingredients. Crit. Rev. Food Sci. Nutr. 2020, 60, 1651–1666. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Costa, A.L.R.; Sobral, P.J.D.A.; Cunha, R.L. Delivering β-carotene from O/W emulsion-based systems: Influence of phase ratio and carrier lipid composition. Food Hydrocoll. Health 2023, 3, 100125. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, Z.; Liu, L.; Li, Y.; Dong, X.; Chen, W. Fabrication of soy protein isolate/kappa-carrageenan hydrogels for release control of hydrophilic compounds: Flax lignans. Int. J. Biol. Macromol. 2022, 223 Pt A, 821–829. [Google Scholar] [CrossRef]
- Lu, Y.; Mao, L.; Zheng, H.; Chen, H.; Gao, Y. Characterization of β-carotene loaded emulsion gels containing de-natured and native whey protein. Food Hydrocoll. 2020, 102, 105600. [Google Scholar] [CrossRef]
- Lin, D.; Kelly, A.L.; Miao, S. Preparation, structure-property relationships and applications of different emulsion gels: Bulk emulsion gels, emulsion gel particles, and fluid emulsion gels. Trends Food Sci. Technol. 2020, 102, 123–137. [Google Scholar] [CrossRef]
- Farjami, T.; Madadlou, A. An overview on preparation of emulsion-filled gels and emulsion particulate gels. Trends Food Sci. Technol. 2019, 86, 85–94. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Gong, Y.; Li, Z.; Guo, Y.; Yu, D.; Pan, M. Emulsion gels stabilized by soybean protein isolate and pectin: Effects of high intensity ultrasound on the gel properties, stability and β-carotene digestive characteristics. Ultrason. Sonochem. 2021, 79, 105756. [Google Scholar] [CrossRef]
- Liang, L.; Line, V.L.S.; Remondetto, G.E.; Subirade, M. In vitro release of alpha-tocopherol from emulsion-loaded beta-lactoglobulin gels. Int. Dairy J. 2010, 20, 176–181. [Google Scholar] [CrossRef]
- Geremias-Andrade, I.M.; Souki, N.P.D.B.G.; Moraes, I.C.F.; Pinho, S.C. Rheological and mechanical characterization of curcumin-loaded emulsion-filled gels produced with whey protein isolate and xanthan gum. Lwt-Food Sci. Technol. 2017, 86, 166–173. [Google Scholar] [CrossRef]
- Mao, L.; Roos, Y.H.; Miao, S. Study on the Rheological Properties and Volatile Release of Cold-Set Emulsion-Filled Protein Gels. J. Agric. Food Chem. 2014, 62, 11420–11428. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, B.; McClements, D.J. Progress in natural emulsifiers for utilization in food emulsions. Curr. Opin. Food Sci. 2016, 7, 1–6. [Google Scholar] [CrossRef]
- Niknam, R.; Mousavi, M.; Kiani, H. Comprehensive evaluation of emulsifying and foaming properties of Gleditsia caspica seed galactomannan as a new source of hydrocolloid: Effect of extraction method. Food Hydrocoll. 2022, 131, 107758. [Google Scholar] [CrossRef]
- Li, P.Y.; Li, X.F.; Nisar, T.; Yang, X.D.; Sun, J.J.; Yang, X.; Guo, Y. Structural characteristics of binary biopoly-mers-based emulsion-filled gels: A case of mixed sodium caseinate/methyl cellulose emulsion gels. Food Struct.-Neth. 2021, 30, 100233. [Google Scholar] [CrossRef]
- O’Sullivan, J.; Murray, B.; Flynn, C.; Norton, I. The effect of ultrasound treatment on the structural, physical and emulsifying properties of animal and vegetable proteins. Food Hydrocoll. 2016, 53, 141–154. [Google Scholar] [CrossRef] [Green Version]
- Le, X.T.; Rioux, L.-E.; Turgeon, S.L. Formation and functional properties of protein–polysaccharide electrostatic hydrogels in comparison to protein or polysaccharide hydrogels. Adv. Colloid Interface Sci. 2017, 239, 127–135. [Google Scholar] [CrossRef]
- Mozafarpour, R.; Koocheki, A.; Sani, M.A.; McClements, D.J.; Mehr, H.M. Ultrasound-modified protein-based colloidal particles: Interfacial activity, gelation properties, and encapsulation efficiency. Adv. Colloid Interface Sci. 2022, 309, 102768. [Google Scholar] [CrossRef]
- Dickinson, E. Emulsion gels: The structuring of soft solids with protein-stabilized oil droplets. Food Hydrocoll. 2012, 28, 224–241. [Google Scholar] [CrossRef]
- Hong, G.P.; Chun, J.Y.; Jo, Y.J.; Choi, M.J. Effects of pH-Shift Processing and Microbial Transglutaminase on the Gel and Emulsion Characteristics of Porcine Myofibrillar System. Korean J. Food Sci. Anim. Resour. 2014, 34, 207–213. [Google Scholar] [CrossRef] [Green Version]
- Kiosseoglou, V. Egg yolk protein gels and emulsions. Curr. Opin. Colloid Interface Sci. 2003, 8, 365–370. [Google Scholar] [CrossRef]
- Li, J.; Dai, Z.C.; Chen, Z.H.; Hao, Y.A.; Wang, S.; Mao, X.Z. Improved gelling and emulsifying properties of myofibrillar protein from frozen shrimp (Litopenaeus vannamei) by high-intensity ultrasound. Food Hydrocoll. 2023, 135, 108188. [Google Scholar] [CrossRef]
- Lingiardi, N.; Galante, M.; de Sanctis, M.; Spelzini, D. Are quinoa proteins a promising alternative to be applied in plant-based emulsion gel formulation? Food Chem. 2022, 394, 133485. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, H.; Hao, R.; Mráz, J.; Pu, Y.; Li, S.; Dong, X.; Pan, J. Gelling and emulsifying properties of tiger puffer (Takifugu rubripes) skin gelatin as manipulated by pH. J. Mol. Liq. 2023, 369, 120886. [Google Scholar] [CrossRef]
- Lin, D.; Kelly, A.L.; Maidannyk, V.; Miao, S. Effect of structuring emulsion gels by whey or soy protein isolate on the structure, mechanical properties, and in-vitro digestion of alginate-based emulsion gel beads. Food Hydrocoll. 2021, 110, 106165. [Google Scholar] [CrossRef]
- Sridharan, S.; Meinders, M.B.J.; Sagis, L.M.C.; Bitter, J.H.; Nikiforidis, C.V. Starch controls brittleness in emulsion-gels stabilized by pea flour. Food Hydrocoll. 2022, 131, 107708. [Google Scholar] [CrossRef]
- Zhang, M.H.; Yin, L.J.; Yan, W.J.; Gao, C.; Jia, X. Preparation and Characterization of a Novel Soy Protein Isolate-Sugar Beet Pectin Emulsion Gel and Its Application as a Multi-Phased Nutrient Carrier. Foods 2022, 11, 469. [Google Scholar] [CrossRef] [PubMed]
- MC Sagis, L.; Yang, J. Protein-stabilized interfaces in multiphase food: Comparing structure-function relations of plant-based and animal-based proteins. Curr. Opin. Food Sci. 2022, 43, 53–60. [Google Scholar] [CrossRef]
- Kim, W.; Wang, Y.; Selomulya, C. Dairy and plant proteins as natural food emulsifiers. Trends Food Sci. Technol. 2020, 105, 261–272. [Google Scholar] [CrossRef]
- Day, L.; Cakebread, J.A.; Loveday, S.M. Food proteins from animals and plants: Differences in the nutritional and functional properties. Trends Food Sci. Technol. 2022, 119, 428–442. [Google Scholar] [CrossRef]
- Chuesiang, P.; Kim, J.T.; Shin, G.H. Observation of curcumin-encapsulated Pickering emulsion stabilized by cellulose nanocrystals-whey protein isolate (CNCs-WPI) complex under in vitro lipid digestion through INFOGEST model. Int. J. Biol. Macromol. 2023, 234, 123679. [Google Scholar] [CrossRef]
- Mohammadian, M.; Salami, M.; Momen, S.; Alavi, F.; Emam-Djomeh, Z. Fabrication of curcumin-loaded whey protein microgels: Structural properties, antioxidant activity, and in vitro release behavior. LWT 2019, 103, 94–100. [Google Scholar] [CrossRef]
- Wu, S.; Wang, L.; Zhao, Y.; Chen, B.; Qiu, D.; Sun, P.; Shao, P.; Feng, S. Fabrication of high strength cold-set sodium alginate/whey protein nanofiber double network hydrogels and their interaction with curcumin. Food Res. Int. 2023, 165, 112490. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, S.; Zhong, M.; Qi, B.; Li, Y. Soy and whey protein isolate mixture/calcium chloride thermally induced emulsion gels: Rheological properties and digestive characteristics. Food Chem. 2022, 380, 132212. [Google Scholar] [CrossRef] [PubMed]
- Casanova, F.; Nascimento, L.G.L.; Silva, N.F.; de Carvalho, A.F.; Gaucheron, F. Interactions between caseins and food-derived bioactive molecules: A review. Food Chem. 2021, 359, 129820. [Google Scholar] [CrossRef] [PubMed]
- Elzoghby, A.O.; El-Fotoh, W.S.A.; Elgindy, N.A. Casein-based formulations as promising controlled release drug delivery systems. J. Control. Release 2011, 153, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Ranadheera, C.S.; Liyanaarachchi, W.S.; Chandrapala, J.; Dissanayake, M.; Vasiljevic, T. Utilizing unique properties of caseins and the casein micelle for delivery of sensitive food ingredients and bioactives. Trends Food Sci. Technol. 2016, 57, 178–187. [Google Scholar] [CrossRef]
- Boland, M. Whey Proteins. In Handbook of Food Proteins; Elsevier: Amsterdam, The Netherlands, 2011; pp. 30–55. [Google Scholar] [CrossRef]
- Falsafi, S.R.; Karaca, A.C.; Deng, L.; Wang, Y.; Li, H.; Askari, G.; Rostamabadi, H. Insights into whey protein-based carriers for targeted delivery and controlled release of bioactive components. Food Hydrocoll. 2022, 133, 108002. [Google Scholar] [CrossRef]
- Nishinari, K.; Fang, Y.; Nagano, T.; Guo, S.; Wang, R. Soy as a Food Ingredient. In Proteins in Food Processing; Woodhead Publishing: Sawston, UK, 2018; pp. 149–186. [Google Scholar]
- Burger, T.G.; Zhang, Y. Recent progress in the utilization of pea protein as an emulsifier for food applications. Trends Food Sci. Technol. 2019, 86, 25–33. [Google Scholar] [CrossRef]
- Mession, J.-L.; Chihi, M.L.; Sok, N.; Saurel, R. Effect of globular pea proteins fractionation on their heat-induced aggregation and acid cold-set gelation. Food Hydrocoll. 2015, 46, 233–243. [Google Scholar] [CrossRef]
- Amagliani, L.; O’Regan, J.; Kelly, A.L.; O’Mahony, J.A. The composition, extraction, functionality and applications of rice proteins: A review. Trends Food Sci. Technol. 2017, 64, 1–12. [Google Scholar] [CrossRef]
- Voci, S.; Fresta, M.; Cosco, D. Gliadins as versatile biomaterials for drug delivery applications. J. Control. Release 2021, 329, 385–400. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, P.; Dowling, K.; McKnight, S.; Barrow, C.J.; Wang, B.; Adhikari, B. Preparation, characterization and functional properties of flax seed protein isolate. Food Chem. 2016, 197, 212–220. [Google Scholar] [CrossRef] [PubMed]
- González-Pérez, S.; Vereijken, J.M. Sunflower proteins: Overview of their physicochemical, structural and functional properties. J. Sci. Food Agric. 2007, 87, 2173–2191. [Google Scholar] [CrossRef]
- Chatsuwan, N.; Nalinanon, S.; Puechkamut, Y.; Lamsal, B.P.; Pinsirodom, P. Characteristics, Functional Properties, and Antioxidant Activities of Water-Soluble Proteins Extracted from Grasshoppers, Patanga succincta and Chondracris roseapbrunner. J. Chem. 2018, 2018, 6528312. [Google Scholar] [CrossRef] [Green Version]
- Azagoh, C.; Ducept, F.; Garcia, R.; Rakotozafy, L.; Cuvelier, M.E.; Keller, S.; Lewandowski, R.; Mezdour, S. Extraction and physicochemical characterization of Tenebrio molitor proteins. Food Res. Int. 2016, 88, 24–31. [Google Scholar] [CrossRef]
- Yi, L.; Lakemond, C.M.; Sagis, L.M.; Eisner-Schadler, V.; van Huis, A.; van Boekel, M.A. Extraction and characterisation of protein fractions from five insect species. Food Chem. 2013, 141, 3341–3348. [Google Scholar] [CrossRef]
- Teuling, E.; Schrama, J.W.; Gruppen, H.; Wierenga, P.A. Characterizing emulsion properties of microalgal and cyanobacterial protein isolates. Algal Res. 2019, 39, 101471. [Google Scholar] [CrossRef]
- Mezzenga, R.; Fischer, P. The self-assembly, aggregation and phase transitions of food protein systems in one, two and three dimensions. Rep. Prog. Phys. 2013, 76, 046601. [Google Scholar] [CrossRef]
- McClements, D.J. Protein-stabilized emulsions. Curr. Opin. Colloid Interface Sci. 2004, 9, 305–313. [Google Scholar] [CrossRef]
- Tang, C.-H. Globular proteins as soft particles for stabilizing emulsions: Concepts and strategies. Food Hydrocoll. 2020, 103, 105664. [Google Scholar] [CrossRef]
- Li, H.; Zhang, L.; Jia, Y.; Yuan, Y.; Li, H.; Cui, W.; Yu, J. Application of whey protein emulsion gel microparticles as fat replacers in low-fat yogurt: Applicability of vegetable oil as the oil phase. J. Dairy Sci. 2022, 105, 9404–9416. [Google Scholar] [CrossRef]
- Li, M.; Feng, L.; Xu, Y.; Nie, M.; Li, D.; Zhou, C.; Dai, Z.; Zhang, Z.; Zhang, M. Rheological property, beta-carotene stability and 3D printing characteristic of whey protein isolate emulsion gels by adding different polysaccharides. Food Chem. 2023, 414, 135702. [Google Scholar] [CrossRef]
- Bryant, C.M.; McClements, D. Molecular basis of protein functionality with special consideration of cold-set gels derived from heat-denatured whey. Trends Food Sci. Technol. 1998, 9, 143–151. [Google Scholar] [CrossRef]
- Li, S.; Chen, G.; Shi, X.; Ma, C.; Liu, F. Comparative Study of Heat- and Enzyme-Induced Emulsion Gels Formed by Gelatin and Whey Protein Isolate: Physical Properties and Formation Mechanism. Gels 2022, 8, 212. [Google Scholar] [CrossRef] [PubMed]
- Loveday, S.M. Food Proteins: Technological, Nutritional, and Sustainability Attributes of Traditional and Emerging Proteins. Annu. Rev. Food Sci. Technol. 2019, 10, 311–339. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, H.; Denon, Q.; Tavernier, I.; Gregersen, S.; Hammershøj, M.; Van der Meeren, P.; Dewettinck, K.; Dalsgaard, T. Improved food functional properties of pea protein isolate in blends and co-precipitates with whey protein isolate. Food Hydrocoll. 2021, 113, 106556. [Google Scholar] [CrossRef]
- Liu, G.N.; Hu, M.; Du, X.Q.; Liao, Y.; Yan, S.Z.; Zhang, S.; Qi, B.K.; Li, Y. Correlating structure and emulsification of soybean protein isolate: Synergism between low-pH-shifting treatment and ultrasonication improves emulsifying properties. Colloids Surf. A-Physicochem. Eng. Asp. 2022, 646, 128963. [Google Scholar] [CrossRef]
- Mozafarpour, R.; Koocheki, A. Effect of ultrasonic pretreatment on the rheology and structure of grass pea (Lathyrus sativus L.) protein emulsion gels induced by transglutaminase. Ultrason. Sonochem. 2023, 92, 106278. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, W.; Feizollahi, E.; Roopesh, M.; Chen, L. Improvement of pea protein gelation at reduced temperature by atmospheric cold plasma and the gelling mechanism study. Innov. Food Sci. Emerg. Technol. 2021, 67, 102567. [Google Scholar] [CrossRef]
- Yang, J.; Kornet, R.; Diedericks, C.F.; Yang, Q.H.Z.; Berton-Carabin, C.C.; Nikiforidis, C.V.; Sagis, L.M.C. Re-thinking plant protein extraction: Albumin-From side stream to an excellent foaming ingredient. Food Struct.-Neth. 2022, 31, 100254. [Google Scholar] [CrossRef]
- Zhao, C.; Chu, Z.; Mao, Y.; Xu, Y.; Fei, P.; Zhang, H.; Xu, X.; Wu, Y.; Zheng, M.; Liu, J. Structural characteristics and acid-induced emulsion gel properties of heated soy protein isolate–soy oligosaccharide glycation conjugates. Food Hydrocoll. 2023, 137, 108408. [Google Scholar] [CrossRef]
- Shen, P.H.; Zhao, M.M.; Zhou, F.B. Design of soy protein/peptide-based colloidal particles and their role in con-trolling the lipid digestion of emulsions. Curr. Opin. Food Sci. 2022, 43, 61–70. [Google Scholar] [CrossRef]
- Nishinari, K.; Fang, Y.; Guo, S.; Phillips, G. Soy proteins: A review on composition, aggregation and emulsification. Food Hydrocoll. 2014, 39, 301–318. [Google Scholar] [CrossRef]
- Tang, C.-H. Nanostructured soy proteins: Fabrication and applications as delivery systems for bioactives (a review). Food Hydrocoll. 2019, 91, 92–116. [Google Scholar] [CrossRef]
- Amagliani, L.; Schmitt, C. Globular plant protein aggregates for stabilization of food foams and emulsions. Trends Food Sci. Technol. 2017, 67, 248–259. [Google Scholar] [CrossRef]
- He, X.-T.; Yuan, D.-B.; Wang, J.-M.; Yang, X.-Q. Thermal aggregation behaviour of soy protein: Characteristics of different polypeptides and sub-units. J. Sci. Food Agric. 2016, 96, 1121–1131. [Google Scholar] [CrossRef]
- Pesic, M.B.; Vucelic-Radovic, B.V.; Barac, M.B.; Stanojevic, S.P. The influence of genotypic variation in protein composition on emulsifying properties of soy proteins. J. Am. Oil Chem. Soc. 2005, 82, 667–672. [Google Scholar] [CrossRef]
- Muhoza, B.; Qi, B.; Harindintwali, J.D.; Koko, M.Y.F.; Zhang, S.; Li, Y. Combined plant protein modification and complex coacervation as a sustainable strategy to produce coacervates encapsulating bioactives. Food Hydrocoll. 2022, 124, 107239. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schlüter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef]
- Gould, J.; Wolf, B. Interfacial and emulsifying properties of mealworm protein at the oil/water interface. Food Hydrocoll. 2018, 77, 57–65. [Google Scholar] [CrossRef]
- Kim, T.-K.; Yong, H.I.; Chun, H.H.; Lee, M.-A.; Kim, Y.-B.; Choi, Y.-S. Changes of amino acid composition and protein technical functionality of edible insects by extracting steps. J. Asia-Pac. Èntomol. 2020, 23, 298–305. [Google Scholar] [CrossRef]
- Ursu, A.-V.; Marcati, A.; Sayd, T.; Sante-Lhoutellier, V.; Djelveh, G.; Michaud, P. Extraction, fractionation and functional properties of proteins from the microalgae Chlorella vulgaris. Bioresour. Technol. 2014, 157, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Schwenzfeier, A.; Helbig, A.; Wierenga, P.A.; Gruppen, H. Emulsion properties of algae soluble protein isolate from Tetraselmis sp. Food Hydrocoll. 2013, 30, 258–263. [Google Scholar] [CrossRef]
- Ebert, S.; Grossmann, L.; Hinrichs, J.; Weiss, J. Emulsifying properties of water-soluble proteins extracted from the microalgae Chlorella sorokiniana and Phaeodactylum tricornutum. Food Funct. 2019, 10, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.H.; Chen, L.; Foegeding, E.A. Mechanical and water-holding properties and microstructures of soy protein isolate emulsion gels induced by CaCl2, glucono-delta-lactone (GDL), and transglutaminase: Influence of thermal treatments before and/or after emulsification. J. Agric. Food Chem. 2011, 59, 4071–4077. [Google Scholar] [CrossRef] [PubMed]
- Chao, D.; Aluko, R.E. Modification of the structural, emulsifying, and foaming properties of an isolated pea protein by thermal pretreatment. CyTA-J. Food 2018, 16, 357–366. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Xu, X.; Wang, S.; Wang, J.; Peng, W. Effects of Microwave Treatment on Structure, Functional Properties and Antioxidant Activities of Germinated Tartary Buckwheat Protein. Foods 2022, 11, 1373. [Google Scholar] [CrossRef]
- Xu, J.; Teng, F.; Wang, B.; Ruan, X.; Ma, Y.; Zhang, D.; Zhang, Y.; Fan, Z.; Jin, H. Gel Property of Soy Protein Emulsion Gel: Impact of Combined Microwave Pretreatment and Covalent Binding of Polyphenols by Alkaline Method. Molecules 2022, 27, 3458. [Google Scholar] [CrossRef]
- Paglarini, C.S.; Martini, S.; Pollonio, M.A.R. Physical properties of emulsion gels formulated with sonicated soy protein isolate. Int. J. Food Sci. Technol. 2019, 54, 451–459. [Google Scholar] [CrossRef]
- Cheng, Y.; Donkor, P.O.; Ren, X.; Wu, J.; Agyemang, K.; Ayim, I.; Ma, H. Effect of ultrasound pretreatment with mono-frequency and simultaneous dual frequency on the mechanical properties and microstructure of whey protein emulsion gels. Food Hydrocoll. 2019, 89, 434–442. [Google Scholar] [CrossRef]
- Wang, S.; Wang, T.; Sun, Y.; Cui, Y.; Yu, G.; Jiang, L. Effects of High Hydrostatic Pressure Pretreatment on the Functional and Structural Properties of Rice Bran Protein Hydrolysates. Foods 2021, 11, 29. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Y.; Zhou, P.; Zhang, X.; Wang, J. Effects of high pressure modification on conformation and gelation properties of myofibrillar protein. Food Chem. 2017, 217, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.; Wang, D.; Dai, L.; Wu, X.; Gao, Y.; Yuan, F. Pickering emulsion gels stabilized by high hydrostatic pressure-induced whey protein isolate gel particles: Characterization and encapsulation of curcumin. Food Res. Int. 2020, 132, 109032. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Dong, S.; Han, F.; Li, Y.; Chen, G.; Li, L.; Chen, Y. Effects of Dielectric Barrier Discharge (DBD) Cold Plasma Treatment on Physicochemical and Functional Properties of Peanut Protein. Food Bioprocess Technol. 2018, 11, 344–354. [Google Scholar] [CrossRef]
- Mehr, H.M.; Koocheki, A. Effect of atmospheric cold plasma on structure, interfacial and emulsifying properties of Grass pea (Lathyrus sativus L.) protein isolate. Food Hydrocoll. 2020, 106, 105899. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Xie, F.; Han, L.; Li, L.; Jiang, L.; Qi, B.; Li, Y. Soy/whey protein isolates: Interfacial properties and effects on the stability of oil-in-water emulsions. J. Sci. Food Agric. 2021, 101, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Chapeau, A.-L.; Tavares, G.M.; Hamon, P.; Croguennec, T.; Poncelet, D.; Bouhallab, S. Spontaneous co-assembly of lactoferrin and β-lactoglobulin as a promising biocarrier for vitamin B9. Food Hydrocoll. 2016, 57, 280–290. [Google Scholar] [CrossRef]
- Bi, C.-H.; Chi, S.-Y.; Wang, X.-Y.; Alkhatib, A.; Huang, Z.-G.; Liu, Y. Effect of flax gum on the functional properties of soy protein isolate emulsion gel. LWT 2021, 149, 111846. [Google Scholar] [CrossRef]
- Felix, M.; Romero, A.; Guerrero, A. Influence of pH and Xanthan Gum on long-term stability of crayfish-based emulsions. Food Hydrocoll. 2017, 72, 372–380. [Google Scholar] [CrossRef]
- Liu, F.; Liang, X.; Yan, J.; Zhao, S.; Li, S.; Liu, X.; Ngai, T.; McClements, D.J. Tailoring the properties of double-crosslinked emulsion gels using structural design principles: Physical characteristics, stability, and delivery of lycopene. Biomaterials 2021, 280, 121265. [Google Scholar] [CrossRef]
- Fan, R.; Zhang, T.; Tai, K.; Yuan, F. Surface properties and adsorption of lactoferrin-xanthan complex in the oil-water interface. J. Dispers. Sci. Technol. 2020, 41, 1037–1044. [Google Scholar] [CrossRef]
- Tavernier, I.; Patel, A.R.; Van der Meeren, P.; Dewettinck, K. Emulsion-templated liquid oil structuring with soy protein and soy protein: κ-carrageenan complexes. Food Hydrocoll. 2017, 65, 107–120. [Google Scholar] [CrossRef]
- Davis, P.J.; Williams, S.C. Protein modification by thermal processing. Allergy 1998, 53, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Nicorescu, I.; Loisel, C.; Vial, C.; Riaublanc, A.; Djelveh, G.; Cuvelier, G.; Legrand, J. Combined effect of dynamic heat treatment and ionic strength on denaturation and aggregation of whey proteins—Part I. Food Res. Int. 2008, 41, 707–713. [Google Scholar] [CrossRef]
- Zink, J.; Wyrobnik, T.; Prinz, T.; Schmid, M. Physical, Chemical and Biochemical Modifications of Protein-Based Films and Coatings: An Extensive Review. Int. J. Mol. Sci. 2016, 17, 1376. [Google Scholar] [CrossRef]
- Tang, C.-H.; Ma, C.-Y. Heat-induced modifications in the functional and structural properties of vicilin-rich protein isolate from kidney (Phaseolus vulgaris L.) bean. Food Chem. 2009, 115, 859–866. [Google Scholar] [CrossRef]
- Basak, S.; Annapure, U.S. Recent trends in the application of cold plasma for the modification of plant proteins—A review. Futur. Foods 2022, 5, 100119. [Google Scholar] [CrossRef]
- Chen, X.X.; Yang, J.; Shen, M.Y.; Chen, Y.; Yu, Q.; Xie, J.H. Structure, function and advance application of microwave-treated polysaccharide: A review. Trends Food Sci. Technol. 2022, 123, 198–209. [Google Scholar] [CrossRef]
- Jin, J.; Ma, H.L.; Wang, B.; Yagoub, A.E.G.A.; Wang, K.; He, R.H.; Zhou, C.S. Effects and mechanism of du-al-frequency power ultrasound on the molecular weight distribution of corn gluten meal hydrolysates. Ultrason. Sonochem. 2016, 30, 44–51. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Y.; Zu, S.; Wu, X.; Shi, A.; Zhang, J.; Wang, Q.; He, N. Effects of High Hydrostatic Pressure on the Conformational Structure and Gel Properties of Myofibrillar Protein and Meat Quality: A Review. Foods 2021, 10, 1872. [Google Scholar] [CrossRef]
- Jiang, Z.M.; Shi, R.J.; Ma, L.; Munkh-Amgalan, G.; Bilawal, A.; Hou, J.C.; Tian, B. Microwave irradiation treatment improved the structure, emulsifying properties and cell proliferation of laccase-crosslinked alpha-lactalbumin. Food Hydrocoll. 2021, 121, 107036. [Google Scholar] [CrossRef]
- Mu, D.; Li, H.; Li, X.; Zhu, J.; Qiao, M.; Wu, X.; Luo, S.; Yang, P.; Zhao, Y.; Liu, F.; et al. Enhancing laccase-induced soybean protein isolates gel properties by microwave pretreatment. J. Food Process. Preserv. 2020, 44, e14386. [Google Scholar] [CrossRef]
- Han, Z.; Cai, M.-J.; Cheng, J.-H.; Sun, D.-W. Effects of electric fields and electromagnetic wave on food protein structure and functionality: A review. Trends Food Sci. Technol. 2018, 75, 1–9. [Google Scholar] [CrossRef]
- Guo, Q.; Sun, D.-W.; Cheng, J.-H.; Han, Z. Microwave processing techniques and their recent applications in the food industry. Trends Food Sci. Technol. 2017, 67, 236–247. [Google Scholar] [CrossRef]
- Fan, D.-M.; Hu, B.; Lin, L.-F.; Huang, L.-L.; Wang, M.-F.; Zhao, J.-X.; Zhang, H. Rice protein radicals: Growth and stability under microwave treatment. RSC Adv. 2016, 6, 97825–97831. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Ma, X. Study on the mechanism of microwave modified wheat protein fiber to improve its mechanical properties. J. Cereal Sci. 2016, 70, 99–107. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Z.; Zhang, C.; Zheng, B.; Tian, Y. Effects of microwave-vacuum pre-treatment with different power levels on the structural and emulsifying properties of lotus seed protein isolates. Food Chem. 2020, 311, 125932. [Google Scholar] [CrossRef]
- Wei, S.; Yang, Y.; Feng, X.; Li, S.; Zhou, L.; Wang, J.; Tang, X. Structures and properties of chicken myofibrillar protein gel induced by microwave heating. Int. J. Food Sci. Technol. 2020, 55, 2691–2699. [Google Scholar] [CrossRef]
- Nasrabadi, M.N.; Doost, A.S.; Mezzenga, R. Modification approaches of plant-based proteins to improve their techno-functionality and use in food products. Food Hydrocoll. 2021, 118, 106789. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Yao, H.; Zhou, J.; Duan, Y.; Zhang, H.; Ma, H. Advances in renewable plant-derived protein source: The structure, physicochemical properties affected by ultrasonication. Ultrason. Sonochem. 2019, 53, 83–98. [Google Scholar] [CrossRef]
- O’Sullivan, J.; Park, M.; Beevers, J. The effect of ultrasound upon the physicochemical and emulsifying properties of wheat and soy protein isolates. J. Cereal Sci. 2016, 69, 77–84. [Google Scholar] [CrossRef]
- Shen, X.; Zhao, C.; Guo, M. Effects of high intensity ultrasound on acid-induced gelation properties of whey protein gel. Ultrason. Sonochem. 2017, 39, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-L.; Hsieh, C.-M. Single-transducer dual-frequency ultrasound generation to enhance acoustic cavitation. Ultrason. Sonochem. 2009, 16, 431–438. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Li, S.; Oladejo, A.O.; Ruan, S.; Wang, Y.; Huang, S.; Ma, H. Effects of ultrasound pretreatment with different frequencies and working modes on the enzymolysis and the structure characterization of rice protein. Ultrason. Sonochem. 2017, 38, 19–28. [Google Scholar] [CrossRef]
- Jin, J.; Ma, H.L.; Wang, K.; Yagoub, A.A.; Owusu, J.; Qu, W.J.; He, R.H.; Zhou, C.S.; Ye, X.F. Effects of mul-ti-frequency power ultrasound on the enzymolysis and structural characteristics of corn gluten meal. Ultrason. Sonochem. 2015, 24, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Devi, A.F.; Buckow, R.; Hemar, Y.; Kasapis, S. Structuring dairy systems through high pressure processing. J. Food Eng. 2013, 114, 106–122. [Google Scholar] [CrossRef]
- Ding, Y.; Ban, Q.; Wu, Y.; Sun, Y.; Zhou, Z.; Wang, Q.; Cheng, J.; Xiao, H. Effect of high hydrostatic pressure on the edible quality, health and safety attributes of plant-based foods represented by cereals and legumes: A review. Crit. Rev. Food Sci. Nutr. 2021, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Liu, X.; Sang, Y.; Tian, G.; Wang, Z.; Hou, Y. Characterization and emulsifying properties of mantle proteins from scallops (Patinopecten yessoensis) treated by high hydrostatic pressure treatment. LWT 2022, 167, 113865. [Google Scholar] [CrossRef]
- Bai, Y.; Zeng, X.; Zhang, C.; Zhang, T.; Wang, C.; Han, M.; Zhou, G.; Xu, X. Effects of high hydrostatic pressure treatment on the emulsifying behavior of myosin and its underlying mechanism. LWT 2021, 146, 111397. [Google Scholar] [CrossRef]
- Wang, X.S.; Tang, C.H.; Li, B.S.; Yang, X.Q.; Li, L.; Ma, C.Y. Effects of high-pressure treatment on some physico-chemical and functional properties of soy protein isolates. Food Hydrocoll. 2008, 22, 560–567. [Google Scholar] [CrossRef]
- Thirumdas, R.; Sarangapani, C.; Annapure, U.S. Cold Plasma: A novel Non-Thermal Technology for Food Processing. Food Biophys. 2015, 10, 1–11. [Google Scholar] [CrossRef]
- Xiang, Q.; Liu, X.; Li, J.; Liu, S.; Zhang, H.; Bai, Y. Effects of dielectric barrier discharge plasma on the inactivation of Zygosaccharomyces rouxii and quality of apple juice. Food Chem. 2018, 254, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Ahmadnia, M.; Sadeghi, M.; Abbaszadeh, R.; Marzdashti, H.R.G. Decontamination of whole strawberry via dielectric barrier discharge cold plasma and effects on quality attributes. J. Food Process. Preserv. 2021, 45, e15019. [Google Scholar] [CrossRef]
- Sadhu, S.; Thirumdas, R.; Deshmukh, R.R.; Annapure, U.S. Influence of cold plasma on the enzymatic activity in germinating mung beans (Vigna radiate). LWT 2017, 78, 97–104. [Google Scholar] [CrossRef]
- Tappi, S.; Berardinelli, A.; Ragni, L.; Rosa, M.D.; Guarnieri, A.; Rocculi, P. Atmospheric gas plasma treatment of fresh-cut apples. Innov. Food Sci. Emerg. Technol. 2014, 21, 114–122. [Google Scholar] [CrossRef]
- Basak, S.; Annapure, U.S. Trends in “green” and novel methods of pectin modification-A review. Carbohydr. Polym. 2022, 278, 118967. [Google Scholar] [CrossRef]
- Sharafodin, H.; Soltanizadeh, N. Potential application of DBD Plasma Technique for modifying structural and physicochemical properties of Soy Protein Isolate. Food Hydrocoll. 2022, 122, 107077. [Google Scholar] [CrossRef]
- Zheng, J.; Tang, C.-H.; Ge, G.; Zhao, M.; Sun, W. Heteroprotein complex of soy protein isolate and lysozyme: Formation mechanism and thermodynamic characterization. Food Hydrocoll. 2020, 101, 105571. [Google Scholar] [CrossRef]
- Alves, A.C.; Tavares, G.M. Mixing animal and plant proteins: Is this a way to improve protein techno-functionalities? Food Hydrocoll. 2019, 97, 105171. [Google Scholar] [CrossRef]
- Boire, A.; Bouchoux, A.; Bouhallab, S.; Chapeau, A.-L.; Croguennec, T.; Ferraro, V.; Lechevalier, V.; Menut, P.; Pézennec, S.; Renard, D.; et al. Proteins for the future: A soft matter approach to link basic knowledge and innovative applications. Innov. Food Sci. Emerg. Technol. 2017, 46, 18–28. [Google Scholar] [CrossRef]
- Kayitmazer, A.B. Thermodynamics of complex coacervation. Adv. Colloid Interface Sci. 2017, 239, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Huang, Q. Assembly of Protein–Polysaccharide Complexes for Delivery of Bioactive Ingredients: A Perspective Paper. J. Agric. Food Chem. 2019, 67, 1344–1352. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.; Sanchez, C.; Desobry-Banon, S.; Hardy, J. Structure and technofunctional properties of protein-polysaccharide complexes: A review. Crit. Rev. Food Sci. Nutr. 1998, 38, 689–753. [Google Scholar] [CrossRef]
- Cui, L.; Guo, J.; Meng, Z. A review on food-grade-polymer-based O/W emulsion gels: Stabilization mechanism and 3D printing application. Food Hydrocoll. 2023, 139, 108588. [Google Scholar] [CrossRef]
- Soltani, S.; Madadlou, A. Two-step sequential cross-linking of sugar beet pectin for transforming zein nanoparticle-based Pickering emulsions to emulgels. Carbohydr. Polym. 2016, 136, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Zhao, S.; Guan, X.; McClements, D.J.; Liu, X.; Liu, F.; Ngai, T. Polysaccharide-based Pickering emulsions: Formation, stabilization and applications. Food Hydrocoll. 2021, 119, 106812. [Google Scholar] [CrossRef]
- Clark, A.; Kavanagh, G.; Ross-Murphy, S. Globular protein gelation—Theory and experiment. Food Hydrocoll. 2001, 15, 383–400. [Google Scholar] [CrossRef]
- Hinderink, E.B.; Boire, A.; Renard, D.; Riaublanc, A.; Sagis, L.M.; Schroën, K.; Bouhallab, S.; Famelart, M.-H.; Gagnaire, V.; Guyomarc’H, F.; et al. Combining plant and dairy proteins in food colloid design. Curr. Opin. Colloid Interface Sci. 2021, 56, 101507. [Google Scholar] [CrossRef]
- Guo, J.; Cui, L.; Meng, Z. Oleogels/emulsion gels as novel saturated fat replacers in meat products: A review. Food Hydrocoll. 2023, 137, 108313. [Google Scholar] [CrossRef]
- Nicolai, T.; Britten, M.; Schmitt, C. beta-Lactoglobulin and WPI aggregates: Formation, structure and applications. Food Hydrocoll. 2011, 25, 1945–1962. [Google Scholar] [CrossRef]
- Liang, X.; Ma, C.; Yan, X.; Zeng, H.; McClements, D.J.; Liu, X.; Liu, F. Structure, rheology and functionality of whey protein emulsion gels: Effects of double cross-linking with transglutaminase and calcium ions. Food Hydrocoll. 2020, 102, 105569. [Google Scholar] [CrossRef]
- Schmitt, C.; Silva, J.V.; Amagliani, L.; Chassenieux, C.; Nicolai, T. Heat-induced and acid-induced gelation of dairy/plant protein dispersions and emulsions. Curr. Opin. Food Sci. 2019, 27, 43–48. [Google Scholar] [CrossRef]
- Guo, Q.; Ye, A.; Lad, M.; Dalgleish, D.; Singh, H. The breakdown properties of heat-set whey protein emulsion gels in the human mouth. Food Hydrocoll. 2013, 33, 215–224. [Google Scholar] [CrossRef]
- He, D.; Yi, X.; Xia, G.; Liu, Z.; Zhang, X.; Li, C.; Shen, X. Effects of fish oil on the gel properties and emulsifying stability of myofibrillar proteins: A comparative study of tilapia, hairtail and squid. LWT 2022, 161, 113373. [Google Scholar] [CrossRef]
- Cui, B.; Mao, Y.; Liang, H.; Li, Y.; Li, J.; Ye, S.; Chen, W.; Li, B. Properties of soybean protein isolate/curdlan based emulsion gel for fat analogue: Comparison with pork backfat. Int. J. Biol. Macromol. 2022, 206, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Geng, M.J.; Wang, Z.K.; Qin, L.; Taha, A.; Du, L.X.; Xu, X.Y.; Pan, S.Y.; Hu, H. Effect of ultrasound and coagulant types on properties of beta-carotene bulk emulsion gels stabilized by soy protein. Food Hydrocoll. 2022, 123, 107146. [Google Scholar] [CrossRef]
- Maltais, A.; Remondetto, G.E.; Gonzalez, R.; Subirade, M. Formation of Soy Protein Isolate Cold-set Comprehensive evaluation of emulsifying and foaming properties of Gleditsia caspica seed galactomannan as a new source of hydrocolloid: Effect of extraction method.Gels: Protein and Salt Effects. J. Food Sci. 2005, 70, C67–C73. [Google Scholar] [CrossRef]
- Yan, C.; Fu, D.W.; McClements, D.J.; Xu, P.; Zou, L.Q.; Zhu, Y.Q.; Cheng, C.; Liu, W. Rheological and micro-structural properties of cold-set emulsion gels fabricated from mixed proteins: Whey protein and lactoferrin. Food Res.-Ternational 2019, 119, 315–324. [Google Scholar] [CrossRef]
- Xi, Z.; Liu, W.; McClements, D.J.; Zou, L. Rheological, structural, and microstructural properties of ethanol induced cold-set whey protein emulsion gels: Effect of oil content. Food Chem. 2019, 291, 22–29. [Google Scholar] [CrossRef]
- Bao, H.; Ni, Y.; Wusigale; Dong, H.; Liang, L. α-Tocopherol and resveratrol in emulsion-filled whey protein gels: Co-encapsulation and in vitro digestion. Int. Dairy J. 2020, 104, 104649. [Google Scholar] [CrossRef]
- Tang, C.-H.; Liu, F. Cold, gel-like soy protein emulsions by microfluidization: Emulsion characteristics, rheological and microstructural properties, and gelling mechanism. Food Hydrocoll. 2013, 30, 61–72. [Google Scholar] [CrossRef]
- Hu, S.; Wu, J.; Zhu, B.; Du, M.; Wu, C.; Yu, C.; Song, L.; Xu, X. Low oil emulsion gel stabilized by defatted Antarctic krill (Euphausia superba) protein using high-intensity ultrasound. Ultrason. Sonochem. 2021, 70, 105294. [Google Scholar] [CrossRef]
- Paradiso, V.M.; Giarnetti, M.; Summo, C.; Pasqualone, A.; Minervini, F.; Caponio, F. Production and characterization of emulsion filled gels based on inulin and extra virgin olive oil. Food Hydrocoll. 2015, 45, 30–40. [Google Scholar] [CrossRef]
- Pei, Z.; Wang, H.; Xia, G.; Hu, Y.; Xue, C.; Lu, S.; Li, C.; Shen, X. Emulsion gel stabilized by tilapia myofibrillar protein: Application in lipid-enhanced surimi preparation. Food Chem. 2023, 403, 134424. [Google Scholar] [CrossRef] [PubMed]
- Belgheisi, S.; Motamedzadegan, A.; Milani, J.M.; Rashidi, L.; Rafe, A. Impact of ultrasound processing parameters on physical characteristics of lycopene emulsion. J. Food Sci. Technol. 2020, 58, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Fu, L.; Zhao, Y.-Y.; Xue, S.-W.; Wang, P.; Xu, X.-L.; Bai, Y.-H. Use of high-intensity ultrasound to improve emulsifying properties of chicken myofibrillar protein and enhance the rheological properties and stability of the emulsion. Food Hydrocoll. 2020, 98, 105275. [Google Scholar] [CrossRef]
- Sun, L.-H.; Lv, S.-W.; Chen, C.-H.; Wang, C. Preparation and characterization of rice bran protein-stabilized emulsion by using ultrasound homogenization. Cereal Chem. 2019, 96, 478–486. [Google Scholar] [CrossRef]
- Du, H.Y.; Zhang, J.; Wang, S.Q.; Manyande, A.; Wang, J. Effect of high-intensity ultrasonic treatment on the phys-icochemical, structural, rheological, behavioral, and foaming properties of pumpkin (Cucurbita moschata Duch.)-seed protein isolates. Lwt-Food Sci. Technol. 2022, 155, 112952. [Google Scholar] [CrossRef]
- Flores-Jiménez, N.T.; Ulloa, J.A.; Silvas, J.E.U.; Ramírez, J.C.R.; Ulloa, P.R.; Rosales, P.U.B.; Carrillo, Y.S.; Leyva, R.G. Effect of high-intensity ultrasound on the compositional, physicochemical, biochemical, functional and structural properties of canola (Brassica napus L.) protein isolate. Food Res. Int. 2019, 121, 947–956. [Google Scholar] [CrossRef]
- Sun, L.-H.; Yu, F.; Wang, Y.-Y.; Lv, S.-W.; He, L.-Y. Effects of ultrasound extraction on the physicochemical and emulsifying properties of rice bran protein. Int. J. Food Eng. 2021, 17, 327–335. [Google Scholar] [CrossRef]
- Geng, M.; Hu, T.; Zhou, Q.; Taha, A.; Qin, L.; Lv, W.; Xu, X.; Pan, S.; Hu, H. Effects of different nut oils on the structures and properties of gel-like emulsions induced by ultrasound using soy protein as an emulsifier. Int. J. Food Sci. Technol. 2021, 56, 1649–1660. [Google Scholar] [CrossRef]
- McClements, D.J. Edible nanoemulsions: Fabrication, properties, and functional performance. Soft Matter. 2011, 7, 2297–2316. [Google Scholar] [CrossRef] [Green Version]
- Soria, A.C.; Villamiel, M. Effect of ultrasound on the technological properties and bioactivity of food: A review. Trends Food Sci. Technol. 2010, 21, 323–331. [Google Scholar] [CrossRef]
- Zhang, R.; Belwal, T.; Li, L.; Lin, X.; Xu, Y.; Luo, Z. Recent advances in polysaccharides stabilized emulsions for encapsulation and delivery of bioactive food ingredients: A review. Carbohydr. Polym. 2020, 242, 116388. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Shi, K.; Liu, D.; Huang, Q. Development of a food-grade organogel with high bioaccessibility and loading of curcuminoids. Food Chem. 2012, 131, 48–54. [Google Scholar] [CrossRef]
- Guo, Q.; Ye, A.; Bellissimo, N.; Singh, H.; Rousseau, D. Modulating fat digestion through food structure design. Prog. Lipid Res. 2017, 68, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Su, J.Q.; Wang, L.L.; Dong, W.X.; Wei, J.; Liu, X.; Yan, J.X.; Ren, F.Z.; Yuan, F.; Wang, P.J. Fabrication and Characterization of Ultra-High-Pressure (UHP)-Induced Whey Protein Isolate/kappa-Carrageenan Composite Emulsion Gels for the Delivery of Curcumin. Front. Nutr. 2022, 9, 839761. [Google Scholar] [CrossRef]
- Guo, Q.; Ye, A.; Lad, M.; Dalgleish, D.; Singh, H. Impact of colloidal structure of gastric digesta on in-vitro intestinal digestion of whey protein emulsion gels. Food Hydrocoll. 2016, 54, 255–265. [Google Scholar] [CrossRef]
- Shao, Y.; Tang, C.H. Gel-like pea protein Pickering emulsions at pH 3.0 as a potential intestine-targeted and sustained-release delivery system for beta-carotene. Food Res. Int. 2016, 79, 64–72. [Google Scholar] [CrossRef]
- Brito-Oliveira, T.C.; Bispo, M.; Moraes, I.C.F.; Campanella, O.H.; Pinho, S.C. Stability of curcumin encapsulated in solid lipid microparticles incorporated in cold-set emulsion filled gels of soy protein isolate and xanthan gum. Food Res. Int. 2017, 102, 759–767. [Google Scholar] [CrossRef]
- Lu, Y.; Mao, L.; Cui, M.; Yuan, F.; Gao, Y. Effect of the Solid Fat Content on Properties of Emulsion Gels and Stability of beta-Carotene. J. Agric. Food Chem. 2019, 67, 6466–6475. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Huang, Z.; Guo, P.P.; Guo, Q.N.; Zhang, H.J.; Jiang, L.W.; Xia, N.; Xiao, B.W. Tuning egg yolk granules/sodium alginate emulsion gel structure to enhance β-carotene stability and in vitro digestion property. Int. J. Biol. Macromol. 2023, 232, 123444. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.W.; Fu, S.Y.; Hou, J.J.; Guo, J.; Wang, J.M.; Yang, X.Q. Zein based oil-in-glycerol emulgels enriched with beta-carotene as margarine alternatives. Food Chem. 2016, 211, 836–844. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Liu, Y.; Zou, L.; McClements, D.J.; Liu, W. A review of recent progress in improving the bioavailability of nutraceutical-loaded emulsions after oral intake. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3963–4001. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Mao, L.; Hou, Z.; Miao, S.; Gao, Y. Development of Emulsion Gels for the Delivery of Functional Food Ingredients: From Structure to Functionality. Food Eng. Rev. 2019, 11, 245–258. [Google Scholar] [CrossRef]


| Protein Sources | Proteins | Protein Structure | Protein Fractions | Isoelectric Point | Molecular Weight (kDa) | Functional Properties | References |
|---|---|---|---|---|---|---|---|
| Dairy products | Casein | Unstructured, flexible random coil protein | αs1-casein | 4.4–4.8 | 23.6 | Excellent interfacial stability, emulsifying, gelling, foaming, and water-binding properties | [28,36] |
| αs2-casein | 4.9 | 25.2 | |||||
| β-casein | 4.8–5.0 | 24.0 | |||||
| κ-casein | ~3.5 | 19.1 | |||||
| Whey protein isolate | Globular protein | β-lactoglobulin | 5.35–5.49 | 18.3 | Excellent emulsifying, gelling, foaming, and water-binding properties | [37,38] | |
| α-lactalbumin | 4.2–4.5 | 14 | |||||
| Serum albumin | 5.5 | 65 | |||||
| Lactoferrin | 9.5–10.0 | 76.5 | |||||
| Legumes | Soybean protein | Complex globular protein | β-conglycinin (7S) | 4.6 | 150–200 | The foaming and emulsifying properties of untreated soy protein isolate are not as good as those of animal proteins | [27,39] |
| Glycine globular protein (11S) | 4.6 | 300–380 | |||||
| Pea protein | Complex globular protein | Legumin 11S | 4.8 | 330–410 | Having functional properties similar to soy protein, such as emulsifying properties | [40,41] | |
| Vicilin/convicilin 7S | 5.5 | 150/180–210 | |||||
| Cereals | Rice protein | Complex globular protein | Albumin | 4.1 | 10–100 | Low foaming capacity but high foaming stability. Emulsifying performance is similar to foaming properties | [42] |
| Globulin | 4.3/7.9 | 16–130 | |||||
| Glutelin | 4.8 | 51–57 | |||||
| Prolamin | 6.0–6.5 | 10–16 | |||||
| Wheat Protein | Complex globular protein | Glutenin | 7.5 | 30–140 | Poor emulsifying properties when compared to other vegetable proteins (such as zein) | [43] | |
| α-gliadin/β-gliadin/ γ-gliadin/ω-gliadin | 25–35/30–35/35–40/55–70 | ||||||
| Seeds | Flaxseed | Complex globular protein | 12S linen | 4.75 | 252–298 | High emulsifying activity and foaming capacity | [44] |
| 2S conlinin | 16–18 | ||||||
| Sunflower | Complex globular protein | 11S globulin (helianthinin) | 4.5 | 300–350 | Poor gel-forming properties and similar emulsifying properties to corresponding soy-based emulsions | [45] | |
| 2S albumin | 10–18 | ||||||
| Insects | Patanga succincta and Chondracris roseapbrunner | ND | ND | 4.0 | 20–250 | Poor emulsifying and foaming properties, but foaming stability was better than bovine serum albumin. | [46] |
| Tenebrio molitor | ND | ND | ND | 14–100 | Good gel-forming capacity | [47,48] | |
| Algae | Arthrospira maxima | ND | ND | ND | 36–112 | Good interfacial stability, and emulsifying properties | [49] |
| Modification Approach | Treatment Condition | Protein Type | Modified Characteristics | References |
|---|---|---|---|---|
| Physical techniques | Heat treatment | Soybean protein isolate |
| [77] |
| Pea protein isolate |
| [78] | ||
| Microwave treatment | Germinated tartary buckwheat protein |
| [79] | |
| Soybean protein isolate |
| [80] | ||
| Ultrasound treatment | Soybean protein isolate |
| [81] | |
| Whey protein |
| [82] | ||
| Soybean protein isolate |
| [8] | ||
| High hydrostatic pressure treatment | Rice bran protein |
| [83] | |
| Myofibrillar protein |
| [84] | ||
| Whey protein isolate |
| [85] | ||
| Cold plasma treatment | Peanut protein |
| [86] | |
| Grass pea protein isolate |
| [87] | ||
| Protein-protein interactions | Electrostatic interactions | Soybean protein isolate–whey protein isolate composite |
| [33,88] |
| Lactoferrin and β-lactoglobulin composite |
| [89] | ||
| Protein-polysaccharide interactions | Thickening with polysaccharides | Soybean protein isolate–flax gum |
| [90] |
| Crayfish protein–xanthan gum |
| [91] | ||
| Whey protein isolate–sodium alginate |
| [92] | ||
| Electrostatic interactions | Lactoferrin-xanthan complex |
| [93] | |
| Soybean protein isolate–κ-carrageenan complex |
| [94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Sun, L.; Zhuang, Y.; Gu, Y.; Cheng, G.; Fan, X.; Ding, Y.; Liu, H. Protein-Stabilized Emulsion Gels with Improved Emulsifying and Gelling Properties for the Delivery of Bioactive Ingredients: A Review. Foods 2023, 12, 2703. https://doi.org/10.3390/foods12142703
Xu Y, Sun L, Zhuang Y, Gu Y, Cheng G, Fan X, Ding Y, Liu H. Protein-Stabilized Emulsion Gels with Improved Emulsifying and Gelling Properties for the Delivery of Bioactive Ingredients: A Review. Foods. 2023; 12(14):2703. https://doi.org/10.3390/foods12142703
Chicago/Turabian StyleXu, Yuan, Liping Sun, Yongliang Zhuang, Ying Gu, Guiguang Cheng, Xuejing Fan, Yangyue Ding, and Haotian Liu. 2023. "Protein-Stabilized Emulsion Gels with Improved Emulsifying and Gelling Properties for the Delivery of Bioactive Ingredients: A Review" Foods 12, no. 14: 2703. https://doi.org/10.3390/foods12142703