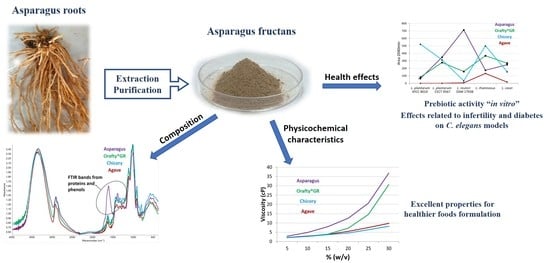
Asparagus Fructans as Emerging Prebiotics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Fructan Extraction from Asparagus Roots
2.3. Purification of Asparagus Fructans by Adsorption Chromatography
2.4. Commercial Fructan Sources
2.5. Determination of Chemical Composition
2.5.1. Moisture
2.5.2. Simple Sugar Composition
2.5.3. Ash
2.5.4. Proteins
2.5.5. Fructans
2.5.6. Phenols
2.6. pH
2.7. Antioxidant Activity
2.8. Degree of Polymerization of Fructans
2.9. Viscosity
2.10. Oil Holding Capacity (OHC)
2.11. Color
2.12. Fourier-Transform Infrared (FTIR) Spectroscopy
2.13. Prebiotic Effect In Vitro
2.14. C. elegans Assays
2.14.1. C. elegans Strains and Maintenance Conditions
2.14.2. Paralysis Assay in GMC101 Strain
2.14.3. Determination of Progeny Production in daf-2(e1370)
2.15. Statistical Analysis
3. Results and Discussion
3.1. Asparagus Inulin Yield and Chemical Composition
3.2. Degree of Polymerization of PAF and Commercial Inulins
3.3. Physicochemical Characteristics of PAF and Other Commercial Inulins
3.4. FT-IR Spectrum for Different Inulins
3.5. Prebiotic Activity of Inulins In Vitro
3.6. Effect of Fructans Extracts against Aβ-Induced Paralysis
3.7. Effect of Fructan Extracts on the Fertility of the Mutant daf-2(e-1370)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hughes, R.L.; Alvarado, D.A.; Swanson, K.S.; Holscher, H.D. The Prebiotic Potential of Inulin-Type Fructans: A Systematic Review. Adv. Nutr. 2022, 13, 492–529. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, M.; Shehzad, A.; Omar, M.; Rakha, A.; Raza, H.; Sharif, H.R.; Shakeel, A.; Ansari, A.; Niazi, S. Inulin: Properties, Health Benefits and Food Applications. Carbohydr. Polym. 2016, 147, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Apolinário, A.C.; de Lima Damasceno, B.P.G.; de Macêdo Beltrão, N.E.; Pessoa, A.; Converti, A.; da Silva, J.A. Inulin-Type Fructans: A Review on Different Aspects of Biochemical and Pharmaceutical Technology. Carbohydr. Polym. 2014, 101, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Lepczyński, A.; Herosimczyk, A.; Barszcz, M.; Ożgo, M.; Michałek, K.; Grabowska, M.; Tuśnio, A.; Szczerbińska, D.; Skomiał, J. Diet Supplemented Either with Dried Chicory Root or Chicory Inulin Significantly Influence Kidney and Liver Mineral Content and Antioxidative Capacity in Growing Pigs. Animal 2021, 15, 100129. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, H.; Nakanishi, N.; Miyoshi, T.; Okamura, T.; Hashimoto, Y.; Senmaru, T.; Majima, S.; Ushigome, E.; Asano, M.; Yamaguchi, M.; et al. Inulin Reduces Visceral Adipose Tissue Mass and Improves Glucose Tolerance through Altering Gut Metabolites. Nutr. Metab. 2022, 19, 50. [Google Scholar] [CrossRef]
- De Giani, A.; Sandionigi, A.; Zampolli, J.; Michelotti, A.; Tursi, F.; Labra, M.; Di Gennaro, P. Effects of Inulin-Based Prebiotics Alone or in Combination with Probiotics on Human Gut Microbiota and Markers of Immune System: A Randomized, Double-Blind, Placebo-Controlled Study in Healthy Subjects. Microorganisms 2022, 10, 1256. [Google Scholar] [CrossRef]
- Mauro, N.; Giammona, G.; Scialabba, C. Inulin for Cancer Therapy: Present and Perspectives. Int. J. Pharma Res. Rev. 2016, 5, 63–69. [Google Scholar]
- Mudannayake, D.C.; Wimalasiri, K.M.S.; Silva, K.F.S.T.; Ajlouni, S. Comparison of Properties of New Sources of Partially Purified Inulin to Those of Commercially Pure Chicory Inylin. J. Food Sci. 2015, 80, C950–C960. [Google Scholar] [CrossRef]
- Alaei, F.; Hojjatoleslamy, M.; Hashemi Dehkordi, S.M. The Effect of Inulin as a Fat Substitute on the Physicochemical and Sensory Properties of Chicken Sausages. Food Sci. Nutr. 2018, 6, 512–519. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Gunenc, A.; Hosseinian, F. Developing Emulsion Gels by Incorporating Jerusalem Artichoke Inulin and Investigating Their Lipid Oxidative Stability. Food Prod. Process Nutr. 2020, 2, 2. [Google Scholar] [CrossRef]
- Benkeblia, N. Fructooligosaccharides and Fructans Analysis in Plants and Food Crops. J. Chrom. A 2013, 1313, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Cuenca, A.; Herrera-Vázquez, S.E.; Condezo-Hoyos, L.; Gómez-Ordóñez, E.; Rupérez, P. Inulin Extraction from Common Inulin-Containing Plant Sources. Ind. Crops Prod. 2021, 170, 113726. [Google Scholar] [CrossRef]
- Bhagia, S.; Akinosho, H.; Ferreira, J.F.S.; Ragauskas, A.J. Biofuel Production from Jerusalem Artichoke Tuber Inulins: A Review. Biofuel Res. J. 2017, 4, 587–599. [Google Scholar] [CrossRef]
- Bouaziz, M.A.; Rassaoui, R.; Besbes, S. Chemical Composition, Functional Properties, and Effect of Inulin from Tunisian Agave Americana L. Leaves on Textural Qualities of Pectin Gel. J. Chem. 2014, 2014, 758697. [Google Scholar] [CrossRef] [Green Version]
- Viera-Alcaide, I.; Hamdi, A.; Guillén-Bejarano, R.; Rodríguez-Arcos, R.; Espejo-Calvo, J.A.; Jiménez-Araujo, A. Asparagus Roots: From an Agricultural By-Product to a Valuable Source of Fructans. Foods 2022, 11, 652. [Google Scholar] [CrossRef]
- Weerasingha, V.; Wimalasiri, K.M.S.; Jayasumana, L. Enhanced Probiotic Activity, Physicochemical and Sensory Properties of Set-Yoghurt Incorporated with Asparagus Officinalis Inulin. J. Diet. Food Technol. 2020, 1, 1–6. [Google Scholar] [CrossRef]
- Sun, Q.; Zhu, L.; Li, Y.; Cui, Y.; Jiang, S.; Tao, N.; Chen, H.; Zhao, Z.; Xu, J.; Dong, C. A Novel Inulin-Type Fructan from Asparagus Cochinchinensis and Its Beneficial Impact on Human Intestinal Microbiota. Carbohydr. Polym. 2020, 247, 116761. [Google Scholar] [CrossRef]
- Singh, R.S.; Dhaliwal, R.; Puri, M. Production of High Fructose Syrup from Asparagus Inulin Using Immobilized Exoinulinase from Kluyveromyces Marxianus YS-1. J. Ind. Microbiol. Biotechnol. 2007, 34, 649–655. [Google Scholar] [CrossRef]
- Jaramillo-Carmona, S.M.; Javier Tejero-Maján, F.; Jiménez-Araujo, A.; Guillén-Bejarano, R.; Rodríguez-Arcos, R. Comparative Analysis of Chemical Compounds Related to Quality of Canned Asparagus. JFNR 2019, 7, 171–182. [Google Scholar] [CrossRef] [Green Version]
- Megazyme Fructan Assay Kit-Measurement of Fructan in Plants Food|Megazyme. Available online: https://www.megazyme.com/fructan-assay-kit (accessed on 27 October 2021).
- Hamdi, A.; Jaramillo-Carmona, S.; Beji, R.; Tej, R.; Zaoui, S.; Rodríguez-Arcos, R.; Jiménez-Araujo, A.; Kasri, M.; Lachaal, M.; Bouraoui, N.K.; et al. The Phytochemical and Bioactivity Profiles of Wild Asparagus Albus L. Plant. Food Res. Int. 2017, 99, 720–729. [Google Scholar] [CrossRef]
- Rodríguez, R.; Jaramillo, S.; Rodríguez, G.; Espejo, J.A.; Guillén, R.; Fernández-Bolaños, J.; Heredia, A.; Jiménez, A. Antioxidant Activity of Ethanolic Extracts from Several Asparagus Cultivars. J. Agric. Food Chem. 2005, 53, 5212–5217. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Alventosa, J.M.; Rodríguez-Gutiérrez, G.; Jaramillo-Carmona, S.; Espejo-Calvo, J.A.; Rodríguez-Arcos, R.; Fernandez-Bolaños, J.; Guillén-Bejarano, R.; Jiménez-Araujo, A. Effect of Extraction Method on Chemical Composition and Functional Characteristics of High Dietary Fibre Powders Obtained from Asparagus By-Products. Food Chem. 2009, 113, 665–671. [Google Scholar] [CrossRef]
- Hornero Méndez, D. Cambios en la Composición de Pigmentos en Frutos de Capsicum annuum Durante la Maduración y Procesado Para Pimentón: Variedades Bola y Agridulce. Doctoral Dissertation, Universidad de Sevilla, Sevilla, Spain, 1994. [Google Scholar]
- García-González, D.L.; Van De Voort, F.R. A Novel Wire Mesh “Cell” for Studying Lipid Oxidative Processes by Fourier Transform Infrared Spectroscopy. Appl. Spectrosc. 2009, 63, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.; Reiner, J.; Fleischhacker, L.; Viernstein, H.; Loeppert, R.; Praznik, W. Growth of Selected Probiotic Strains with Fructans from Different Sources Relating to Degree of Polymerization and Structure. J. Funct. Foods 2016, 24, 264–275. [Google Scholar] [CrossRef]
- Sulston, J.; Hodgkin, J. The Nematode Caenorhabditis Elegans; Wood, W., Ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1988. [Google Scholar]
- Viera-Alcaide, I.; Hamdi, A.; Rodríguez-Arcos, R.; Guillén-Bejarano, R.; Jiménez-Araujo, A. Asparagus Cultivation Co-Products: From Waste to Chance. FSN 2020, 6, 57. [Google Scholar] [CrossRef]
- Hamdi, A.; Jaramillo-Carmona, S.; Rodríguez-Arcos, R.; Jiménez-Araujo, A.; Lachaal, M.; Karray-Bouraoui, N.; Guillén-Bejarano, R. Phytochemical Characterization and Bioactivity of Asparagus Acutifolius: A Focus on Antioxidant, Cytotoxic, Lipase Inhibitory and Antimicrobial Activities. Molecules 2021, 26, 3328. [Google Scholar] [CrossRef]
- Hamdi, A.; Jiménez-Araujo, A.; Rodríguez-Arcos, R.; Jaramillo-Carmona, S.; Lachaal, M.; Bouraoui, N.K.; Guillén-Bejarano, R. Asparagus Saponins; Chemical Characterization, Bioavailability and Intervention in Human Health. Nutr. Food Sci. Int. J. 2018, 7, 555704. [Google Scholar] [CrossRef]
- Jaramillo, S.; Muriana, F.J.G.; Guillén, R.; Jiménez-Araujo, A.; Rodríguez-Arcos, R.; López, S. Saponins from Edible Spears of Wild Asparagus Inhibit AKT, P70S6K, and ERK Signalling, and Induce Apoptosis through G0/G1 Cell Cycle Arrest in Human Colon Cancer HCT-116 Cells. J. Funct. Foods 2016, 26, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Verma, D.K.; Patel, A.R.; Thakur, M.; Singh, S.; Tripathy, S.; Srivastav, P.P.; Chávez-González, M.L.; Gupta, A.K.; Aguilar, C.N. A Review of the Composition and Toxicology of Fructans, and Their Applications in Foods and Health. J. Food Compos. Anal. 2021, 99, 103884. [Google Scholar] [CrossRef]
- Tewari, S.; Ramalakshmi, K.; Methre, L.; Mohan Rao, L.J. Microwave-Assisted Extraction of Inulin from Chicory Roots Using Response Surface Methodology. J. Nutr. Food Sci. 2015, 5, 342. [Google Scholar] [CrossRef] [Green Version]
- Monti, A.; Amaducci, M.T.; Pritoni, G.; Venturi, G. Growth, Fructan Yield, and Quality of Chicory (Cichorium Intybus L.) as Related to Photosynthetic Capacity, Harvest Time, and Water Regime. J. Exp. Bot. 2005, 56, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Lingyun, W.; Jianhua, W.; Xiaodong, Z.; Da, T.; Yalin, Y.; Chenggang, C.; Tianhua, F.; Fan, Z. Studies on the Extracting Technical Conditions of Inulin from Jerusalem Artichoke Tubers. J. Food Eng. 2007, 79, 1087–1093. [Google Scholar] [CrossRef]
- Puangbut, D.; Jogloy, S.; Vorasoot, N.; Srijaranai, S.; Holbrook, C.C.; Patanothai, A. Variation of Inulin Content, Inulin Yield and Water Use Efficiency for Inulin Yield in Jerusalem Artichoke Genotypes under Different Water Regimes. Agric. Water Manag. 2015, 152, 142–150. [Google Scholar] [CrossRef]
- Flores-Girón, E.; Salazar-Montoya, J.A.; Ramos-Ramírez, E.G. Application of a Box-Behnken Design for Optimizing the Extraction Process of Agave Fructans ( Agave Tequilana Weber Var. Azul): Box-Behnken Design for Optimizing the Extraction Process of Agave Fructans. J. Sci. Food Agric. 2016, 96, 3860–3866. [Google Scholar] [CrossRef] [PubMed]
- Velázquez-Martínez, J.; González-Cervantes, R.; Hernández-Gallegos, M.; Mendiola, R.; Aparicio, A.; Ocampo, M. Prebiotic Potential of Agave Angustifolia Haw Fructans with Different Degrees of Polymerization. Molecules 2014, 19, 12660–12675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeaiter, Z.; Regonesi, M.E.; Cavini, S.; Labra, M.; Sello, G.; Di Gennaro, P. Extraction and Characterization of Inulin-Type Fructans from Artichoke Wastes and Their Effect on the Growth of Intestinal Bacteria Associated with Health. BioMed Res. Int. 2019, 2019, 1083952. [Google Scholar] [CrossRef] [Green Version]
- Roldán-Marín, E.; Jensen, R.I.; Krath, B.N.; Kristensen, M.; Poulsen, M.; Cano, M.P.; Sánchez-Moreno, C.; Dragsted, L.O. An Onion Byproduct Affects Plasma Lipids in Healthy Rats. J. Agric. Food Chem. 2010, 58, 5308–5314. [Google Scholar] [CrossRef]
- Duarte, F.N.D.; Rodrigues, J.B.; da Costa Lima, M.; dos S. Lima, M.; Pacheco, M.T.B.; Pintado, M.M.E.; de Souza Aquino, J.; de Souza, E.L. Potential Prebiotic Properties of Cashew Apple (Anacardium Occidentale L.) Agro-Industrial Byproduct on Lactobacillus Species: Prebiotic Effects of Cashew Byproduct on Lactobacilli. J. Sci. Food Agric. 2017, 97, 3712–3719. [Google Scholar] [CrossRef]
- Li, W.; Zhang, J.; Yu, C.; Li, Q.; Dong, F.; Wang, G.; Gu, G.; Guo, Z. Extraction, Degree of Polymerization Determination and Prebiotic Effect Evaluation of Inulin from Jerusalem Artichoke. Carbohyd. Polym. 2015, 121, 315–319. [Google Scholar] [CrossRef]
- Singh, D.K.; Gulati, K.; Ray, A. Effects of Chelidonic Acid, a Secondary Plant Metabolite, on Mast Cell Degranulation and Adaptive Immunity in Rats. Int. Immunopharmacol. 2016, 40, 229–234. [Google Scholar] [CrossRef]
- Kim, D.-S.; Kim, S.-J.; Kim, M.-C.; Jeon, Y.-D.; Um, J.; Hong, S.-H. The Therapeutic Effect of Chelidonic Acid on Ulcerative Colitis. Biol. Pharm. Bull. 2012, 35, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zheng, Y.; Li, C.; Zheng, Y.; Wang, D.; Wu, Z.; Huang, L.; Wang, Y.; Li, P.; Peng, W.; et al. Discovery of Anti-Inflammatory Ingredients in Chinese Herbal Formula Kouyanqing Granule Based on Relevance Analysis between Chemical Characters and Biological Effects. Sci. Rep. 2015, 5, 18080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGaw, L.J.; Eloff, J.N. Ethnoveterinary Use of Southern African Plants and Scientific Evaluation of Their Medicinal Properties. J. Ethnopharmacol. 2008, 119, 559–574. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Ahmed, S.; Elasbali, A.M.; Adnan, M.; Alam, S.; Hassan, M.I.; Pasupuleti, V.R. Therapeutic Implications of Caffeic Acid in Cancer and Neurological Diseases. Front. Oncol. 2022, 12, 860508. [Google Scholar] [CrossRef]
- Beneo Inulin from Orafti®|BENEO Prebiotic, Dietary Chicory Root Fibre. Available online: https://www.beneo.com/ingredients/human-nutrition/functional-fibres/inulin (accessed on 27 October 2021).
- Yoshida, M. Fructan Structure and Metabolism in Overwintering Plants. Plants 2021, 10, 933. [Google Scholar] [CrossRef]
- Shiomi, N. Structure of Fructopolysaccharide (Asparagosin) from Roots of Asparagus (Asparagus Officinalis L.). New Phytol. 1993, 123, 263–270. [Google Scholar] [CrossRef]
- Shiomi, N.; Yamada, J.; Izawa, M. Isolation and Identification of Fructo-Oligosaccharides in Roots of Asparagus (Asparagus Officinalis L.). Agric. Biol. Chem. 1976, 40, 567–575. [Google Scholar] [CrossRef]
- Witzel, K.; Matros, A. Fructans Are Differentially Distributed in Root Tissues of Asparagus. Cells 2020, 9, 1943. [Google Scholar] [CrossRef]
- Nobre, C.; Teixeira, J.A.; Rodrigues, L.R. New Trends and Technological Challenges in the Industrial Production and Purification of Fructo-Oligosaccharides. Crit. Rev. Food Sci. 2015, 55, 1444–1455. [Google Scholar] [CrossRef]
- Oligofructose the 100% Natural Sugar Replacer|BENEO. Available online: https://www.beneo.com/ingredients/human-nutrition/functional-fibres/oligofructose (accessed on 25 May 2022).
- Roberfroid, M.B.; Van Loo, J.A.E.; Gibson, G.R. The Bifidogenic Nature of Chicory Inulin and Its Hydrolysis Products. J. Nutr. 1998, 128, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Hong, T.; Iwashita, K.; Shiraki, K. Viscosity Control of Protein Solution by Small Solutes: A Review. CPPS 2018, 19, 746–758. [Google Scholar] [CrossRef] [PubMed]
- Minton, A.P. Influence of Macromolecular Crowding upon the Stability and State of Association of Proteins: Predictions and Observations. J. Pharm. Sci. 2005, 94, 1668–1675. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Woods, C.E.; Litowski, J.R.; Roschen, L.A.; Gadgil, H.S.; Razinkov, V.I.; Kerwin, B.A. Effect of Sugar Molecules on the Viscosity of High Concentration Monoclonal Antibody Solutions. Pharm. Res. 2011, 28, 1552–1560. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D.; Bayarri, S.; Tárrega, A.; Costell, E. Inulin as Texture Modifier in Dairy Products. Food Hydrocoll. 2011, 25, 1881–1890. [Google Scholar] [CrossRef]
- Yousaf, M.S.; Yusof, S.; Manap, M.Y.B.A.; Abd-Aziz, S. Storage Stabiliy of Clarified Banana Juice Fortified with Inulin and Oligofructose. J. Food Process. Pres. 2010, 34, 599–610. [Google Scholar] [CrossRef]
- Cardarelli, H.R.; Buriti, F.C.A.; Castro, I.A.; Saad, S.M.I. Inulin and Oligofructose Improve Sensory Quality and Increase the Probiotic Viable Count in Potentially Synbiotic Petit-Suisse Chesse. LWT-Food Sci. Technol. 2008, 41, 1037–1046. [Google Scholar] [CrossRef]
- Abou-Arab, A.A.; Talaat, H.A.; Abu-Salem, F.M. Physico-Chemical Properties of Inulin Produced from Jerusalem Artichoke Tubers on Bench and Pilot Plant Scale. Aust. J. Basic Appl. Sci. 2011, 5, 1297–1309. [Google Scholar]
- Barhatova, T.-; Nazarenko, M.-; Koguhova, M.-; Hripko, I. Obtaining and identification of inulin from jerusalem artichoke (Helianthus tuberosus) tubers. Foods Raw Mater. 2015, 3, 13–22. [Google Scholar] [CrossRef]
- Oliveira, R.N.; Mancini, M.C.; de Oliveira, F.C.S.; Passos, T.M.; Quilty, B.; Thiré, R.M.D.S.M.; McGuinness, G.B. FTIR Analysis and Quantification of Phenols and Flavonoids of Five Commercially Available Plants Extracts Used in Wound Healing. Matéria 2016, 21, 767–779. [Google Scholar] [CrossRef] [Green Version]
- Wongsa, P.; Phatikulrungsun, P.; Prathumthong, S. FT-IR Characteristics, Phenolic Profiles and Inhibitory Potential against Digestive Enzymes of 25 Herbal Infusions. Sci. Rep. 2022, 12, 6631. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, S.; Khor, B.-K.; Khaw, K.-Y.; Murugaiyah, V.; Chan, K.-L. Cholinesterase Inhibitory Potential of Dillenia Suffruticosa Chemical Constituents and Protective Effect against Aβ−induced Toxicity in Transgenic Caenorhabditis Elegans Model. Phytomed. Plus 2021, 1, 100022. [Google Scholar] [CrossRef]
- Yang, T.; Fang, L.; Lin, T.; Li, J.; Zhang, Y.; Zhou, A.; Xie, J. Ultrasonicated Sour Jujube Seed Flavonoids Extract Exerts Ameliorative Antioxidant Capacity and Reduces Aβ-Induced Toxicity in Caenorhabditis Elegans. J. Ethnopharmacol. 2019, 239, 111886. [Google Scholar] [CrossRef] [PubMed]
- Yuen, C.W.; Murugaiyah, V.; Najimudin, N.; Azzam, G. Danshen (Salvia Miltiorrhiza) Water Extract Shows Potential Neuroprotective Effects in Caenorhabditis Elegans. J. Ethnopharmacol. 2021, 266, 113418. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Li, Q.; Yang, T.; Qi, W.; Zhang, Y.; Wang, L.; Sun, X.; Xie, J. Flavonoid Glucosides from Ziziphus Jujuba Seeds Improve Learning and Memory in Mice. Rev. Bras. Farmacogn. 2022, 32, 99–110. [Google Scholar] [CrossRef]
- Kang, N.; Luan, Y.; Jiang, Y.; Cheng, W.; Liu, Y.; Su, Z.; Liu, Y.; Tan, P. Neuroprotective Effects of Oligosaccharides in Rehmanniae Radix on Transgenic Caenorhabditis Elegans Models for Alzheimer’s Disease. Front. Pharmacol. 2022, 13, 878631. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, R.; Li, H.; Xiang, Y.; Xiao, L.; Hu, M.; Ma, F.; Ma, C.W.; Huang, Z. Antioxidant and Neuroprotective Effects of Dictyophora Indusiata Polysaccharide in Caenorhabditis Elegans. J. Ethnopharmacol. 2016, 192, 413–422. [Google Scholar] [CrossRef]
- Taniguchi, C.M.; Emanuelli, B.; Kahn, C.R. Critical Nodes in Signalling Pathways: Insights into Insulin Action. Nat. Rev. Mol. Cell Biol. 2006, 7, 85–96. [Google Scholar] [CrossRef]
- Lindhurst, M.J.; Parker, V.E.R.; Payne, F.; Sapp, J.C.; Rudge, S.; Harris, J.; Witkowski, A.M.; Zhang, Q.; Groeneveld, M.P.; Scott, C.E.; et al. Mosaic Overgrowth with Fibroadipose Hyperplasia Is Caused by Somatic Activating Mutations in PIK3CA. Nat. Genet. 2012, 44, 928–933. [Google Scholar] [CrossRef]
- Iravani, S. Green Synthesis of Metal Nanoparticles Using Plants. Green Chem. 2011, 13, 2638. [Google Scholar] [CrossRef]
- Mohammadi Arvanag, F.; Bayrami, A.; Habibi-Yangjeh, A.; Rahim Pouran, S. A Comprehensive Study on Antidiabetic and Antibacterial Activities of ZnO Nanoparticles Biosynthesized Using Silybum Marianum L Seed Extract. Mater. Sci. Eng. C 2019, 97, 397–405. [Google Scholar] [CrossRef]
- Hussein, J.; Attia, M.F.; El Bana, M.; El-Daly, S.M.; Mohamed, N.; El-Khayat, Z.; El-Naggar, M.E. Solid State Synthesis of Docosahexaenoic Acid-Loaded Zinc Oxide Nanoparticles as a Potential Antidiabetic Agent in Rats. Int. J. Biol. Macromol. 2019, 140, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, S.; Ruvkun, G. Daf-2, Daf-16 and Daf-23: Genetically Interacting Genes Controlling Dauer Formation in Caenorhabditis Elegans. Genetics 1994, 137, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Gems, D.; Sutton, A.J.; Sundermeyer, M.L.; Albert, P.S.; King, K.V.; Edgley, M.L.; Larsen, P.L.; Riddle, D.L. Two Pleiotropic Classes of Daf-2 Mutation Affect Larval Arrest, Adult Behavior, Reproduction and Longevity in Caenorhabditis Elegans. Genetics 1998, 150, 129–155. [Google Scholar] [CrossRef] [PubMed]
- Oghbaei, H.; Rastgar Rezaei, Y.; Nikanfar, S.; Zarezadeh, R.; Sadegi, M.; Latifi, Z.; Nouri, M.; Fattahi, A.; Ahmadi, Y.; Bleisinger, N. Effects of Bacteria on Male Fertility: Spermatogenesis and Sperm Function. Life Sci. 2020, 256, 117891. [Google Scholar] [CrossRef] [PubMed]
- Tissenbaum, H.A.; Ruvkun, G. An Insulin-like Signaling Pathway Affects Both Longevity and Reproduction in Caenorhabditis Elegans. Genetics 1998, 148, 703–717. [Google Scholar] [CrossRef]
- Lakra, A.K.; Ramatchandirane, M.; Kumar, S.; Suchiang, K.; Arul, V. Physico-Chemical Characterization and Aging Effects of Fructan Exopolysaccharide Produced by Weissella Cibaria MD2 on Caenorhabditis Elegans. LWT 2021, 143, 111100. [Google Scholar] [CrossRef]
- Rasulova, M.; Zečić, A.; Monje Moreno, J.M.; Vandemeulebroucke, L.; Dhondt, I.; Braeckman, B.P. Elevated Trehalose Levels in C. elegans Daf-2 Mutants Increase Stress Resistance, Not Lifespan. Metabolites 2021, 11, 105. [Google Scholar] [CrossRef]
- Chandrashekar, V.; Bartke, A. The Impact of Altered Insulin-like Growth Factor-I Secretion on the Neuroendocrine and Testicular Functions. Minerva. Ginecol. 2005, 57, 87–97. [Google Scholar]
- Hamidi, M.; Ziaee, M.; Delashoub, M.; Marjani, M.; Karimitabar, F.; Khorami, A.; Ahmadi, N.A. The Effects of Essential Oil of Lavandula Angustifolia on Sperm Parameters Quality and Reproductive Hormones in Rats Exposed to Cadmium. J. Rep. Pharma. Sci. 2015, 4, 135–142. [Google Scholar]
- Keyhanmanesh, R.; Hamidian, G.; Alipour, M.R.; Ranjbar, M.; Oghbaei, H. Protective Effects of Sodium Nitrate against Testicular Apoptosis and Spermatogenesis Impairments in Streptozotocin-Induced Diabetic Male Rats. Life Sci. 2018, 211, 63–73. [Google Scholar] [CrossRef]
- Nef, S.; Verma-Kurvari, S.; Merenmies, J.; Vassalli, J.-D.; Efstratiadis, A.; Accili, D.; Parada, L.F. Testis Determination Requires Insulin Receptor Family Function in Mice. Nature 2003, 426, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Vikram, A.; Kushwaha, S.; Jena, G.B. Relative Influence of Testosterone and Insulin in the Regulation of Prostatic Cell Proliferation and Growth. Steroids 2011, 76, 416–423. [Google Scholar] [CrossRef]
- Brüning, J.C.; Gautam, D.; Burks, D.J.; Gillette, J.; Schubert, M.; Orban, P.C.; Klein, R.; Krone, W.; Müller-Wieland, D.; Kahn, C.R. Role of Brain Insulin Receptor in Control of Body Weight and Reproduction. Science 2000, 289, 2122–2125. [Google Scholar] [CrossRef] [PubMed]
- Pitetti, J.-L.; Calvel, P.; Zimmermann, C.; Conne, B.; Papaioannou, M.D.; Aubry, F.; Cederroth, C.R.; Urner, F.; Fumel, B.; Crausaz, M.; et al. An Essential Role for Insulin and IGF1 Receptors in Regulating Sertoli Cell Proliferation, Testis Size, and FSH Action in Mice. Mol. Endocrinol. 2013, 27, 814–827. [Google Scholar] [CrossRef] [PubMed]
- Hammoud, A.; Meikle, A.; Reis, L.; Gibson, M.; Peterson, C.; Carrell, D. Obesity and Male Infertility: A Practical Approach. Semin. Reprod. Med. 2012, 30, 486–495. [Google Scholar] [CrossRef]
- Khorrami, A.; Ghanbarzadeh, S.; Ziaee, M.; Arami, S.; Vajdi, R.; Garjani, A. Dietary Cholesterol and Oxidised Cholesterol: Effects on Sperm Characteristics, Antioxidant Status and Hormonal Profile in Rats. Andrologia 2015, 47, 310–317. [Google Scholar] [CrossRef]
- Liu, F.; Prabhakar, M.; Ju, J.; Long, H.; Zhou, H.-W. Effect of Inulin-Type Fructans on Blood Lipid Profile and Glucose Level: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Eur. J. Clin. Nutr. 2017, 71, 9–20. [Google Scholar] [CrossRef]
- Guess, N.D.; Dornhorst, A.; Oliver, N.; Frost, G.S. A Randomised Crossover Trial: The Effect of Inulin on Glucose Homeostasis in Subtypes of Prediabetes. Ann. Nutr. Metab. 2016, 68, 26–34. [Google Scholar] [CrossRef]







| Moisture | Simple Sugars | Ash | Protein | Fructans | |
|---|---|---|---|---|---|
| Asparagus | 9.00 ± 0.13 c | 4.19 ± 0.43 b | 6.69 ± 0.06 | 17.50 ± 0.83 | 57.94 ± 0.41 a |
| Orafti-GR | 4.61 ± 0.37 a | 1.99 ± 0.01 a | t | n.d. | 78.11 ± 0.51 b |
| Chicory | 6.00 ± 0.57 b | 8.48 ± 0.59 d | t | n.d. | 58.96 ± 1.44 a |
| Agave | 4.60 ± 0.11 a | 7.05 ± 0.39 c | t | n.d. | 80.65 ± 2.47 b |
| mg/g Asparagus Fructan | |
| Chelidonic acid | 1.13 ± 0.07 |
| Caffeic acid glycoside | 0.30 ± 0.00 |
| Caffeic acid | 0.64 ± 0.03 |
| p-Coumaric acid | 0.07 ± 0.00 |
| t-Ferulic acid | 0.11 ± 0.01 |
| Total | 2.25 ± 0.03 |
| μmols TE/g asparagus fructan | |
| Antioxidant activity | 43.62 ± 3.16 |
| Color | |||||
|---|---|---|---|---|---|
| pH | OHC | L* | a* | b* | |
| Asparagus | 6.45 ± 0.01 d | 93.63 ± 1.41 b | 50.5203 | 3.9032 | 17.3581 |
| Orafti®GR | 6.04 ± 0.02 c | 78.46 ± 3.01 a | 88.8906 | −0.3873 | 2.6834 |
| Chicory | 5.12 ± 0.03 b | 95.15 ± 4.76 b | 86.9714 | −0.1935 | 3.039 |
| Agave | 4.59 ± 0.23 a | 79.93 ± 2.78 a | 88.1635 | −0.6182 | 4.652 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamdi, A.; Viera-Alcaide, I.; Guillén-Bejarano, R.; Rodríguez-Arcos, R.; Muñoz, M.J.; Monje Moreno, J.M.; Jiménez-Araujo, A. Asparagus Fructans as Emerging Prebiotics. Foods 2023, 12, 81. https://doi.org/10.3390/foods12010081
Hamdi A, Viera-Alcaide I, Guillén-Bejarano R, Rodríguez-Arcos R, Muñoz MJ, Monje Moreno JM, Jiménez-Araujo A. Asparagus Fructans as Emerging Prebiotics. Foods. 2023; 12(1):81. https://doi.org/10.3390/foods12010081
Chicago/Turabian StyleHamdi, Amel, Isabel Viera-Alcaide, Rafael Guillén-Bejarano, Rocío Rodríguez-Arcos, Manuel Jesús Muñoz, Jose Manuel Monje Moreno, and Ana Jiménez-Araujo. 2023. "Asparagus Fructans as Emerging Prebiotics" Foods 12, no. 1: 81. https://doi.org/10.3390/foods12010081