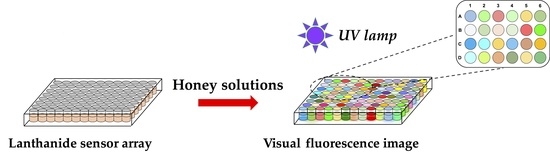
Dipicolinic Acid-Tb3+/Eu3+ Lanthanide Fluorescence Sensor Array for Rapid and Visual Discrimination of Botanical Origin of Honey
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of DPA-Tb3+/Eu3+ Complex Sensors
2.3. Effects of Honey on the Fluorescence of DPA-Tb3+/Eu3+ Complex
2.4. Cross-Reactive Response of DPA-Tb3+/Eu3+ Complex to Honey Constituents
2.5. Sensor Array Measured by Microplate Reader
2.6. Visual Discrimination Using Sensor Array
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bogdanov, S.; Jurendic, T.; Sieber, R.; Gallmann, P. Honey for Nutrition and Health: A Review. J. Am. Coll. Nutr. 2008, 27, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Valverde, S.; Ares, A.M.; Elmore, J.S.; Bernal, J. Recent trends in the analysis of honey constituents. Food Chem. 2022, 387, 132920. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.; Amaral, J.S.; Oliveira, M.B.P.P.; Mafra, I.A. Comprehensive Review on the Main Honey Authentication Issues: Production and Origin. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1072–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsagkaris, A.S.; Koulis, G.A.; Danezis, G.P.; Martakos, I.; Dasenaki, M.; Georgiou, C.A.; Thomaidis, N.S. Honey Authenticity: Analytical Techniques, State of the Art and Challenges. RSC Adv. 2021, 11, 11273–11294. [Google Scholar] [CrossRef]
- Pires, J.; Estevinho, M.L.; Feás, X.; Cantalapiedra, J.; Iglesias, A. Pollen Spectrum and Physico-Chemical Attributes of Heather (Erica Sp.) Honeys of North Portugal: Attributes of Heather Honeys from North Portugal. J. Sci. Food Agric. 2009, 89, 1862–1870. [Google Scholar] [CrossRef] [Green Version]
- Cuevas-Glory, L.F.; Pino, J.A.; Santiago, L.S.; Sauri-Duch, E. A Review of Volatile Analytical Methods for Determining the Botanical Origin of Honey. Food Chem. 2007, 103, 1032–1043. [Google Scholar] [CrossRef]
- Machado, A.M.; Miguel, M.G.; Vilas-Boas, M.; Figueiredo, A.C. Honey Volatiles as a Fingerprint for Botanical Origin—A Review on Their Occurrence on Monofloral Honeys. Molecules 2020, 25, 374. [Google Scholar] [CrossRef] [Green Version]
- Gliszczyńska-Świgło, A.; Chmielewski, J. Electronic Nose as a Tool for Monitoring the Authenticity of Food. A Review. Food Anal. Methods 2017, 10, 1800–1816. [Google Scholar] [CrossRef] [Green Version]
- Bong, J.; Middleditch, M.; Loomes, K.M.; Stephens, J.M. Proteomic Analysis of Honey. Identification of Unique Peptide Markers for Authentication of NZ Mānuka (Leptospermum scoparium) Honey. Food Chem. 2021, 350, 128442. [Google Scholar] [CrossRef]
- Mato, I.; Huidobro, J.F.; Simal-Lozano, J.; Sancho, M.T. Significance of Nonaromatic Organic Acids in Honey. J. Food Prot. 2003, 66, 2371–2376. [Google Scholar] [CrossRef]
- Ciulu, M.; Spano, N.; Pilo, M.; Sanna, G. Recent Advances in the Analysis of Phenolic Compounds in Unifloral Honeys. Molecules 2016, 21, 451. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Fan, C.; Chang, Q.; Pang, G.; Hu, X.; Lu, M.; Wang, W. Chemometric Determination of the Botanical Origin for Chinese Honeys on the Basis of Mineral Elements Determined by ICP-MS. J. Agric. Food Chem. 2014, 62, 2443–2448. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, H.; Fan, C.; Gao, S.; Zhang, Z.; Bo, L. Classification of the Botanical and Geographical Origins of Chinese Honey Based on 1H NMR Profile with Chemometrics. Food Res. Int. 2020, 137, 109714. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Song, H.; Guo, L.; Guang, P.; Yang, X.; Li, L.; Zhao, H.; Yang, M. Detection of Adulteration in Chinese Honey Using NIR and ATR-FTIR Spectral Data Fusion. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 235, 118297. [Google Scholar] [CrossRef] [PubMed]
- Tahir, H.E.; Xiaobo, Z.; Tinting, S.; Jiyong, S.; Mariod, A.A. Near-Infrared (NIR) Spectroscopy for Rapid Measurement of Antioxidant Properties and Discrimination of Sudanese Honeys from Different Botanical Origin. Food Anal. Methods 2016, 9, 2631–2641. [Google Scholar] [CrossRef]
- Antônio, D.C.; de Assis, D.C.S.; Botelho, B.G.; Sena, M.M. Detection of Adulterations in a Valuable Brazilian Honey by Using Spectrofluorimetry and Multiway Classification. Food Chem. 2022, 370, 131064. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Li, J.; Liu, X.; Yuan, J.; Yuan, W.; Tian, Y.; Xuan, H. Authentication of Acacia Honey Using Fluorescence Spectroscopy. Food Control 2021, 130, 108327. [Google Scholar] [CrossRef]
- Lippolis, V.; De Angelis, E.; Fiorino, G.M.; Di Gioia, A.; Arlorio, M.; Logrieco, A.F.; Monaci, L. Geographical Origin Discrimination of Monofloral Honeys by Direct Analysis in Real Time Ionization-High Resolution Mass Spectrometry (DART-HRMS). Foods 2020, 9, 1205. [Google Scholar] [CrossRef]
- Smith, D.G.; Topolnicki, I.L.; Zwicker, V.E.; Jolliffe, K.A.; New, E.J. Fluorescent Sensing Arrays for Cations and Anions. Analyst 2017, 142, 3549–3563. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, J.; Han, L.; Wang, X.; Li, W.; Guo, H.; Wei, H. Nanozyme Sensor Arrays Based on Heteroatom-Doped Graphene for Detecting Pesticides. Anal. Chem. 2020, 92, 7444–7452. [Google Scholar] [CrossRef]
- Lin, D.; Li, L.; Song, X.; Xu, S.; Zhang, Q.; Hu, Z.; Yang, L.; Jiang, C. “Light Up” Fluorescence Visual Sensitive Detection of Organophosphorus with a Smartphone-Based Platform Utilizing a Composite Rhodamine B-Ag@Au Nanoprobe. ACS Sustain. Chem. Eng. 2021, 9, 14579–14587. [Google Scholar] [CrossRef]
- Chen, F.; Qin, M.; Liu, W.; Wang, F.; Ren, W.; Xu, H.; Li, F. Snake Venom Identification via Fluorescent Discrimination. Anal. Chem. 2021, 93, 14025–14030. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, L.; Shen, C.; Timmins, H.C.; Park, S.B.; New, E.J. A Versatile Fluorescent Sensor Array for Platinum Anticancer Drug Detection in Biological Fluids. ACS Sens. 2021, 6, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Ma, C.; Wang, B.; Bender, M.; Bojanowski, M.; Hergert, M.; Seehafer, K.; Herrmann, A.; Bunz, U.H.F. A Hypothesis-Free Sensor Array Discriminates Whiskies for Brand, Age, and Taste. Chem 2017, 2, 817–824. [Google Scholar] [CrossRef] [Green Version]
- Clear, K.J.; Stroud, S.; Smith, B.D. Dual Colorimetric and Luminescent Assay for Dipicolinate, a Biomarker of Bacterial Spores. Analyst 2013, 138, 7079–7082. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, M.D.; Oktem, H.A. Eriochrome Black T-Eu3+ Complex as a Ratiometric Colorimetric and Fluorescent Probe for the Detection of Dipicolinic Acid, a Biomarker of Bacterial Spores. Anal. Chem. 2018, 90, 4221–4225. [Google Scholar] [CrossRef]
- Gao, N.; Zhang, Y.; Huang, P.; Xiang, Z.; Wu, F.-Y.; Mao, L. Perturbing Tandem Energy Transfer in Luminescent Heterobinuclear Lanthanide Coordination Polymer Nanoparticles Enables Real-Time Monitoring of Release of the Anthrax Biomarker from Bacterial Spores. Anal. Chem. 2018, 90, 7004–7011. [Google Scholar] [CrossRef]
- Ali, H.; Khan, S.; Ullah, R.; Khan, B. Fluorescence Fingerprints of Sidr Honey in Comparison with Uni/Polyfloral Honey Samples. Eur. Food Res. Technol. 2020, 246, 1829–1837. [Google Scholar] [CrossRef]
- Suto, M.; Kawashima, H.; Nakamura, Y. Determination of Organic Acids in Honey by Liquid Chromatography with Tandem Mass Spectrometry. Food Anal. Methods 2020, 13, 2249–2257. [Google Scholar] [CrossRef]
- Mato, I.; Huidobro, J.F.; Simal-Lozano, J.; Sancho, M.T. Rapid Determination of Nonaromatic Organic Acids in Honey by Capillary Zone Electrophoresis with Direct Ultraviolet Detection. J. Agric. Food Chem. 2006, 54, 1541–1550. [Google Scholar] [CrossRef]
- Gheldof, N.; Wang, X.-H.; Engeseth, N.J. Identification and Quantification of Antioxidant Components of Honeys from Various Floral Sources. J. Agric. Food Chem. 2002, 50, 5870–5877. [Google Scholar] [CrossRef] [PubMed]
- Wabaidur, S.M.; Ahmed, Y.B.H.; Alothman, Z.A.; Obbed, M.S.; AL-Harbi, N.M.; AL-Turki, T.M. Ultra High Performance Liquid Chromatography with Mass Spectrometry Method for the Simultaneous Determination of Phenolic Constituents in Honey from Various Floral Sources Using Multiwalled Carbon Nanotubes as Extraction Sorbents: Liquid Chromatography. J. Sep. Sci. 2015, 38, 2597–2606. [Google Scholar] [CrossRef] [PubMed]
- Truzzi, C.; Annibaldi, A.; Illuminati, S.; Finale, C.; Scarponi, G. Determination of Proline in Honey: Comparison between Official Methods, Optimization and Validation of the Analytical Methodology. Food Chem. 2014, 150, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Pita-Calvo, C.; Guerra-Rodríguez, M.E.; Vázquez, M. Analytical Methods Used in the Quality Control of Honey. J. Agric. Food Chem. 2017, 65, 690–703. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, X.; Tao, Y.; Chen, J.; Du, C.; Jin, Q.; He, Y.; Yang, J.; Huang, S.; Chen, W. Dipicolinic Acid-Tb3+/Eu3+ Lanthanide Fluorescence Sensor Array for Rapid and Visual Discrimination of Botanical Origin of Honey. Foods 2022, 11, 3388. https://doi.org/10.3390/foods11213388
Tu X, Tao Y, Chen J, Du C, Jin Q, He Y, Yang J, Huang S, Chen W. Dipicolinic Acid-Tb3+/Eu3+ Lanthanide Fluorescence Sensor Array for Rapid and Visual Discrimination of Botanical Origin of Honey. Foods. 2022; 11(21):3388. https://doi.org/10.3390/foods11213388
Chicago/Turabian StyleTu, Xijuan, Yunmin Tao, Jiaxu Chen, Chunping Du, Qian Jin, Yuchang He, Ji Yang, Shaokang Huang, and Wenbin Chen. 2022. "Dipicolinic Acid-Tb3+/Eu3+ Lanthanide Fluorescence Sensor Array for Rapid and Visual Discrimination of Botanical Origin of Honey" Foods 11, no. 21: 3388. https://doi.org/10.3390/foods11213388