Effects of Boiling Processing on Texture of Scallop Adductor Muscle and Its Mechanism
Abstract
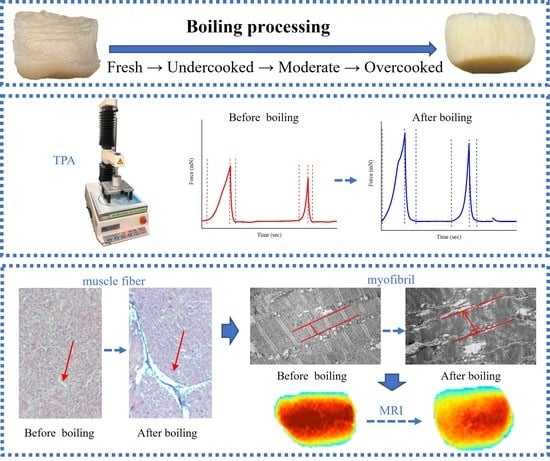
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Boiling Processing
2.3. Thermal Transition Measurement
2.4. Shear Force and Texture Profile Analysis
2.5. Sensory Evaluation
2.6. MP Extraction Rate
2.7. Determination of Total Soluble Matter
2.8. Water-Soluble Protein Content
2.9. Determination of Amino Acid of Water-Soluble Fraction
2.10. Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis
2.11. Water-Soluble Hydroxyproline Content
2.12. Fluorescence Microscopy
2.13. Light Microscopy
2.14. Transmission Electron Microscopy
2.15. Water-Phase Distribution
2.16. Statistical Analysis
3. Results and Discussion
3.1. Determination of Boiling Conditions
3.2. Changes in Proteins of Scallop Adductor Muscles
3.2.1. Changes in Water-Soluble Proteins
3.2.2. Changes in Myofibrillar Proteins
3.2.3. Changes in Collagen
3.3. Changes in the Protein Structure
3.4. Changes in Microstructure of Scallop Adductor Muscles
3.5. Changes in Water-Phase Distribution
3.6. Changes in Texture Properties and Their Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization. FIGIS List of Species for Fishery Global Production Statistics. Available online: http://www.fao.org/fishery/statistics/en (accessed on 22 February 2022).
- Odeleye, T.; White, W.L.; Lu, J. Extraction techniques and potential health benefits of bioactive compounds from marine molluscs: A review. Food Funct. 2019, 10, 2278–2289. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, A.; Heia, K.; Lindberg, S.K.; Nilsen, H. Spectroscopic Techniques for Monitoring Thermal Treatments in Fish and Other Seafood: A Review of Recent Developments and Applications. Foods 2020, 9, 767. [Google Scholar] [CrossRef] [PubMed]
- Mizuta, S.; Isobe, S.; Yoshinaka, R. Existence of two molecular species of collagen in the muscle layer of the ascidian (Halocynthia roretzi). Food Chem. 2002, 79, 9–13. [Google Scholar] [CrossRef]
- He, S.; Sun, X.; Du, M.; Chen, H.; Tan, M.; Sun, H.; Zhu, B. Effects of muscle protein denaturation and water distribution on the quality of false abalone (Volutharpa ampullacea perryi) during wet heating. J. Food Process Eng. 2019, 42, e12932. [Google Scholar] [CrossRef] [Green Version]
- Vilgis, T.A. Soft matter food physics--the physics of food and cooking. Rep. Prog. Phys. 2015, 78, 124602. [Google Scholar] [CrossRef]
- Wright, D.J.; Leach, I.B.; Wilding, P. Differential Scanning Calorimetric Studies of Muscle and Its Constituent Proteins. J. Sci. Food Agric. 1977, 28, 557–564. [Google Scholar] [CrossRef]
- Abe, T.; Miyashita, K. Heat treatment of scallop adductor muscle using superheated steam. J. Food Sci. 2007, 72, E345–E350. [Google Scholar] [CrossRef]
- Dong, X.F.; Fu, H.; Chang, S.J.; Zhang, X.Y.; Sun, H.; He, B.Y.; Jiang, D.; Yu, C.X.; Qi, H. Textural and biochemical changes of scallop Patinopecten yessoensis adductor muscle during low-temperature long-time (LTLT) processing. Int. J. Food Prop. 2018, 20, S2495–S2507. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Kanno, M.; Kijima, A. Genetic diversity of wild populations of Chlamys farreri in Japan and their genetic relationship with cultured stocks in China. Aquaculture 2012, 370, 109–122. [Google Scholar] [CrossRef]
- Liu, Y.X.; Zhou, D.Y.; Ma, D.D.; Liu, Z.Q.; Liu, Y.F.; Song, L.; Dong, X.P.; Li, D.M.; Zhu, B.W.; Konno, K.; et al. Effects of endogenous cysteine proteinases on structures of collagen fibres from dermis of sea cucumber (Stichopus japonicus). Food Chem. 2017, 232, 10–18. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Z.Q.; Li, D.Y.; Yu, M.M.; Liu, Y.X.; Qin, L.; Zhou, D.Y.; Shahidi, F.; Zhu, B.W. Action of endogenous proteases on texture deterioration of the bay scallop (Argopecten irradians) adductor muscle during cold storage and its mechanism. Food Chem. 2020, 323, 126790. [Google Scholar] [CrossRef] [PubMed]
- Kulawik, P.; Migdal, W.; Tkaczewska, J.; Oezogul, F. Assessment of Color and Sensory Evaluation of Frozen Fillets from Pangasius Catfish and Nile Tilapia Imported to European Countries. Int. J. Food Prop. 2016, 19, 1439–1446. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Takahashi, K.; Kurose, K.; Okazaki, E.; Osako, K. Effect of various protease inhibitors on heat-induced myofibrillar protein degradation and gel-forming ability of red tilefish (Branchiostegus japonicus) meat. LWT Food Sci. Technol. 2016, 68, 717–723. [Google Scholar] [CrossRef]
- Yang, J.F.; Gao, R.C.; Wu, H.T.; Li, P.F.; Hu, X.S.; Zhou, D.Y.; Zhu, B.W.; Su, Y.C. Analysis of Apoptosis in Ultraviolet-Induced Sea Cucumber (Stichopus japonicus) Melting Using Terminal Deoxynucleotidyl-Transferase-Mediated dUTP Nick End-Labeling Assay and Cleaved Caspase-3 Immunohistochemistry. J. Agric. Food Chem. 2015, 63, 9601–9608. [Google Scholar] [CrossRef]
- Santé-Lhoutellier, V.; Astruc, T.; Marinova, P.; Greve, E.; Gatellier, P. Effect of Meat Cooking on Physicochemical State and in Vitro Digestibility of Myofibrillar Proteins. J. Agric. Food Chem. 2008, 56, 1488–1494. [Google Scholar] [CrossRef]
- Liu, Y.X.; Liu, Z.Q.; Song, L.; Ma, Q.R.; Zhou, D.Y.; Zhu, B.W.; Shahidi, F. Effects of collagenase type I on the structural features of collagen fibres from sea cucumber (Stichopus japonicus) body wall. Food Chem. 2019, 301, 125302. [Google Scholar] [CrossRef]
- Scab, C.; Wab, C.X.; Rlab, C.; Hyab, C.; Hwab, C.; Hwab, C.; Mtab, C. Influence of multiple freeze-thaw cycles on quality characteristics of beef semimembranous muscle: With emphasis on water status and distribution by LF-NMR and MRI. Meat Sci. 2019, 147, 44–52. [Google Scholar]
- Paredi, M.E.; Tomas, M.C.; Crupkin, M. Thermal denaturation of myofibrillar proteins of striated and smooth adductor muscles of scallop (Zygochlamys patagonica). A differential scanning calorimetric study. J. Agric. Food Chem. 2002, 50, 830–834. [Google Scholar] [CrossRef]
- Ovissipour, M.; Rasco, B.; Tang, J.; Sablani, S. Kinetics of Protein Degradation and Physical Changes in Thermally Processed Atlantic Salmon (Salmo salar). Food Bioprocess Technol. 2017, 10, 1865–1882. [Google Scholar] [CrossRef]
- Wang, W.; Xia, W.; Gao, P.; Xu, Y.; Jiang, Q. Proteolysis during fermentation of Suanyu as a traditional fermented fish product of China. Int. J. Food Prop. 2017, 20, S166–S176. [Google Scholar] [CrossRef] [Green Version]
- Wattanachant, S.; Benjakul, S.; Ledward, D.A. Effect of heat treatment on changes in texture, structure and properties of Thai indigenous chicken muscle. Food Chem. 2005, 93, 337–348. [Google Scholar] [CrossRef]
- Gauza-Wodarczyk, M.; Kubisz, L.; Wodarczyk, D. Amino acid composition in determination of collagen origin and assessment of physical factors effects—ScienceDirect. Int. J. Biol. Macromol. 2017, 104, 987–991. [Google Scholar] [CrossRef]
- Demeule, B.; Gurny, R.; Arvinte, T. Detection and characterization of protein aggregates by fluorescence microscopy. Int. J. Pharm. 2007, 329, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Bhat, Z.F.; Morton, J.D.; Bekhit, A.E.D.A.; Kumar, S.; Bhat, H.F. Thermal processing implications on the digestibility of meat, fish and seafood proteins. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4511–4548. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.H.; Sun, D.W.; Han, Z.; Zeng, X.A. Texture and Structure Measurements and Analyses for Evaluation of Fish and Fillet Freshness Quality: A Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 52–61. [Google Scholar] [CrossRef]
- Boland, M.; Kaur, L.; Chian, F.M.; Astruc, T. Muscle Proteins. Encyclopedia of Food Chemistry; Melton, L., Shahidi, F., Varelis, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 164–179. [Google Scholar]
- Palka, K.; Daun, H. Changes in texture, cooking losses, and myofibrillar structure of bovine M. semitendinosus during heating. Meat Sci. 1999, 51, 237–243. [Google Scholar] [CrossRef]
- Nakamura, Y.N.; Tsuneishi, E.; Kamiya, M.; Yamada, A. Histological contribution of collagen architecture to beef toughness. J. Food Sci. 2010, 75, E73–E77. [Google Scholar] [CrossRef]
- Llave, Y.; Morinaga, K.; Fukuoka, M.; Sakai, N. Characterization of ohmic heating and sous-vide treatment of scallops: Analysis of electrical conductivity and the effect of thermal protein denaturation on quality attribute changes. Innov. Food Sci. Emerg. Technol. 2018, 50, 112–123. [Google Scholar] [CrossRef]
- Bao, Y.; Ertbjerg, P. Effects of protein oxidation on the texture and water-holding of meat: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 3564–3578. [Google Scholar] [CrossRef]
- Hughes, J.M.; Oiseth, S.K.; Purslow, P.P.; Warner, R.D. A structural approach to understanding the interactions between colour, water-holding capacity and tenderness. Meat Sci. 2014, 98, 520–532. [Google Scholar] [CrossRef]
- Moczkowska, M.; Półtorak, A.; Montowska, M.; Pospiech, E.; Wierzbicka, A. The effect of the packaging system and storage time on myofibrillar protein degradation and oxidation process in relation to beef tenderness. Meat Sci. 2017, 130, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Jongberg, S.; Wen, J.; Tørngren, M.A.; Lund, M.N. Effect of high-oxygen atmosphere packaging on oxidative stability and sensory quality of two chicken muscles during chill storage. Food Packag. Shelf Life 2014, 1, 38–48. [Google Scholar] [CrossRef]
- Purslow, P.P. Intramuscular connective tissue and its role in meat quality. Meat Sci. 2005, 70, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Tian, Y.; Yamashita, T.; Ishimura, G.; Sasaki, K.; Niu, Y.; Yuan, C. Effects of thawing methods on the biochemical properties and microstructure of pre-rigor frozen scallop striated adductor muscle. Food Chem. 2020, 319, 126559. [Google Scholar] [CrossRef]
- Vaskoska, R.; Vénien, A.; Ha, M.; White, J.D.; Unnithan, R.R.; Astruc, T.; Warner, R.D. Thermal denaturation of proteins in the muscle fibre and connective tissue from bovine muscles composed of type I (masseter) or type II (cutaneous trunci) fibres: DSC and FTIR microspectroscopy study. Food Chem. 2021, 343, 128544. [Google Scholar] [CrossRef]
- Huff-Lonergan, E.; Lonergan, S.M. Mechanisms of water-holding capacity of meat: The role of postmortem biochemical and structural changes. Meat Sci. 2005, 71, 194–204. [Google Scholar] [CrossRef]
- Sun, H.; Huang, F.; Ding, Z.; Zhang, C.; Zhang, L.; Zhang, H. Changes in tenderness and water-holding capacity and underlying mechanism during beef stewing. Shipin Kexue/Food Sci. 2018, 39, 84–90. [Google Scholar]
- Pearce, K.L.; Rosenvold, K.; Andersen, H.J.; Hopkins, D.L. Water distribution and mobility in meat during the conversion of muscle to meat and ageing and the impacts on fresh meat quality attributes—A review. Meat Sci. 2011, 89, 111–124. [Google Scholar] [CrossRef]
- Bertram, H.C.; Aaslyng, M.D.; Andersen, H.J. Elucidation of the relationship between cooking temperature, water distribution and sensory attributes of pork–a combined NMR and sensory study. Meat Sci. 2005, 70, 75–81. [Google Scholar] [CrossRef]
- Bertram, H.C.; Straadt, I.K.; Jensen, J.A.; Aaslyng, M.D. Relationship between water mobility and distribution and sensory attributes in pork slaughtered at an age between 90 and 180 days. Meat Sci. 2007, 77, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Suriaatmaja, D. Mechanism of Meat Tenderization by Long-Time Low-Temperature Heating. Master’s Thesis, North Carolina State Universit, Raleigh, NC, USA, 2013. [Google Scholar]
- Sikorski, Z.E.; Scott, D.N.; Buisson, D.H.; Love, R.M. The role of collagen in the quality and processing of fish. Crit. Rev. Food Sci. Nutr. 1984, 20, 301–343. [Google Scholar] [CrossRef] [PubMed]
- Ertbjerg, P.; Puolanne, E. Muscle structure, sarcomere length and influences on meat quality: A review. Meat Sci. 2017, 132, 139–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.-X.; Fan, Y.-C.; Guo, C.; Liu, Y.-X.; Li, D.-Y.; Jiang, P.-F.; Qin, L.; Bai, Y.-H.; Zhou, D.-Y. Effects of Boiling Processing on Texture of Scallop Adductor Muscle and Its Mechanism. Foods 2022, 11, 1947. https://doi.org/10.3390/foods11131947
Wu Z-X, Fan Y-C, Guo C, Liu Y-X, Li D-Y, Jiang P-F, Qin L, Bai Y-H, Zhou D-Y. Effects of Boiling Processing on Texture of Scallop Adductor Muscle and Its Mechanism. Foods. 2022; 11(13):1947. https://doi.org/10.3390/foods11131947
Chicago/Turabian StyleWu, Zi-Xuan, Ying-Chen Fan, Chao Guo, Yu-Xin Liu, De-Yang Li, Peng-Fei Jiang, Lei Qin, Yan-Hong Bai, and Da-Yong Zhou. 2022. "Effects of Boiling Processing on Texture of Scallop Adductor Muscle and Its Mechanism" Foods 11, no. 13: 1947. https://doi.org/10.3390/foods11131947