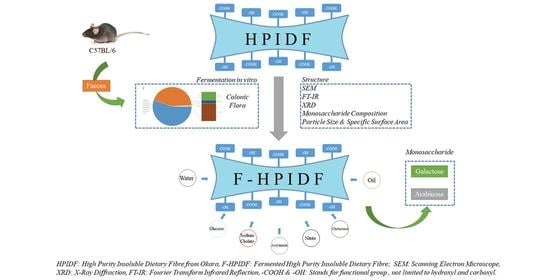
Changes of High-Purity Insoluble Fiber from Soybean Dregs (Okara) after Being Fermented by Colonic Flora and Its Adsorption Capacity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Okara-HPIDF
2.2. The Fermentation of Okara-HPIDF
2.3. Bacterial 16S rDNA Sequencing
2.4. Simulated Fermentation of Okara-HPIDF In Vitro
2.5. Structure of Okara-HPIDF before/after Fermentation
2.5.1. Scanning Electron Microscopy (SEM)
2.5.2. Fourier Infrared Spectrum (FT-IR)
2.5.3. X-ray Diffraction (XRD)
2.5.4. Particle Size and Specific Surface Area
2.6. Monosaccharide Composition of Okara-HPIDF before/after Fermentation and the Hydrolyzed
2.7. Adsorption Capacity in the Colon of Okara-HPIDF before/after Fermentation
2.7.1. Basic Characteristics of HPIDF/F-HPIDF
2.7.2. Heavy Metals-Adsorption Capacity (HMAC)
2.7.3. Potentially Harmful Substances-Adsorption Capacity
- GAC 0.1 g HPIDF/F-HPIDF, mixed with 10 mL 100 mmol/L glucose solution at 37 ℃ for 16 h, centrifuged at 4000 rpm for 20 min, and the supernatant was taken to determine the glucose concentration using a glucose kit (hexokinase method, A154-2-1, Nanjing Jiancheng Bioengineering Institute).
- CAC 1 mg/mL cholesterol in ethanol solution was prepared. About 0.2 g HPIDF/F-HPIDF was mixed in 10 mL cholesterol solution. The adsorption and centrifugation conditions were the same as the above. Total cholesterol assay kit (A111-1-1, Nanjing Jiancheng Bioengineering Institute) was used to determine the cholesterol content in the supernatant.
- SCAC 0.1 g HPIDF/F-HPIDF was mixed with 10 mL sodium cholate standard solution (0.2 g sodium cholate + 15 mmol/L NaCl aq 100 mL). The adsorption and centrifugation conditions were the same as 2.7.3-1. Furfural-sulfuric acid process [23] was used to determine the content of sodium cholate in the supernatant.
- AAC 0.5 g HPIDF/F-HPIDF was mixed with 50 mL 15 mmol/L acrylamide solution. The adsorption and centrifugation conditions were the same as 2.7.3-1. HPLC was used to determine the concentration of acrylamide in the supernatant according to the method described in GB 5009.204-2014 by 1260 HPLC (Agilent, Lexington, MA, USA).
- NAC 0.5 g HPIDF/F-HPIDF was mixed with 50 mL 100 mmol/L nitrite (Sodium nitrite) solution. The adsorption and centrifugation conditions were the same as the above. Ultraviolet spectrophotometry method (described in GB 5009.23-2010) was used to determine the concentration of sodium nitrite in the supernatant.
2.8. Statistical Analysis
3. Results and Discussion
3.1. The Gut Microbiota Structure in the Feces from C57BL/6 Mice
3.2. The Structural Changes of HPIDF after Being Fermented
3.2.1. Scanning Electron Micrograph (SEM)
3.2.2. X-ray Diffraction (XRD)
3.2.3. Fourier Transform Infrared Spectroscopy (FT-IR)
3.2.4. Particle Size and Specific Surface Area
3.3. The Monosaccharide Composition of HPIDF, F-HPIDF, and Hydrolysate
3.4. The Adsorption Capacity of HPIDF/F-HPIDF
3.4.1. WHC, WSC, and OHC of HPIDF/F-HPIDF
3.4.2. HMAC of HPIDF/F-HPIDF
3.4.3. Potentially Harmful Substances-Adsorption Capacity of HPIDF/F-HPIDF
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hua, M.; Lu, J.; Qu, D.; Liu, C.; Zhang, L.; Li, S.; Chen, J.; Sun, Y. Structure, physicochemical properties and adsorption function of insoluble dietary fiber from ginseng residue: A potential functional ingredient. Food Chem. 2019, 286, 522–529. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, R.; Dong, L.; Huang, F.; Tang, X.; Wei, Z.; Zhang, M. Particle size of insoluble dietary fiber from rice bran affects its phenolic profile, bioaccessibility and functional properties. LWT 2018, 87, 450–456. [Google Scholar] [CrossRef]
- Meng, X.; Liu, F.; Xiao, Y.; Cao, J.; Wang, M.; Duan, X. Alterations in physicochemical and functional properties of buckwheat straw insoluble dietary fiber by alkaline hydrogen peroxide treatment. Food Chem. X 2019, 3, 100029. [Google Scholar] [CrossRef]
- Hino, S.; Takemura, N.; Sonoyama, K.; Morita, A.; Kawagishi, H.; Aoe, S.; Morita, T. Small intestinal goblet cell proliferation induced by ingestion of soluble and insoluble dietary fiber is characterized by an increase in sialylated mucins in rats. J. Nutr. 2012, 142, 1429–1436. [Google Scholar] [CrossRef]
- McIntosh, G.; Jorgensen, L.; Royle, P. The potential of an insoluble dietary fiber-rich source from barley to protect from DMH-induced intestinal tumors in rats. Nutr. Cancer 1993, 19, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Chen, D.; Tian, G.; Zheng, P.; Mao, X.; Yu, J.; He, J.; Huang, Z.; Luo, Y.; Luo, J. Effects of soluble and insoluble dietary fiber supplementation on growth performance, nutrient digestibility, intestinal microbe and barrier function in weaning piglet. Anim. Feed. Sci. Technol. 2020, 260, 114335. [Google Scholar] [CrossRef]
- Wilberts, B.L.; Arruda, P.H.; Kinyon, J.M.; Frana, T.S.; Wang, C.; Magstadt, D.R.; Madson, D.M.; Patience, J.F.; Burrough, E.R. Investigation of the impact of increased dietary insoluble fiber through the feeding of distillers dried grains with solubles (DDGS) on the incidence and severity of Brachyspira-associated colitis in pigs. PLoS ONE 2014, 9, e114741. [Google Scholar] [CrossRef] [PubMed]
- Miles, J.P.; Zou, J.; Kumar, M.-V.; Pellizzon, M.; Ulman, E.; Ricci, M.; Gewirtz, A.T.; Chassaing, B. Supplementation of low-and high-fat diets with fermentable fiber exacerbates severity of DSS-induced acute colitis. Inflamm. Bowel Dis. 2017, 23, 1133–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, V.; San Yeoh, B.; Walker, R.E.; Xiao, X.; Saha, P.; Golonka, R.M.; Cai, J.; Bretin, A.C.A.; Cheng, X.; Liu, Q. Microbiota fermentation-NLRP3 axis shapes the impact of dietary fibres on intestinal inflammation. Gut 2019, 68, 1801–1812. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, H.; Mander, I.; Zhang, Z.; Armstrong, D.; Wine, E. Not All Fibers Are Born Equal; Variable Response to Dietary Fiber Subtypes in IBD. Front. Pediatr. 2021, 8, 924. [Google Scholar] [CrossRef]
- Vong, W.C.; Liu, S.-Q. Biovalorisation of okara (soybean residue) for food and nutrition. Trends Food Sci. Technol. 2016, 52, 139–147. [Google Scholar] [CrossRef]
- Lu, F.; Liu, Y.; Li, B. Okara dietary fiber and hypoglycemic effect of okara foods. Bioact. Carbohydr. Diet. Fibre 2013, 2, 126–132. [Google Scholar] [CrossRef]
- Jiménez-Escrig, A.; Tenorio, M.D.; Espinosa-Martos, I.; Rupérez, P. Health-promoting effects of a dietary fiber concentrate from the soybean byproduct okara in rats. J. Agric. Food Chem. 2008, 56, 7495–7501. [Google Scholar] [CrossRef]
- Fan, X.; Chang, H.; Lin, Y.; Zhao, X.; Zhang, A.; Li, S.; Feng, Z.; Chen, X. Effects of ultrasound-assisted enzyme hydrolysis on the microstructure and physicochemical properties of okara fibers. Ultrason. Sonochem. 2020, 69, 105247. [Google Scholar] [CrossRef] [PubMed]
- Lazarin, R.A.; Falcão, H.G.; Ida, E.I.; Berteli, M.N.; Kurozawa, L.E. Rotating-Pulsed Fluidized Bed Drying of Okara: Evaluation of Process Kinetic and Nutritive Properties of Dried Product. Food Bioprocess Technol. 2020, 13, 1611–1620. [Google Scholar] [CrossRef]
- Bo, L.; Huan, W.; Swallah, M.S.; Hongling, F.; Yue, S.; Zengwang, G.; Xiaohong, T.; Yang, L.; Hansong, Y.; Lianzhou, J. Structure, Properties and Potential Bioactivities of High-purity Insoluble Fibre from Soybean Dregs (Okara). Food Chem. 2021, 364, 130402. [Google Scholar]
- Wang, S.; Sun, W.; Swallah, M.S.; Amin, K.; Lyu, B.; Fan, H.; Zhang, Z.; Yu, H. Preparation and characterization of soybean insoluble dietary fiber and its prebiotic effect on dyslipidemia and hepatic steatosis in high fat-fed C57BL/6J mice. Food Funct. 2021, 12, 8760–8773. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yu, H.; He, Y.; Wen, L.; Gu, J.; Wang, X.; Miao, X.; Qiu, G.; Wang, H. Effect of soybean insoluble dietary fiber on prevention of obesity in high-fat diet fed mice via regulation of the gut microbiota. Food Funct. 2021, 12, 7923–7937. [Google Scholar] [CrossRef]
- Gaddum, J.H. Hawley’s Condensed Chemical Dictionary; United States Pharmacopeia: Rockville, MD, USA, 2007. [Google Scholar]
- Wu, C.; Teng, F.; McClements, D.J.; Zhang, S.; Li, Y.; Wang, Z. Effect of cavitation jet processing on the physicochemical properties and structural characteristics of okara dietary fiber. Food Res. Int. 2020, 134, 109251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yu, L.; Bi, H.; Li, X.; Ni, W.; Han, H.; Li, N.; Wang, B.; Zhou, Y.; Tai, G. Total fractionation and characterization of the water-soluble polysaccharides isolated from Panax ginseng CA Meyer. Carbohydr. Polym. 2009, 77, 544–552. [Google Scholar] [CrossRef]
- Zhang, W.; Zeng, G.; Pan, Y.; Chen, W.; Huang, W.; Chen, H.; Li, Y. Properties of soluble dietary fiber-polysaccharide from papaya peel obtained through alkaline or ultrasound-assisted alkaline extraction. Carbohydr. Polym. 2017, 172, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Wang, Y.; Wu, J.; Wang, Z. Functional properties and morphological characters of soluble dietary fibers in different edible parts of Angelica keiskei. J. Food Sci. 2016, 81, C2189–C2198. [Google Scholar] [CrossRef] [PubMed]
- Lkhagva, E.; Chung, H.-J.; Hong, J.; Tang, W.H.W.; Lee, S.-I.; Hong, S.-T.; Lee, S. The regional diversity of gut microbiome along the GI tract of male C57BL/6 mice. BMC Microbiol. 2021, 21, 44. [Google Scholar] [CrossRef] [PubMed]
- Mohtar, S.S.; Busu, T.N.Z.T.M.; Noor, A.M.M.; Shaari, N.; Mat, H. An ionic liquid treatment and fractionation of cellulose, hemicellulose and lignin from oil palm empty fruit bunch. Carbohydr. Polym. 2017, 166, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Alba, K.; Macnaughtan, W.; Laws, A.; Foster, T.J.; Campbell, G.; Kontogiorgos, V. Fractionation and characterisation of dietary fibre from blackcurrant pomace. Food Hydrocoll. 2018, 81, 398–408. [Google Scholar] [CrossRef] [Green Version]
- Ullah, I.; Yin, T.; Xiong, S.; Zhang, J.; Din, Z.-u.; Zhang, M. Structural characteristics and physicochemical properties of okara (soybean residue) insoluble dietary fiber modified by high-energy wet media milling. LWT-Food Sci. Technol. 2017, 82, 15–22. [Google Scholar] [CrossRef]
- Sharma, V.; Smolin, J.; Nayak, J.; Ayala, J.E.; Scott, D.A.; Peterson, S.N.; Freeze, H.H. Mannose alters gut microbiome, prevents diet-induced obesity, and improves host metabolism. Cell Rep. 2018, 24, 3087–3098. [Google Scholar] [CrossRef] [Green Version]
- Mudgil, D.; Barak, S. Composition, properties and health benefits of indigestible carbohydrate polymers as dietary fiber: A review. Int. J. Biol. Macromol. 2013, 61, 1–6. [Google Scholar] [CrossRef]
- Reddy, N.R.; Palmer, J.K.; Pierson, M.D.; Bothast, R.J. Wheat straw hemicelluloses: Composition and fermentation by human colon Bacteroides. J. Agric. Food Chem. 1983, 31, 1308–1313. [Google Scholar] [CrossRef]
- Garde, A.; Jonsson, G.; Schmidt, A.S.; Ahring, B.K. Lactic acid production from wheat straw hemicellulose hydrolysate by Lactobacillus pentosus and Lactobacillus brevis. Bioresour. Technol. 2002, 81, 217–223. [Google Scholar] [CrossRef]
- Moldes, A.; Torrado, A.; Converti, A.; Dominguez, J. Complete bioconversion of hemicellulosic sugars from agricultural residues into lactic acid by Lactobacillus pentosus. Appl. Biochem. Biotechnol. 2006, 135, 219–227. [Google Scholar] [CrossRef]
- Duncan, S.H.; Barcenilla, A.; Stewart, C.S.; Pryde, S.E.; Flint, H.J. Acetate utilization and butyryl coenzyme A (CoA): Acetate-CoA transferase in butyrate-producing bacteria from the human large intestine. Appl. Environ. Microbiol. 2002, 68, 5186–5190. [Google Scholar] [CrossRef] [Green Version]
- Rafter, J.J. The role of lactic acid bacteria in colon cancer prevention. Scand. J. Gastroenterol. 1995, 30, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Rivenson, A.; Tomita, M.; Shimamura, S.; Ishibashi, N.; Reddy, B.S. Bifidobacterium longum, a lactic acid-producing intestinal bacterium inhibits colon cancer and modulates the intermediate biomarkers of colon carcinogenesis. Carcinogenesis 1997, 18, 833–841. [Google Scholar] [CrossRef]
- Lin, J.; Nafday, S.M.; Chauvin, S.N.; Magid, M.S.; Pabbatireddy, S.; Holzman, I.R.; Babyatsky, M.W. Variable effects of short chain fatty acids and lactic acid in inducing intestinal mucosal injury in newborn rats. J. Pediatric Gastroenterol. Nutr. 2002, 35, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Torres, D.P.; Gonçalves, M.d.P.F.; Teixeira, J.A.; Rodrigues, L.R. Galacto-oligosaccharides: Production, properties, applications, and significance as prebiotics. Compr. Rev. Food Sci. Food Saf. 2010, 9, 438–454. [Google Scholar] [CrossRef] [Green Version]
- Evans, R.C.; Fear, S.; Ashby, D.; Hackett, A.; Williams, E.; Van Der Vliet, M.; Dunstan, F.D.; Rhodes, J.M. Diet and colorectal cancer: An investigation of the lectin/galactose hypothesis. Gastroenterology 2002, 122, 1784–1792. [Google Scholar] [CrossRef]
- Li, Y.; Pan, H.; Liu, J.; Li, T.; Liu, S.; Shi, W.; Sun, C.; Fan, M.; Xue, L.; Wang, Y. l-Arabinose inhibits colitis by modulating gut microbiota in mice. J. Agric. Food Chem. 2019, 67, 13299–13306. [Google Scholar] [CrossRef]
- Luo, X.; Wang, Q.; Zheng, B.; Lin, L.; Chen, B.; Zheng, Y.; Xiao, J. Hydration properties and binding capacities of dietary fibers from bamboo shoot shell and its hypolipidemic effects in mice. Food Chem. Toxicol. 2017, 109, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Niu, M.; Zhang, B.; Zhao, S.; Xiong, S. Structural characteristics and functional properties of rice bran dietary fiber modified by enzymatic and enzyme-micronization treatments. LWT 2017, 75, 344–351. [Google Scholar] [CrossRef]
- Wang, S.; Ru, B.; Lin, H.; Sun, W. Pyrolysis behaviors of four O-acetyl-preserved hemicelluloses isolated from hardwoods and softwoods. Fuel 2015, 150, 243–251. [Google Scholar] [CrossRef]
- Kachenpukdee, N.; Santerre, C.R.; Ferruzzi, M.G.; Oonsivilai, R. Modified Dietary Fiber from Cassava Pulp and Assessment of Mercury Bioaccessibility and Intestinal Uptake Using an In Vitro Digestion/Caco-2 Model System. J. Food Sci. 2016, 81, T1854–T1863. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huo, X.; Lu, X.; Zeng, Z.; Faas, M.M.; Xu, X. Exposure to multiple heavy metals associate with aberrant immune homeostasis and inflammatory activation in preschool children. Chemosphere 2020, 257, 127257. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Ren, B.; Diao, Z.; Chen, Y.; Qiao, Q.; Liu, X. Physicochemical properties and intestinal protective effect of ultra-micro ground insoluble dietary fibre from carrot pomace. Food Funct. 2016, 7, 3902–3909. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, Q.; Wang, L.; Zha, S.; Zhang, L.; Zhao, B. Physicochemical and functional properties of dietary fiber from maca (Lepidium meyenii Walp.) liquor residue. Carbohydr. Polym. 2015, 132, 509–512. [Google Scholar] [CrossRef]
- Yao, S.-L.; Xu, Y.; Zhang, Y.-Y.; Lu, Y.-H. Black rice and anthocyanins induce inhibition of cholesterol absorption in vitro. Food Funct. 2013, 4, 1602–1608. [Google Scholar] [CrossRef]
- Morita, S.; Ikeda, Y.; Tsuji, T.; Terada, T. Molecular mechanisms for protection of hepatocytes against bile salt cytotoxicity. Chem. Pharm. Bull. 2019, 67, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Labbé, A.; Ganopolsky, J.G.; Martoni, C.J.; Prakash, S.; Jones, M.L. Bacterial bile metabolising gene abundance in Crohn’s, ulcerative colitis and type 2 diabetes metagenomes. PLoS ONE 2014, 9, e115175. [Google Scholar] [CrossRef]
- Tabernero, M.; Venema, K.; Maathuis, A.J.; Saura-Calixto, F.D. Metabolite production during in vitro colonic fermentation of dietary fiber: Analysis and comparison of two European diets. J. Agric. Food Chem. 2011, 59, 8968–8975. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.; Jiang, H.; Lin, Z.; Xu, S.; Xie, J.; Zhang, A. Preparation of acrylamide/acrylic acid cellulose hydrogels for the adsorption of heavy metal ions. Carbohydr. Polym. 2019, 224, 115022. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, E.; Ranucci, E.; Edlund, U.; Albertsson, A.C. Design of renewable poly (amidoamine)/hemicellulose hydrogels for heavy metal adsorption. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Kundu, D.; Mondal, S.K.; Banerjee, T. Development of β-cyclodextrin-cellulose/hemicellulose-based hydrogels for the removal of Cd (II) and Ni (II): Synthesis, kinetics, and adsorption aspects. J. Chem. Eng. Data 2019, 64, 2601–2617. [Google Scholar] [CrossRef]








| D10 (μm) | D25 (μm) | D50 (μm) | D75 (μm) | Specific Surface Area (m2/kg) | |
|---|---|---|---|---|---|
| HPIDF | 8.52 ± 0.08 a | 24.59 ± 0.58 a | 53.33 ± 1.45 a | 102.24 ± 5.69 a | 90.24 ± 1.27 a |
| F-HPIDF | 6.02 ± 0.05 b | 11.25 ± 0.12 b | 30.00 ± 0.39 b | 64.60 ± 1.48 b | 135.75 ± 1.36 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, B.; Wang, Y.; Zhang, X.; Chen, Y.; Fu, H.; Liu, T.; Hao, J.; Li, Y.; Yu, H.; Jiang, L. Changes of High-Purity Insoluble Fiber from Soybean Dregs (Okara) after Being Fermented by Colonic Flora and Its Adsorption Capacity. Foods 2021, 10, 2485. https://doi.org/10.3390/foods10102485
Lyu B, Wang Y, Zhang X, Chen Y, Fu H, Liu T, Hao J, Li Y, Yu H, Jiang L. Changes of High-Purity Insoluble Fiber from Soybean Dregs (Okara) after Being Fermented by Colonic Flora and Its Adsorption Capacity. Foods. 2021; 10(10):2485. https://doi.org/10.3390/foods10102485
Chicago/Turabian StyleLyu, Bo, Yi Wang, Xin Zhang, Yuxi Chen, Hongling Fu, Tong Liu, Jianyu Hao, Yang Li, Hansong Yu, and Lianzhou Jiang. 2021. "Changes of High-Purity Insoluble Fiber from Soybean Dregs (Okara) after Being Fermented by Colonic Flora and Its Adsorption Capacity" Foods 10, no. 10: 2485. https://doi.org/10.3390/foods10102485