Antihypertensive and Antioxidant Activity of Chia Protein Techno-Functional Extensive Hydrolysates
Abstract
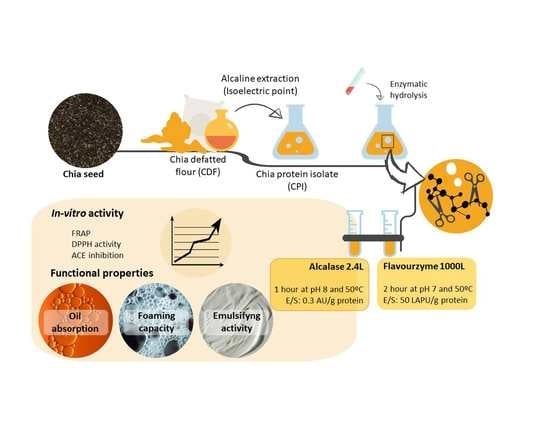
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Analysis of Amino Acid Composition by Ultra-High-Performance Liquid Chromatography (UHPLC)
2.2.2. Molecular Weights (MWs) by Fast Performance Liquid Chromatography (FPLC)
2.2.3. Determination of Isoelectric Point of Chia Protein
2.2.4. Generation of CPI
2.2.5. Generation of Chia Protein Hydrolysates
2.2.6. Determination of the Hydrolysis Degree (HD)
2.2.7. Determination of the Angiotensin-Converting Enzyme (ACE) Inhibitory Activity
2.2.8. Determination of Antioxidant Activity
Determination of the Antioxidant Activity by β-Carotene-Linoleic Acid Assay
Ferric Reducing Antioxidant Activity
DPPH Radical Scavenging Power of Protein Products
2.2.9. Functional Properties
Solubility
Oil Absorption
Foaming Capacity and Stability
Emulsifying Activity and Stability
2.2.10. Statistical Analysis
3. Results and Discussion
3.1. Generation of Chia Protein Isolate
3.2. Generation of Chia Protein Hydrolysates
3.3. ACE Inhibitory Activity of Chia Protein Hydrolysates
3.4. Antioxidant Activity of Chia Protein Hydrolysates
3.5. Chemical Characterization of Chia Protein Products
3.6. Amino Acid Composition of Chia Protein Products
3.7. Functional Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tichy, H.-V.; Bruhs, A.; Palisch, A. Development of Real-Time Polymerase Chain Reaction Systems for the Detection of So-Called “Superfoods” Chia and Quinoa in Commercial Food Products. J. Agric. Food Chem. 2020, 68, 14334–14342. [Google Scholar] [CrossRef]
- Miranda-Ramos, K.; Millan-Linares, M.C.; Haros, C.M. Effect of Chia as Breadmaking Ingredient on Nutritional Quality, Mineral Availability, and Glycemic Index of Bread. Foods 2020, 9, 663. [Google Scholar] [CrossRef]
- Melo, D.; Machado, T.B.; Oliveira, M.B.P.P. Chia seeds: An ancient grain trending in modern human diets. Food Funct. 2019, 10, 3068–3089. [Google Scholar] [CrossRef] [PubMed]
- Turck, D.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; et al. Safety of chia seeds (Salvia hispanica L.) as a novel food for extended uses pursuant to Regulation (EU) 2015/2283. EFSA J. 2019, 17, e05657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, L.; Xiang, H.; Sun-Waterhouse, D.; Cui, C.; Lin, J. Enhancing the Usability of Pea Protein Isolate in Food Applications through Modifying Its Structural and Sensory Properties via Deamidation by Glutaminase. J. Agric. Food Chem. 2020, 68, 1691–1697. [Google Scholar] [CrossRef]
- Rodriguez-Martin, N.M.; Toscano, R.; Villanueva, A.; Pedroche, J.; Millan, F.; Montserrat-de la Paz, S.; Millan-Linares, M.C. Neuroprotective protein hydrolysates from hemp (Cannabis sativa L.) seeds. Food Funct. 2019, 10, 6732–6739. [Google Scholar] [CrossRef]
- Segura-Campos, M.R. Isolation and functional caharacterization of chia (Salvia hispanica L.) proteins. Food Sci. Technol. 2020, 40, 334–339. [Google Scholar] [CrossRef] [Green Version]
- Yathusha, U.G.; Ishani, B.; Iddya, K.; Mamatha, B.S. Antihypertensive activity of fish protein hydrolysates and its peptides. Crit. Rev. Food Sci. Nutr. 2019, 15, 2363–2374. [Google Scholar] [CrossRef]
- Montone, C.M.; Zenezini-Chiozzi, R.; Marchetti, N.; Cerrato, A.; Antobelli, M.; Capriotti, A.L.; Cavaliere, C.; Piovesana, S.; Laganà, A. Peptidomic Approach for the Identifucation of Peptides with Potential Antioxidant and Anti-hypertensive Effects Derived From Asparagus by-Products. Molecules 2019, 24, 3627. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Martin, N.M.; Montserrat-de la Paz, S.; Toscano, R.; Grao-Cruces, E.; Villanueva, A.; Pedroche, J.; Millan, F.; Millan-Linares, M.C. Hemp (Cannabis sativa L.) Protein Hydrolysates Promote Anti-Inflammatory Response in Primary Human Monocytes. Biomolecules 2020, 10, 803. [Google Scholar] [CrossRef]
- Arihara, K.; Yokoyama, I.; Ohata, M. Bioactivities generated from meat proteins by enzimatic hydrolysis and the Maillard reaction. Meat Sci. 2021, 180, 108561. [Google Scholar] [CrossRef] [PubMed]
- Montserrat-de la Paz, S.; Rodriguez-Martin, N.M.; Villanueva, A.; Pedroche, J.; Cruz-Chamorro, I.; Millan, F.; Millan-Linares, M.C. Evaluation of Anti-Inflammatory and Atheroprotective Properties of Wheat Gluten Protein Hydrolysates in Primary Human Monocytes. Foods 2020, 9, 854. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Prosky, L.; De Vries, J.W. Determination of Total, Soluble, and Insoluble Dietary Fiber in Foods—Enzymatic-Gravimetric Method, MES-TRIS Buffer: Collaborative Study. J. AOAC Int. 1992, 75, 395–416. [Google Scholar] [CrossRef]
- Moores, R.G.; McDermott, D.L.; Wood, T.R. Determination of Chlorogenic Acid in Coffee. Anal. Chem. 1948, 20, 620–624. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Alaiz, M.; Navarro, J.L.; Girón, J.; Vioque, E. Amino acid analysis by high-performance liquid chromatography after derivatization with diethyl ethoxymethylenemalonate. J. Chromatogr. A 1992, 591, 181–186. [Google Scholar] [CrossRef]
- Yust, M.M.; Pedroche, J.; Girón-Calle, J.; Vioque, J.; Millán, F.; Alaiz, M. Determination of tryptophan by high-performance liquid chromatography of alkaline hydrolysates with spectrophotometric detection. Food Chem. 2004, 85, 317–320. [Google Scholar] [CrossRef]
- Salcedo-Chávez, B.; Osuna-Castro, J.A.; Guevara-Lara, F.; Domínguez-Domínguez, J.; Paredes-López, O. Optimization of the Isoelectric Precipitation Method To Obtain Protein Isolates from Amaranth (Amaranthus cruentus L.) Seeds. J. Agric. Food Chem. 2002, 50, 6515–6520. [Google Scholar] [CrossRef]
- Lqari, H.; Vioque, J.; Pedroche, J.; Millán, F. Lupinus angustifolius protein isolates: Chemical composition, functional properties and protein characterization. Food Chem. 2002, 76, 349–356. [Google Scholar] [CrossRef]
- Adler-Nissen, J. Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid. J. Agric. Food Chem. 1979, 27, 1256–1262. [Google Scholar] [CrossRef]
- Sentandreu, M.A.; Toldrá, F. A fluorescence-based protocol for quantifying angiotensin-converting enzyme activity. Nat. Protoc. 2006, 1, 2423–2427. [Google Scholar] [CrossRef]
- Marco, G.J. A rapid method for evaluation of antioxidants. J. Am. Oil Chem. Soc. 1968, 45, 594–598. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction. Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.-C.; Chen, H.-M.; Shiau, C.-Y. Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus L.). Food Res. Int. 2003, 36, 949–957. [Google Scholar] [CrossRef]
- Lin, M.J.Y.; Humbert, E.S.; Sosulski, F.W. Certain functional properties of sunflower meal products. J. Food Sci. 1974, 39, 368–370. [Google Scholar] [CrossRef]
- Fuhrmeister, H.; Meuser, F. Impact of processing on functional properties of protein products from wrinkled peas. J. Food Eng. 2003, 56, 119–129. [Google Scholar] [CrossRef]
- Bejosano, F.P.; Corke, H. Properties of protein concentrates and hydrolysates from Amaranthus and Buckwheat. Ind. Crops Prod. 1999, 10, 175–183. [Google Scholar] [CrossRef]
- Thrane, M.; Paulsen, P.V.; Orcutt, M.W.; Krieger, T.M. Soy protein: Impacts, production and, applications. In Sustainable Protein Sources; Nadathur, S.R., Wanasundaea, J.P.D., Scanlin, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Chapter 2; pp. 23–45. [Google Scholar] [CrossRef]
- Dakhili, S.; Abdolalizadeh, L.; Hosseini, S.M.; Shojaee-Aliabadi, S.; Mirmoghtadaie, L. Quinoa protein: Composition, structure and functional properties. Food Chem. 2019, 299, 125161. [Google Scholar] [CrossRef]
- Joint FAO/WHO Expert Committee on Food Additives Compendium of Food Additive Specifications FAO JECFA Monographs. 2006. Available online: http://fao.org/documents/card/es/c/df059734-d593-541e-bce0-c35cd4fcc541/ (accessed on 24 August 2021).
- General Standard for Food Additives. 1995. Available online: http://www.fao.org/food/food-safety-quality/scientific-advice/jecfa/jecfa-additives/en/ (accessed on 24 August 2021).
- Qamar, S.; Manrique, Y.J.; Parekh, H.; Falconer, J.R. Nuts, cereals, seeds and legumes proteins derived emulsifiers as a source of plant protein beverages: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 2742–2762. [Google Scholar] [CrossRef]
- Piñuel, L.; Boeri, P.; Zubillaga, F.; Barrio, D.A.; Torreta, J.; Cruz, A.; Vásquez, G.; Pinto, A.; Carrillo, W. Production of White, Red and Black Quinoa (Chenopodium quinoa Willd Var. Real) Protein Isolates and Its Hydrolysates in Germinated and Non-Germinated Quinoa Samples and Antioxidant Activity Evaluation. Plants 2019, 8, 257. [Google Scholar] [CrossRef] [Green Version]
- Chalamaiah, M.; Yu, W.; Wu, J. Immunomodulatory and anticancer protein hydrolysates (peptides) from food proteins: A review. Food Chem. 2018, 245, 205–222. [Google Scholar] [CrossRef]
- Daskaya-Dikmen, C.; Yucetepe, A.; Karbancioglu-Guler, F.; Daskaya, H.; Ozcelik, B. Angiotensin-I-Converting Enzyme (ACE)-Inhibitory Peptides from Plants. Nutrients 2017, 9, 316. [Google Scholar] [CrossRef] [PubMed]
- Segura-Campos, M.R.; Salazar-Vega, I.M.; Chel-Guerrero, L.A.; Betancur-Ancona, D.A. Biological Potential of Chia (Salvia Hispanica L.) Protein Hydrolysates and Their Incorporation into Functional Foods. LWT—Food Sci Tech. 2013, 50, 723–731. [Google Scholar] [CrossRef]
- Rudolph, S.; Lunow, D.; Kaiser, S.; Henle, T. Identification and quantification of ACE-inhibiting peptides in enzymatic hydrolysates of plant proteins. Food Chem. 2017, 224, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Bhagyawant, S.S. Impact of hydrolysis on functional properties, antioxidant, ACE-I inhibitory and antiproliferative activity of Cicer arietinum and Cicer reticulatum hydrolysates. Nutrire 2019, 44, 5. [Google Scholar] [CrossRef]
- Irondi, E.; Agboola, S.; Oboh, G.; Boligon, A. Inhibitory effect of leaves extracts of Ocimum basilicum and Ocimum gratissimum on two key enzymes involved in obesity and hypertension in vitro. J. Intercult. Ethnopharmacol. 2016, 5, 396. [Google Scholar] [CrossRef]
- Kheeree, N.; Sangtanoo, P.; Srimongkol, P.; Saisavoey, T.; Reamtong, O.; Choowongkomon, K.; Karnchanatat, A. ACE inhibitory peptides derived from de-fatted lemon basil seeds: Optimization, purification, identification, structure–activity relationship and molecular docking analysis. Food Funct. 2020, 11, 8161–8178. [Google Scholar] [CrossRef]
- Sung, W.C.; Chiu, E.T.; Sun, A.; Hsiao, H.I. Incorporation of chia seed flour into gluten-free rice layer cake: Effects on nutritional quality and physicochemical properties. J. Food Sci. 2020, 85, 545–555. [Google Scholar] [CrossRef]
- Cruz-Chamorro, I.; Álvarez-Sánchez, N.; Santos-Sánchez, G.; Pedroche, J.; Fernández-Pachón, M.-S.; Millán, F.; Millán-Linares, M.C.; Lardone, P.J.; Bejarano, I.; Guerrero, J.M.; et al. Immunomodulatory and Antioxidant Properties of Wheat Gluten Protein Hydrolysates in Human Peripheral Blood Mononuclear Cells. Nutrients 2020, 12, 1673. [Google Scholar] [CrossRef]
- Silveira Coehlo, M.; de Araujo Aquino, S.; Machado Latorres, J.; Salas-Mellado, M.M. In vitro and in vivo antioxidant capacity of chia protein hydrolysates and peptides. Food Hydrocoll. 2019, 91, 19–25. [Google Scholar] [CrossRef]
- Valdivia-López, M.Á.; Tecante, A. Chia (Salvia hispanica L.): A Review of native Mexican seed and its nutritional and functional properties. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2015; Volume 75, pp. 53–75. [Google Scholar] [CrossRef]
- FAO. Dietary protein quality evaluation in human nutrition. In FAO Food and Nutrition; Paper No. 92; FAO: Rome, Italy, 2017. [Google Scholar]
- Colovic, M.B.; Vasic, V.M.; Djuric, D.M.; Krstic, D.Z. Sulphur-containing Amino Acids: Protective Role against Free Radicals and Heavy Metals. Curr. Med. Chem. 2018, 25, 324–335. [Google Scholar] [CrossRef]
- Hong, G.-P.; Min, S.-G.; Jo, Y.-J. Anti-Oxidative and Anti-Aging Activities of Porcine By-Product Collagen Hydrolysates Produced by Commercial Proteases: Effect of Hydrolysis and Ultrafiltration. Molecules 2019, 24, 1104. [Google Scholar] [CrossRef] [Green Version]
- Nasri, M. Protein Hydrolysates and Biopeptides: Production, biological activities, and applications in foods and health benefits. A review. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2017; Volume 81, pp. 109–159. [Google Scholar] [CrossRef]
- Polanco-Lugo, E.; Dávila-Ortiz, G.; Betancur-Ancona, D.A.; Chel-Guerrero, L.A. Effects of sequential enzymatic hydrolysis on structural, bioactive and functional properties of Phaseolus lunatus protein isolate. Food Sci. Technol. 2014, 34, 441–448. [Google Scholar] [CrossRef] [Green Version]
- Yust, M.M.; Pedroche, J.; Millán-Linares, M.C.; Alcaide-Hidalgo, J.M.; Millán, F. Improvement of functional properties of chickpea proteins by hydrolysis with immobilised Alcalase. Food Chem. 2010, 122, 1212–1217. [Google Scholar] [CrossRef]
- Muñoz, L.A.; Cobos, A.; Diaz, O.; Aguilera, J.M. Chia seeds: Microstructure, mucilage extraction and hydration. J. Food Eng. 2012, 108, 216–224. [Google Scholar] [CrossRef]
- Liu, W.C.M.; Peng, Q.; Zhong, J.Z.; Liu, W.; Zhong, Y.J.; Wang, F. Molecular and Functional Properties of Protein Fractions and Isolate from Cashew Nut (Anacardium occidentale L.). Molecules 2018, 23, 393. [Google Scholar] [CrossRef] [Green Version]
- Ha, H.-K.; Lee, W.-J. Milk Protein-Stabilized Emulsion Delivery System and Its Application to Foods. J. Dairy Sci. Biotechnol. 2020, 38, 189–196. [Google Scholar] [CrossRef]
- Wouters, A.G.B.; Rombouts, I.; Fierens, E.; Brijs, K.; Delcour, J.A. Relevance of the Functional Properties of Enzymatic Plant Protein Hydrolysates in Food Systems. Compr. Rev. Food Sci. Food Saf. 2016, 15, 786–800. [Google Scholar] [CrossRef] [Green Version]
- Mirzanajafi-Zanjani, M.; Yousefi, M.; Ehsani, A. Challenges and approaches for production of a healthy and functional mayonnaise sauce. Food Sci. Nutr. 2019, 7, 2471–2484. [Google Scholar] [CrossRef]




| Hydrolysis Time (min) | CPH Flavourzyme | CPH Alcalase | |
|---|---|---|---|
| ACE Inhibition (%) | ACE Inhibition (%) | IC50 (µg/mL) | |
| 0 | 15.92 ± 0.27 | 12.33 ± 0.54 | |
| 15 | 42.19 ± 0.53 | 82.85 ± 0.42 | 78.84 ± 1.21 |
| 30 | 36.17 ± 0.83 | 80.13 ± 0.40 | 74.63 ± 0.53 |
| 45 | 40.50 ± 0.40 | 82.16 ± 0.25 | 67.50 ± 0.44 |
| 60 | 36.66 ± 0.62 | 82.84 ± 0.13 | 68.06 ± 0.67 |
| 75 | 28.79 ± 0.74 | ||
| 90 | 34.33 ± 0.84 | ||
| 105 | 30.36 ± 0.61 | ||
| 120 | 31.83 ± 2.04 | ||
| CDF | CPI | CPH15A | |
|---|---|---|---|
| Moisture | 7.86 ± 0.07 | 4.80 ± 0.30 | 7.32 ± 0.08 |
| Ash | 6.72 ± 0.05 | 0.13 ± 0.09 | 6.45 ± 0.17 |
| Proteins | 34.95 ± 0.43 | 82.85 ± 0.11 | 75.03 ± 0.45 |
| Fiber | 50.46 ± 1.98 | 11.01 ± 0.80 | 11.20 ± 0.70 |
| Amino Acid Protein | CDF | CPI | CPH 15A | 2007 FAO/WHO/UNU a,b |
|---|---|---|---|---|
| Histidine | 43.9 ± 1.6 | 37.9 ± 2.9 | 39.2 ± 0.5 | 15 |
| Isoleucine | 32.9 ± 0.8 | 35.2 ± 0.3 | 35.1 ± 0.0 | 30 |
| Leucine | 67.5 ± 0.7 | 70.4 ± 4.6 | 72.2 ± 0.7 | 59 |
| Lysine | 48.0 ± 1.1 | 46.4 ± 1.4 | 48.1 ± 0.6 | 45 |
| Methionine + cysteine | 44.9 ± 0.9 | 40.5 ± 1.9 | 40.3 ± 1.2 | 22 |
| Methionine | 24.4 ± 0.5 | 21.9 ± 1.8 | 21.1 ± 2.1 | 16 |
| Cysteine | 20.5 ± 1.3 | 18.6 ± 1.9 | 19.2 ± 0.4 | 6 |
| Phenylalanine + tyrosine | 147.3 ± 0.2 | 136.7 ± 1.9 | 135.5 ± 1.1 | 38 |
| Threonine | 37.2 ± 1.1 | 38.4 ± 2.1 | 38.0 ± 0.3 | 23 |
| Tryptophan | 47.3 ± 0.7 | 48.1 ± 0.6 | 49.3 ± 0.3 | 6 |
| Valine | 44.8 ± 0.7 | 46.9 ± 0.9 | 47.6 ± 0.8 | 39 |
| Total indispensable aminoacids | 513.3 | 500.5 | 505.3 | 277 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villanueva-Lazo, A.; Paz, S.M.-d.l.; Rodriguez-Martin, N.M.; Millan, F.; Carrera, C.; Pedroche, J.J.; Millan-Linares, M.d.C. Antihypertensive and Antioxidant Activity of Chia Protein Techno-Functional Extensive Hydrolysates. Foods 2021, 10, 2297. https://doi.org/10.3390/foods10102297
Villanueva-Lazo A, Paz SM-dl, Rodriguez-Martin NM, Millan F, Carrera C, Pedroche JJ, Millan-Linares MdC. Antihypertensive and Antioxidant Activity of Chia Protein Techno-Functional Extensive Hydrolysates. Foods. 2021; 10(10):2297. https://doi.org/10.3390/foods10102297
Chicago/Turabian StyleVillanueva-Lazo, Alvaro, Sergio Montserrat-de la Paz, Noelia Maria Rodriguez-Martin, Francisco Millan, Cecilio Carrera, Justo Javier Pedroche, and Maria del Carmen Millan-Linares. 2021. "Antihypertensive and Antioxidant Activity of Chia Protein Techno-Functional Extensive Hydrolysates" Foods 10, no. 10: 2297. https://doi.org/10.3390/foods10102297