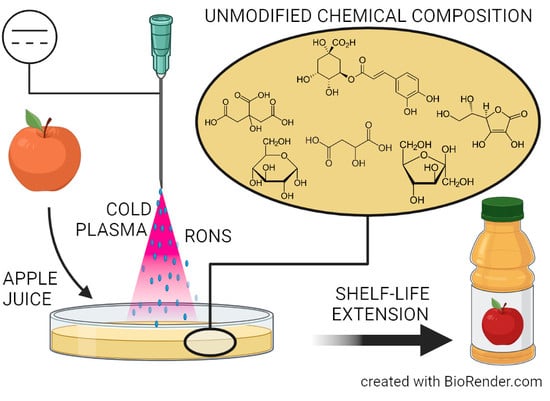
Chemical and Antimicrobial Effects of Air Non-Thermal Plasma Processing of Fresh Apple Juice with Focus on Safety Aspects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Set-Up of Air Transient Spark Discharge for Juice Treatment
2.2. Juice Preparation and Treatment Conditions
2.3. Detection of Reactive Species
2.4. pH, Conductivity, Transmittance, and °Brix Measurement
2.5. Quantification of the Organic Components of the Juice
2.6. Peroxidase Assay
2.7. Antimicrobial Effect of Transient Spark Treatment
2.8. Data Analysis
3. Results and Discussion
3.1. Production of Reactive Oxygen and Nitrogen Species in the Air-Plasma-Treated Apple Juice
3.2. Effect of the Air Cold Plasma on Selected Sensory Apple Juice Properties—pH, Conductivity, °Brix, and the Enzymatic Activity
3.3. Effect of Cold Air Plasma on the Organic Components of the Apple Juice
3.4. Inactivation Rate of Model Microorganisms in Fresh Apple Juice by Air Cold Plasma
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deak, T. Thermal Treatment. In Food Safety Management: A Practical Guide for the Food Industry; Motarjemi, Y., Lelieveld, H., Eds.; Academic Press: New York, NY, USA, 2014; pp. 423–442. ISBN 9780123815040. [Google Scholar]
- Novel Food Preservation and Microbial Assesment Techniques; Boziaris, I.S. (Ed.) CRC Press: Boca Raton, FL, USA; Taylor and Francis Group: Boca Raton, FL, USA, 2014; ISBN 978-1-4665-8076-3. [Google Scholar]
- Misra, N.N.; Keener, K.M.; Bourke, P.; Mosnier, J.P.; Cullen, P.J. In-package atmospheric pressure cold plasma treatment of cherry tomatoes. J. Biosci. Bioeng. 2014, 118, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Ziuzina, D.; Patil, S.; Cullen, P.J.; Keener, K.M.; Bourke, P. Atmospheric cold plasma inactivation of Escherichia coli, Salmonella enterica serovar Typhimurium and Listeria monocytogenes inoculated on fresh produce. Food Microbiol. 2014, 42, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Ramazzina, I.; Berardinelli, A.; Rizzi, F.; Tappi, S.; Ragni, L.; Sacchetti, G.; Rocculi, P. Effect of cold plasma treatment on physico-chemical parameters and antioxidant activity of minimally processed kiwifruit. Postharvest Biol. Technol. 2015, 107, 55–65. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Keener, K.M. Cold Plasma Processing of Fruit Juices. In Fruit Juices: Extraction, Composition, Quality and Analysis; Rajauria, G., Tiwari, B., Eds.; Academic Press: London, UK, 2018; pp. 529–537. ISBN 9780128024911. [Google Scholar]
- Ozen, E.; Singh, R.K. Atmospheric cold plasma treatment of fruit juices: A review. Trends Food Sci. Technol. 2020, 103, 144–151. [Google Scholar] [CrossRef]
- Hertwig, C.; Leslie, A.; Meneses, N.; Reineke, K.; Rauh, C.; Schlüter, O. Inactivation of Salmonella Enteritidis PT30 on the surface of unpeeled almonds by cold plasma. Innov. Food Sci. Emerg. Technol. 2017, 44, 242–248. [Google Scholar] [CrossRef]
- Sen, Y.; Onal-Ulusoy, B.; Mutlu, M. Aspergillus decontamination in hazelnuts: Evaluation of atmospheric and low-pressure plasma technology. Innov. Food Sci. Emerg. Technol. 2019, 54, 235–242. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, E.J.; Choi, E.H.; Kim, Y.J. Inactivation of Staphylococcus aureus on the beef jerky by radio-frequency atmospheric pressure plasma discharge treatment. Innov. Food Sci. Emerg. Technol. 2014, 22, 124–130. [Google Scholar] [CrossRef]
- Fröhling, A.; Durek, J.; Schnabel, U.; Ehlbeck, J.; Bolling, J.; Schlüter, O. Indirect plasma treatment of fresh pork: Decontamination efficiency and effects on quality attributes. Innov. Food Sci. Emerg. Technol. 2012, 16, 381–390. [Google Scholar] [CrossRef]
- Albertos, I.; Martín-Diana, A.B.; Cullen, P.J.; Tiwari, B.K.; Ojha, S.K.; Bourke, P.; Álvarez, C.; Rico, D. Effects of dielectric barrier discharge (DBD) generated plasma on microbial reduction and quality parameters of fresh mackerel (Scomber scombrus) fillets. Innov. Food Sci. Emerg. Technol. 2017, 44, 117–122. [Google Scholar] [CrossRef]
- Gurol, C.; Ekinci, F.Y.; Aslan, N.; Korachi, M. Low Temperature Plasma for decontamination of E. coli in milk. Int. J. Food Microbiol. 2012, 157, 1–5. [Google Scholar] [CrossRef]
- Yong, H.I.; Kim, H.J.; Park, S.; Alahakoon, A.U.; Kim, K.; Choe, W.; Jo, C. Evaluation of pathogen inactivation on sliced cheese induced by encapsulated atmospheric pressure dielectric barrier discharge plasma. Food Microbiol. 2015, 46, 46–50. [Google Scholar] [CrossRef]
- Yong, H.I.; Kim, H.J.; Park, S.; Kim, K.; Choe, W.; Yoo, S.J.; Jo, C. Pathogen inactivation and quality changes in sliced cheddar cheese treated using flexible thin-layer dielectric barrier discharge plasma. Food Res. Int. 2015, 69, 57–63. [Google Scholar] [CrossRef]
- Shi, X.M.; Zhang, G.J.; Wu, X.L.; Li, Y.X.; Ma, Y.; Shao, X.J. Effect of low-temperature plasma on microorganism inactivation and quality of freshly squeezed orange juice. IEEE Trans. Plasma Sci. 2011, 39, 1591–1597. [Google Scholar] [CrossRef]
- Xu, L.; Garner, A.L.; Tao, B.; Keener, K.M. Microbial Inactivation and Quality Changes in Orange Juice Treated by High Voltage Atmospheric Cold Plasma. Food Bioprocess Technol. 2017, 10, 1778–1791. [Google Scholar] [CrossRef]
- Xiang, Q.; Liu, X.; Li, J.; Liu, S.; Zhang, H.; Bai, Y. Effects of dielectric barrier discharge plasma on the inactivation of Zygosaccharomyces rouxii and quality of apple juice. Food Chem. 2018, 254, 201–207. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, T.; Yuan, Y.; Fan, Y.; Guo, K.; Yue, T. Inactivation of yeast in apple juice using gas-phase surface discharge plasma treatment with a spray reactor. LWT—Food Sci. Technol. 2018, 97, 530–536. [Google Scholar] [CrossRef]
- Ali, M.; Cheng, J.H.; Sun, D.W. Effects of dielectric barrier discharge cold plasma treatments on degradation of anilazine fungicide and quality of tomato (Lycopersicon esculentum Mill) juice. Int. J. Food Sci. Technol. 2021, 56, 69–75. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Zhu, X.; Yuan, Y.; Gao, Z.; Yue, T. Application of electrical discharge plasma on the inactivation of Zygosaccharomyces rouxii in apple juice. LWT—Food Sci. Technol. 2020, 121, 108974. [Google Scholar] [CrossRef]
- Elez Garofulić, I.; Režek Jambrak, A.; Milošević, S.; Dragović-Uzelac, V.; Zorić, Z.; Herceg, Z. The effect of gas phase plasma treatment on the anthocyanin and phenolic acid content of sour cherry Marasca (Prunus cerasus var. Marasca) juice. LWT—Food Sci. Technol. 2015, 62, 894–900. [Google Scholar] [CrossRef]
- Herceg, Z.; Kovačević, D.B.; Kljusurić, J.G.; Jambrak, A.R.; Zorić, Z.; Dragović-Uzelac, V. Gas phase plasma impact on phenolic compounds in pomegranate juice. Food Chem. 2016, 190, 665–672. [Google Scholar] [CrossRef]
- Dasan, B.G.; Boyaci, I.H. Effect of Cold Atmospheric Plasma on Inactivation of Escherichia coli and Physicochemical Properties of Apple, Orange, Tomato Juices, and Sour Cherry Nectar. Food Bioprocess Technol. 2018, 11, 334–343. [Google Scholar] [CrossRef]
- Surowsky, B.; Fröhling, A.; Gottschalk, N.; Schlüter, O.; Knorr, D. Impact of cold plasma on Citrobacter freundii in apple juice: Inactivation kinetics and mechanisms. Int. J. Food Microbiol. 2014, 174, 63–71. [Google Scholar] [CrossRef]
- Starek, A.; Sagan, A.; Andrejko, D.; Chudzik, B.; Kobus, Z.; Kwiatkowski, M.; Terebun, P.; Pawłat, J. Possibility to extend the shelf life of NFC tomato juice using cold atmospheric pressure plasma. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Dzimitrowicz, A.; Bielawska-Pohl, A.; Pohl, P.; Cyganowski, P.; Motyka-Pomagruk, A.; Klis, T.; Policht, M.; Klimczak, A.; Jamroz, P. Comprehensive studies on the properties of apple juice treated by non-thermal atmospheric plasma in a flow-through system. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Montenegro, J.; Ruan, R.; Ma, H.; Chen, P. Inactivation of E. coli O157: H7 using a pulsed nonthermal plasma system. J. Food Sci. 2002, 67, 646–648. [Google Scholar] [CrossRef]
- Kumar, S.; Estifaee, P.; Rogers, S.; Mededovic, S. Application of high voltage electrical discharge plasma for the inactivation of Escherichia coli ATCC 700891 in tangerine juice. LWT—Food Sci. Technol. 2018, 90, 180–185. [Google Scholar] [CrossRef]
- Vukušić Pavičić, T.; Šeremet, D.; Maltar- Strmečki, N.; Stulić, V.; Zorić, Z.; Herceg, Z. Impact of heat-assisted HVED plasma treatment on quality of apple juice. Croat. J. Food Technol. Biotechnol. Nutr. 2021, 15, 107–114. [Google Scholar] [CrossRef]
- Liao, X.; Li, J.; Idris Muhammad, A.; Suo, Y.; Chen, S.; Ye, X.; Liu, D.; Ding, T. Application of a Dielectric Barrier Discharge Atmospheric Cold Plasma (Dbd-Acp) for Escherichia Coli Inactivation in Apple Juice. J. Food Sci. 2018, 83, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Mahnot, N.K.; Mahanta, C.L.; Keener, K.M.; Misra, N.N. Strategy to achieve a 5-log Salmonella inactivation in tender coconut water using high voltage atmospheric cold plasma (HVACP). Food Chem. 2019, 284, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Chutia, H.; Mahanta, C.L.; Ojah, N.; Choudhury, A.J. Fuzzy logic approach for optimization of blended beverage of cold plasma treated TCW and orange juice. J. Food Meas. Charact. 2020, 14, 1926–1938. [Google Scholar] [CrossRef]
- Mahnot, N.K.; Mahanta, C.L.; Farkas, B.E.; Keener, K.M.; Misra, N.N. Atmospheric cold plasma inactivation of Escherichia coli and Listeria monocytogenes in tender coconut water: Inoculation and accelerated shelf-life studies. Food Control 2019, 106, 106678. [Google Scholar] [CrossRef]
- Pu, L.; Bi, Y.; Long, H.; Xue, H.; Lu, J.; Zong, Y.; Kankam, F. Glow Discharge Plasma Efficiently Degrades T-2 Toxin in Aqueous Solution and Patulin in Apple Juice. Adv. Tech. Biol. Med. 2017, 5, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Castro, D.R.G.; Mar, J.M.; da Silva, L.S.; da Silva, K.A.; Sanches, E.A.; de Araújo Bezerra, J.; Rodrigues, S.; Fernandes, F.A.N.; Campelo, P.H. Improvement of the Bioavailability of Amazonian Juices Rich in Bioactive Compounds Using Glow Plasma Technique. Food Bioprocess Technol. 2020, 13, 670–679. [Google Scholar] [CrossRef]
- Bursać Kovačević, D.; Putnik, P.; Dragović-Uzelac, V.; Pedisić, S.; Režek Jambrak, A.; Herceg, Z. Effects of cold atmospheric gas phase plasma on anthocyanins and color in pomegranate juice. Food Chem. 2016, 190, 317–323. [Google Scholar] [CrossRef]
- Alves Filho, E.G.; Almeida, F.D.L.; Cavalcante, R.S.; De Brito, E.S.; Cullen, P.J.; Frias, J.M.; Bourke, P.; Fernandes, F.A.N.; Rodrigues, S. 1 H NMR spectroscopy and chemometrics evaluation of non-thermal processing of orange juice. Food Chem. 2016, 204, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wang, R.; Gan, Z.; Shao, T.; Zhang, X.; He, M.; Sun, A. Effect of cold plasma on blueberry juice quality. Food Chem. 2019, 290, 79–86. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Yuan, Y.; Gao, Z.; Guo, K.; Yue, T. Application of gas phase surface discharge plasma with a spray reactor for Zygosaccharomyces rouxii LB inactivation in apple juice. Innov. Food Sci. Emerg. Technol. 2019, 52, 450–456. [Google Scholar] [CrossRef]
- Farias, T.R.B.; Rodrigues, S.; Fernandes, F.A.N. Effect of dielectric barrier discharge plasma excitation frequency on the enzymatic activity, antioxidant capacity and phenolic content of apple cubes and apple juice. Food Res. Int. 2020, 136, 109617. [Google Scholar] [CrossRef]
- Illera, A.E.; Chaple, S.; Sanz, M.T.; Ng, S.; Lu, P.; Jones, J.; Carey, E.; Bourke, P. Effect of cold plasma on polyphenol oxidase inactivation in cloudy apple juice and on the quality parameters of the juice during storage. Food Chem. X 2019, 3, 100049. [Google Scholar] [CrossRef]
- Graves, D.B. Low temperature plasma biomedicine: A tutorial review. Phys. Plasmas 2014, 21, 080901. [Google Scholar] [CrossRef] [Green Version]
- Alves Filho, E.G.; Silva, L.M.A.; de Brito, E.S.; Wurlitzer, N.J.; Fernandes, F.A.N.; Rabelo, M.C.; Fonteles, T.V.; Rodrigues, S. Evaluation of thermal and non-thermal processing effect on non-prebiotic and prebiotic acerola juices using 1H qNMR and GC–MS coupled to chemometrics. Food Chem. 2018, 265, 23–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janda, M.; Machala, Z.; Niklová, A.; Martišovitš, V. The streamer-to-spark transition in a transient spark: A dc-driven nanosecond-pulsed discharge in atmospheric air. Plasma Sources Sci. Technol. 2012, 21, 045006. [Google Scholar] [CrossRef] [Green Version]
- Machala, Z.; Tarabová, B.; Hensel, K.; Spetlikova, E.; Sikurova, L.; Lukeš, P. Formation of ROS and RNS in water electro-sprayed through transient spark discharge in air and their bactericidal effects. Plasma Process. Polym. 2013, 10, 649–659. [Google Scholar] [CrossRef]
- Machala, Z.; Tarabová, B.; Sersenová, D.; Janda, M.; Hensel, K. Chemical and antibacterial effects of plasma activated water: Correlation with gaseous and aqueous reactive oxygen and nitrogen species, plasma sources and air flow conditions. J. Phys. D. Appl. Phys. 2019, 52, 17pp. [Google Scholar] [CrossRef]
- Tarabová, B.; Lukeš, P.; Janda, M.; Hensel, K.; Šikurová, L.; Machala, Z. Specificity of detection methods of nitrites and ozone in aqueous solutions activated by air plasma. Plasma Process. Polym. 2018, 15, e1800030. [Google Scholar] [CrossRef]
- Machala, Z.; Janda, M.; Hensel, K.; Jedlovský, I.; Leštinská, L.; Foltin, V.; Martišovitš, V.; Morvová, M. Emission spectroscopy of atmospheric pressure plasmas for bio-medical and environmental applications. J. Mol. Spectrosc. 2007, 243, 194–201. [Google Scholar] [CrossRef]
- Chinnici, F.; Spinabelli, U.; Riponi, C.; Amati, A. Optimization of the determination of organic acids and sugars in fruit juices by ion-exclusion liquid chromatography. J. Food Compos. Anal. 2005, 18, 121–130. [Google Scholar] [CrossRef]
- Ramirez-Ambrosi, M.; Abad-Garcia, B.; Viloria-Bernal, M.; Garmon-Lobato, S.; Berrueta, L.A.; Gallo, B. A new ultrahigh performance liquid chromatography with diode array detection coupled to electrospray ionization and quadrupole time-of-flight mass spectrometry analytical strategy for fast analysis and improved characterization of phenolic compounds in apple products. J. Chromatogr. A 2013, 1316, 78–91. [Google Scholar] [CrossRef]
- Lu, Y.; Foo, L.Y. Identification and quantification of major polyphenols in apple pomace. Food Chem. 1997, 59, 187–194. [Google Scholar] [CrossRef]
- Tsao, R.; Yang, R.; Young, J.C.; Zhu, H. Polyphenolic profiles in eight apple cultivars using high-performance liquid chromatography (HPLC). J. Agric. Food Chem. 2003, 51, 6347–6353. [Google Scholar] [CrossRef]
- Eisele, T.A.; Drake, S.R. The partial compositional characteristics of apple juice from 175 apple varieties. J. Food Compos. Anal. 2005, 18, 213–221. [Google Scholar] [CrossRef]
- Wu, J.; Gao, H.; Zhao, L.; Liao, X.; Chen, F.; Wang, Z.; Hu, X. Chemical compositional characterization of some apple cultivars. Food Chem. 2007, 103, 88–93. [Google Scholar] [CrossRef]
- Belitz, H.-D.; Grosch, W.; Schieberle, P. Food Chemistry, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 9780128117378. [Google Scholar]
- Maehly, A.C.; Chance, B. The Assay of Catalases and Peroxidases. Methods Biochem. Anal. 1954, 1, 358–423. [Google Scholar] [CrossRef]
- Scientific Committee for Food. Reports of the Scientific Committee for Food; European Commission: Brussels, Belgium, 1997; Volume 38. [Google Scholar]
- Food and Drug Administration. Code of Federal Regulations (21CFR184.1366); Food and Drug Administration: Washington, DC, USA, 2001. [Google Scholar]
- European Parliament and Council. EC Regulation No. 1223/2009 on Cosmetic Products; European Parliament and Council: Brussels, Belgium, 2009. [Google Scholar]
- European Union Commission. Regulation (EU) No. 528/2012 Concerning the Making Available on the Market and Use of Biocidal Products. Hydrogen Peroxide; European Union Commission: Helsinki, Finland, 2015. [Google Scholar]
- Lukeš, P.; Doležalová, E.; Sisrova, I.; Clupek, M. Aqueous-phase chemistry and bactericidal effects from an air discharge plasma in contact with water: Evidence for the formation of peroxynitrite through a pseudo-second-order post-discharge reaction of H2O2 and HNO2. Plasma Sources Sci. Technol. 2014, 23, 015019. [Google Scholar] [CrossRef]
- Han, Y.; Cheng, J.H.; Sun, D.W. Activities and conformation changes of food enzymes induced by cold plasma: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 794–811. [Google Scholar] [CrossRef]
- Commision Regulation (EC). No. 2073/2005 on microbiological criteria for foodstuff. Off. J. Eur. Union 2005, 26. [Google Scholar] [CrossRef]








| Control | BS | ES | BS (+6 Days) | ES (+6 Days) | |
|---|---|---|---|---|---|
| pH | 3.23 ± 0.02 | 3.22 ± 0.01 | 3.23 ± 0.01 | 3.22 ± 0.01 | 3.21 ± 0.01 |
| σ (mS/cm) | 2.03 ± 0.01 | 2.12 ± 0.03 | 2.15 ± 0.03 | 2.10 ± 0.03 | 2.14 ± 0.03 |
| Sugar content (°Brix) | 12.03 ± 0.32 | 11.6 ± 0.37 | 12.03 ± 0.32 | - | - |
| POD activity (%) | 100 | 29 ± 6.1 | 46.9 ± 6.9 | - | - |
| Concentration | Decomposition (%) | ||
|---|---|---|---|
| Sugars | Control (mM) | BS | ES |
| Fructose | 369 ± 3 | 1 | 0.3 |
| Glucose | 90 ± 3 | 7 | 0 |
| Saccharose | 45.7 ± 1.3 | 0 | 0 |
| Organic Acids | Control (mM) | BS | ES |
| Malic acid | 80 ± 4 | 0 | 4 |
| Acetic acid | 57 ± 2 | 12 | 0 |
| Citric acid | 0.4 ± 0.03 | 0 | 5 |
| Ascorbic acid | 0.19 ± 0.04 | 10 | 42 |
| Polyphenols | Control (µM) | BS | ES |
| Chlorogenic acid | 475 ± 13 | 2 | 4 |
| Epicatechin | 36.7 ± 1.2 | 22 | 46 |
| Phloridzin | 29 ± 0.4 | 7 | 23 |
| Initial Concentration | Decomposition (%) | ||
|---|---|---|---|
| Sugars | (mM) | BS | ES |
| Fructose | 200 | 0.41 | 0 |
| Glucose | 200 | 4.3 | 3.9 |
| Saccharose | 200 | 3.0 | 0.2 |
| Organic Acids | (mM) | BS | ES |
| Malic acid | 10 | 0 | 0 |
| Citric acid | 10 | 0 | 0 |
| Ascorbic acid | 0.5 | 100 | 100 |
| Polyphenols | (µM) | BS | ES |
| Chlorogenic acid | 50 | 88 | 87 |
| Epicatechin | 50 | 38 | 48 |
| Phloridzin | 50 | 20 | 40 |
| Authors | Type of Apple Juice, Treated Volume | Plasma Source | Conditions of Treatment | Microorganism | Logarithmic Reduction | Ref. |
|---|---|---|---|---|---|---|
| Montenegro et al. | Generic supermarket brand, 0.8 mL | Pulsed in-liquid plasma system | Direct: 9000 V, f < 100 Hz, 4000 pulses | Escherichia coli O157:H7 | 7 log | [28] |
| Dasan et al. | Commercial clear juice, 11 mL | Atmospheric pressure plasma jet | Indirect: dry air (3000 L/h), 650 W, 120 s | Escherichia coli ATCC 25922 | 4 log | [24] |
| Liao et al. | Commercial juice, 3 mL | Dielectric barrier discharge plasma | Indirect: ambient air, 50 W, 30 s | mix of E.coli O157:H7, CICC 23429 and 10305 | 4.34 log | [31] |
| Surowsky et al. | Commercial juice (Granini), 2 mL | kINPen 09 | Direct: Ar + 0.1% O2 (5 slm), 480 s | Citrobacter freundii | 4.4 log | [25] |
| Xiang et al. | Commercial concentrated juice, 3 mL | Dielectric barrier discharge plasma | Direct: dry air, 90 W, 140 s | Zygosaccharomyces rouxii (GIM2.173) | 5 log | [18] |
| Wang et al. | Commercially available juice, 500 mL | Gas-phase surface discharge plasma spray reactor | Indirect: dry air, 21.3 kV, 30 min | Zygosaccharomyces rouxii LB and IFO1130 | 6.8 log | [19] |
| Wang et al. | Commercial concentrated juice, 500 mL | Gas-phase surface discharge plasma spray reactor | Indirect: dry air (150 L/h), juice flow rate 9 L/h, 30 min | Zygosaccharomyces rouxii LB | 5.6 log | [40] |
| Wang et al. | Commercial concentrated juice, 500 mL | Gas-phase surface discharge with active bubbling through liquid | Direct: dry air (150 L/h), 21 kV, 30 min | Zygosaccharomyces rouxii LB | 5.6 log | [21] |
| Vukušić Pavičić et al | Commercial concentrated juice, 200 mL | Hybrid above and in-liquid (Ar 4 L/min) plasma | Direct: pre-heating to 40 °C, 120 Hz, 9 min | Saccharomyces cerevisiae ATCC 204508 | 6.6 log | [30] |
| Tarabová et al. | Fresh squeezed juice, 5 mL | Transient spark with electrospray or above the liquid surface | Direct: ambient air, 5 mL/1 min, 1 kHz | E. coli ATCC 25922, S. cerevisiae S228C | 5–6 log 1 log | This publication |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarabová, B.; Tampieri, F.; Maran, E.; Marotta, E.; Ostrihoňová, A.; Krewing, M.; Machala, Z. Chemical and Antimicrobial Effects of Air Non-Thermal Plasma Processing of Fresh Apple Juice with Focus on Safety Aspects. Foods 2021, 10, 2055. https://doi.org/10.3390/foods10092055
Tarabová B, Tampieri F, Maran E, Marotta E, Ostrihoňová A, Krewing M, Machala Z. Chemical and Antimicrobial Effects of Air Non-Thermal Plasma Processing of Fresh Apple Juice with Focus on Safety Aspects. Foods. 2021; 10(9):2055. https://doi.org/10.3390/foods10092055
Chicago/Turabian StyleTarabová, Barbora, Francesco Tampieri, Elisabetta Maran, Ester Marotta, Andrea Ostrihoňová, Marco Krewing, and Zdenko Machala. 2021. "Chemical and Antimicrobial Effects of Air Non-Thermal Plasma Processing of Fresh Apple Juice with Focus on Safety Aspects" Foods 10, no. 9: 2055. https://doi.org/10.3390/foods10092055