Sensory and Compositional Properties Affecting the Likeability of Commercially Available Australian Honeys
Abstract
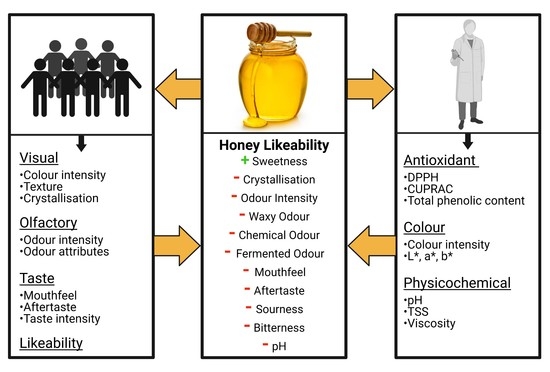
:1. Introduction
2. Materials and Methods
2.1. Honey Samples
2.2. Chemicals
2.3. Panellist Characteristics
2.3.1. Panellist Recruitment and Training
2.3.2. Bitter Taste Endophenotype (6-n-Propylthiouracil Test)
2.3.3. Honey Consumption Food Frequency Questionnaire
2.4. Honey Sensory Analysis
2.4.1. Analysis Conditions
2.4.2. Visual, Olfactory, and Taste Characteristics of Selected Honeys
2.5. Antioxidant and Physicochemcial Characteristics of Selected Honeys
2.5.1. Antioxidant and Total Phenolic Composition
2.5.2. Colour Analysis
2.5.3. Physicochemical Properties
2.6. Statistical Analysis
3. Results and Discussion
3.1. Panel Attributes
3.1.1. Panellist’s Usual Honey Preferences and Consumption Habits
3.1.2. Bitter Taste Endophenotype Evaluation
3.2. Sensory Analysis
3.3. Likeability of the Honey Samples
3.4. Relationships between Sensory and In Vitro Characteristics
3.5. Limitations and Future Directions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Codex Alimentarius Commission. Revised Codex Standard for Honey. Available online: http://www.fao.org/input/download/standards/310/cxs_012e.pdf (accessed on 31 July 2021).
- Geană, E.-I.; Ciucure, C.T.; Costinel, D.; Ionete, R.E. Evaluation of honey in terms of quality and authenticity based on the general physicochemical pattern, major sugar composition and δ13C signature. Food Control 2020, 109, 106919. [Google Scholar] [CrossRef]
- Da Silva, P.M.; Gonzaga, L.V.; de Azevedo, M.S.; Biluca, F.C.; Schulz, M.; Costa, A.C.O.; Fett, R. Stability of volatile compounds of honey during prolonged storage. J. Food Sci. Technol. 2020, 57, 1167–1182. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.S.; Abdul Aziz, A.; Kong, K.W.; Hamid, M.S.A.; Cheong, J.P.G.; Hamzah, S.H. Dose–response effect of Tualang honey on postprandial antioxidant activity and oxidative stress in female athletes: A pilot study. J. Altern. Complement. Med. 2017, 23, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Schramm, D.D.; Karim, M.; Schrader, H.R.; Holt, R.R.; Cardetti, M.; Keen, C.L. Honey with high levels of antioxidants can provide protection to healthy human subjects. J. Agric. Food Chem. 2003, 51, 1732–1735. [Google Scholar] [CrossRef]
- El-Haskoury, R.; Al-Waili, N.; Kamoun, Z.; Makni, M.; Al-Waili, H.; Lyoussi, B. Antioxidant activity and protective effect of carob honey in CCl4-induced kidney and liver injury. Arch. Med. Res. 2018, 49, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Combarros-Fuertes, P.; Fresno, J.M.; Estevinho, M.M.; Sousa-Pimenta, M.; Tornadijo, M.E.; Estevinho, L.M. Honey: Another Alternative in the Fight against Antibiotic-Resistant Bacteria? Antibiotics 2020, 9, 774. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, K. A current perspective on hydrogen peroxide production in honey. A review. Food Chem. 2020, 332, 127229. [Google Scholar] [CrossRef] [PubMed]
- Hadagali, M.D.; Chua, L.S. The anti-inflammatory and wound healing properties of honey. Eur. Food Res. Technol. 2014, 239, 1003–1014. [Google Scholar] [CrossRef]
- Hunter, M.; Kellett, J.; D’Cunha, N.M.; Toohey, K.; McKune, A.; Naumovski, N. The Effect of Honey as a Treatment for Oral Ulcerative Lesions: A Systematic Review. Explor. Res. Hypothesis Med. 2020, 5, 27–37. [Google Scholar] [CrossRef]
- Bogdanov, S.; Jurendic, T.; Sieber, R.; Gallmann, P. Honey for nutrition and health: A review. J. Am. Coll. Nutr. 2008, 27, 677–689. [Google Scholar] [CrossRef]
- Batt, P.J.; Liu, A. Consumer behaviour towards honey products in Western Australia. Br. Food J. 2012, 114, 285–297. [Google Scholar] [CrossRef] [Green Version]
- Escriche, I.; Kadar, M.; Juan-Borrás, M.; Domenech, E. Suitability of antioxidant capacity, flavonoids and phenolic acids for floral authentication of honey. Impact of industrial thermal treatment. Food Chem. 2014, 142, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Campbell, L.; Blair, S.; Carter, D. The effect of standard heat and filtration processing procedures on antimicrobial activity and hydrogen peroxide levels in honey. Front. Microbiol. 2012, 3, 265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcazzan, G.L.; Mucignat-Caretta, C.; Marina Marchese, C.; Piana, M.L. A review of methods for honey sensory analysis. J. Apic. Res. 2018, 57, 75–87. [Google Scholar] [CrossRef]
- Tuorila, H.; Monteleone, E. Sensory food science in the changing society: Opportunities, needs, and challenges. Trends Food Sci. Technol. 2009, 20, 54–62. [Google Scholar] [CrossRef]
- Piana, M.L.; Oddo, L.P.; Bentabol, A.; Bruneau, E.; Bogdanov, S.; Declerck, C.G. Sensory analysis applied to honey: State of the art. Apidologie 2004, 35, S26–S37. [Google Scholar] [CrossRef] [Green Version]
- Cosmina, M.; Gallenti, G.; Marangon, F.; Troiano, S. Reprint of “Attitudes towards honey among Italian consumers: A choice experiment approach”. Appetite 2016, 106, 110–116. [Google Scholar] [CrossRef] [Green Version]
- Gyau, A.; Akalakou, C.; Degrande, A.; Biloso, A. Determinants of consumer preferences for honey in the Democratic Republic of Congo. J. Food Prod. Mark. 2014, 20, 476–490. [Google Scholar] [CrossRef]
- Kortesniemi, M.; Rosenvald, S.; Laaksonen, O.; Vanag, A.; Ollikka, T.; Vene, K.; Yang, B. Sensory and chemical profiles of Finnish honeys of different botanical origins and consumer preferences. Food Chem. 2018, 246, 351–359. [Google Scholar] [CrossRef]
- Murphy, M.; Cowan, C.; Henchion, M.; O’Reilly, S. Irish consumer preferences for honey: A conjoint approach. Br. Food J. 2000, 102, 585–598. [Google Scholar] [CrossRef]
- Di Pizio, A.; Ben Shoshan-Galeczki, Y.; Hayes, J.E.; Niv, M.Y. Bitter and sweet tasting molecules: It's complicated. Neurosci. Lett. 2019, 700, 56–63. [Google Scholar] [CrossRef]
- Escuredo, O.; Dobre, I.; Fernández-González, M.; Seijo, M.C. Contribution of botanical origin and sugar composition of honeys on the crystallization phenomenon. Food Chem. 2014, 149, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Beretta, G.; Granata, P.; Ferrero, M.; Orioli, M.; Maffei Facino, R. Standardization of antioxidant properties of honey by a combination of spectrophotometric/fluorimetric assays and chemometrics. Anal. Chim. Acta 2005, 533, 185–191. [Google Scholar] [CrossRef]
- Kumar, A.; Gill, J.P.S.; Bedi, J.S.; Manav, M.; Ansari, M.J.; Walia, G.S. Sensorial and physicochemical analysis of Indian honeys for assessment of quality and floral origins. Food Res. Int. 2018, 108, 571–583. [Google Scholar] [CrossRef]
- Ciappini, M.C.; Di Vito, M.; Gatti, M.; Calviño, A.M. Development of a quantitative descriptive sensory honey analysis: Application to eucalyptus and clover honeys. Adv. J. Food Sci. Technol. 2013, 5, 829–838. [Google Scholar] [CrossRef]
- Anupama, D.; Bhat, K.K.; Sapna, V.K. Sensory and physico-chemical properties of commercial samples of honey. Food Res. Int. 2003, 36, 183–191. [Google Scholar] [CrossRef]
- Jani, R.; Byrne, R.; Love, P.; Agarwal, C.; Peng, F.; Yew, Y.W.; Panagiotakos, D.; Naumovski, N. The environmental and bitter taste endophenotype determinants of picky eating in Australian school-aged children 7–12 years—A cross-sectional pilot study protocol. Int. J. Environ. Res. Public Health 2020, 17, 1573. [Google Scholar] [CrossRef] [Green Version]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.-P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef]
- Bertoncelj, J.; Doberšek, U.; Jamnik, M.; Golob, T. Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey. Food Chem. 2007, 105, 822–828. [Google Scholar] [CrossRef]
- Szabó, R.T.; Mézes, M.; Szalai, T.; Zajácz, E.; Kovács-Weber, M. Colour identification of honey and methodical development of its instrumental measuring. Columella J. Agric. Environ. Sci. 2016, 3, 29–36. [Google Scholar] [CrossRef]
- Aumeeruddy, M.Z.; Aumeeruddy-Elalfi, Z.; Neetoo, H.; Zengin, G.; Blom van Staden, A.; Fibrich, B.; Lambrechts, I.A.; Rademan, S.; Szuman, K.M.; Lall, N.; et al. Pharmacological activities, chemical profile, and physicochemical properties of raw and commercial honey. Biocatal. Agric. Biotechnol. 2019, 18, 101005. [Google Scholar] [CrossRef]
- Chan, B.K.; Haron, H.; Talib, R.A.; Subramaniam, P. Physical properties, antioxidant content and anti-oxidative activities of Malaysian stingless kelulut (Trigona spp.) Honey. J. Agric. Sci. 2017, 9, 32–40. [Google Scholar] [CrossRef] [Green Version]
- Yücel, Y.; Sultanog, P. Characterization of honeys from Hatay Region by their physicochemical properties combined with chemometrics. Food Biosci. 2013, 1, 16–25. [Google Scholar] [CrossRef]
- Stolzenbach, S.; Bredie, W.L.P.; Byrne, D.V. Consumer concepts in new product development of local foods: Traditional versus novel honeys. Food Res. Int. 2013, 52, 144–152. [Google Scholar] [CrossRef]
- Tarragon, E.; Moreno, J.J. Polyphenols and taste 2 receptors. Physiological, pathophysiological and pharmacological implications. Biochem. Pharmacol. 2020, 178, 114086. [Google Scholar] [CrossRef] [PubMed]
- Gašić, U.M.; Milojković-Opsenica, D.M.; Tešić, Ž.L. Polyphenols as possible markers of botanical origin of honey. J. AOAC Int. 2017, 100, 852–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melis, M.; Tomassini Barbarossa, I. Taste perception of sweet, sour, salty, bitter, and umami and changes due to l-arginine supplementation, as a function of genetic ability to taste 6-n-propylthiouracil. Nutrients 2017, 9, 541. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, I.; Cámara-Martos, F.; Flores, J.M.; Serrano, S. Spanish avocado (Persea americana Mill.) honey: Authentication based on its composition criteria, mineral content and sensory attributes. LWT Food Sci. Technol. 2019, 111, 561–572. [Google Scholar] [CrossRef]
- Biehler, E.; Mayer, F.; Hoffmann, L.; Krause, E.; Bohn, T. Comparison of 3 spectrophotometric methods for carotenoid determination in frequently consumed fruits and vegetables. J. Food Sci. 2010, 75, C55–C61. [Google Scholar] [CrossRef] [PubMed]


| Rank | Honey | Minimum–Maximum (Range) | Mean ± SD | Difference | |
|---|---|---|---|---|---|
| U | Significance | ||||
| 1 | C4 | 4.6–14.5 (9.9) | 10.6 ± 2.84 | - | - |
| 2 | R1 | 5.1–14.5 (9.4) | 9.39 ± 2.85 | 224.0 | 0.187 |
| 3 | B6 | 0.0–14.4 (14.4) | 9.28 ± 3.96 | 235.5 | 0.279 |
| 4 | AE3 | 3.3–14.5 (11.2) | 9.26 ± 2.84 | 208.0 | 0.099 |
| 5 | I5 | 2.4–14.5 (12.1) | 9.13 ± 3.24 | 213.5 | 0.124 |
| 6 | Y3 | 2.0–14.5 (12.5) | 9.09 ± 3.80 | 219.0 | 0.155 |
| 7 | J6 | 0.7–14.5 (13.8) | 9.05 ± 3.50 | 217.5 | 0.146 |
| 8 | S1 | 0.8–14.5 (13.7) | 8.86 ± 4.01 | 220.0 | 0.161 |
| 9 | AF3 | 3.5–13.7 (10.2) | 8.83 ± 2.61 | 176.5 | 0.021 * |
| 10 | D5 | 2.9–14.5 (11.6) | 8.81 ± 2.92 | 181.0 | 0.027 * |
| 11 | W4 | 2.3–14.2 (11.9) | 8.70 ± 3.31 | 196.5 | 0.059 |
| 12 | V6 | 1.5–14.3 (12.8) | 8.48 ± 3.11 | 179.5 | 0.025 * |
| 13 | H4 | 2.1–14.5 (12.4) | 8.46 ± 3.10 | 176.5 | 0.021 * |
| 14 | K5 | 2.0–14.2 (12.2) | 8.38 ± 3.49 | 178.5 | 0.024* |
| 15 | AD2 | 0.0–14.4 (14.4) | 8.35 ± 3.67 | 186.5 | 0.036 * |
| 16 | O6 | 0.4–14.4 (14.0) | 8.31 ± 3.73 | 178.0 | 0.023 * |
| 17 | G5 | 0.5–13.5 (13.0) | 8.26 ± 3.20 | 161.0 | 0.009 * |
| 18 | X3 | 1.8–14.4 (12.6) | 8.22 ± 3.54 | 179.5 | 0.025 * |
| 19 | F4 | 1.8–14.1 (12.3) | 8.15 ± 3.60 | 170.5 | 0.015 * |
| 20 | L3 | 1.3–12.9 (11.6) | 8.11 ± 3.42 | 170.0 | 0.015 * |
| 21 | Q1 | 2.2–14.0 (11.8) | 8.08 ± 3.26 | 165.0 | 0.011 * |
| 22 | P5 | 0.4–14.1 (13.7) | 7.53 ± 3.36 | 137.5 | 0.002 * |
| 23 | N4 | 0.0–13.3 (13.3) | 7.51 ± 3.80 | 158.0 | 0.007 * |
| 24 | A4 | 3.1–12.1 (9.0) | 7.45 ± 2.81 | 129.5 | 0.001 * |
| 25 | AA3 | 1.3–14.4 (13.1) | 7.32 ± 3.43 | 133.0 | 0.001 * |
| 26 | U1 | 0.0–13.0 (13.0) | 7.20 ± 3.23 | 122.5 | 0.001 * |
| 27 | Z3 | 1.2–12.3 (11.1) | 6.90 ± 3.02 | 110.5 | 0.000 * |
| 28 | T1 | 0.0–14.5 (14.5) | 6.80 ± 4.60 | 143.0 | 0.003 * |
| 29 | M1 | 0.0–14.5 (14.5) | 6.56 ± 4.56 | 138.0 | 0.002 * |
| 30 | AC2 | 0.0–12.0 (12.0) | 6.23 ± 3.76 | 106.5 | 0.000 * |
| 31 | AB4 | 0.0–14.5 (14.5) | 6.12 ± 4.66 | 133.5 | 0.001 * |
| 32 | E6 | 0.0–12.2 (12.2) | 5.75 ± 3.71 | 86.5 | 0.000 * |
| Crystallisation | Odour Intensity | Mouthfeel | Aftertaste | Sweetness | Bitterness | Likeability | DPPH Inhibition (%) | CUPRAC | TPC | ABS450 | pH | AUD/100 g | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystallisation | 1 | ||||||||||||
| Odour Intensity | 0.135 | 1 | |||||||||||
| Mouthfeel | 0.550 ** | 0.148 | 1 | ||||||||||
| Aftertaste | −0.039 | 0.256 * | −0.004 | 1 | |||||||||
| Sweetness | −0.176 | −0.218 | −0.104 | −0.260 * | 1 | ||||||||
| Bitterness | 0.289 * | 0.262 * | 0.221 | 0.203 | −0.271 * | 1 | |||||||
| Likeability | −0.260 * | −0.297 * | −0.288* | −0.435 ** | 0.353 ** | −0.252 * | 1 | ||||||
| DPPH Inhibition (%) | −0.162 | 0.059 | −0.077 | 0.379 * | −0.059 | 0.028 | −0.202 | 1 | |||||
| CUPRAC | −0.125 | 0.276 * | −0.089 | 0.315 * | −0.131 | 0.118 | −0.177 | 0.476 ** | 1 | ||||
| TPC | −0.141 | 0.147 | −0.077 | 0.234 | −0.042 | 0.057 | −0.105 | 0.468 ** | 0.677 ** | 1 | |||
| ABS450 | −0.113 | 0.139 | −0.012 | 0.290 * | 0.006 | 0.061 | −0.097 | 0.500 ** | 0.556 ** | 0.573 ** | 1 | ||
| pH | 0.181 | 0.415 ** | 0.165 | 0.311 * | −0.224 | 0.149 | −0.437 ** | 0.169 | 0.339 ** | 0.270 * | 0.209 | 1 | |
| AUD/100 g | −0.025 | 0.137 | 0.157 | 0.193 | −0.187 | 0.254 * | −0.184 | 0.111 | 0.271 * | 0.316 * | 0.225 | 0.155 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hunter, M.; Kellett, J.; Toohey, K.; Naumovski, N. Sensory and Compositional Properties Affecting the Likeability of Commercially Available Australian Honeys. Foods 2021, 10, 1842. https://doi.org/10.3390/foods10081842
Hunter M, Kellett J, Toohey K, Naumovski N. Sensory and Compositional Properties Affecting the Likeability of Commercially Available Australian Honeys. Foods. 2021; 10(8):1842. https://doi.org/10.3390/foods10081842
Chicago/Turabian StyleHunter, Maddison, Jane Kellett, Kellie Toohey, and Nenad Naumovski. 2021. "Sensory and Compositional Properties Affecting the Likeability of Commercially Available Australian Honeys" Foods 10, no. 8: 1842. https://doi.org/10.3390/foods10081842