Physical and Thermal Evaluation of Olive Oils from Minor Italian Cultivars
Abstract
:1. Introduction
2. Materials and Methods
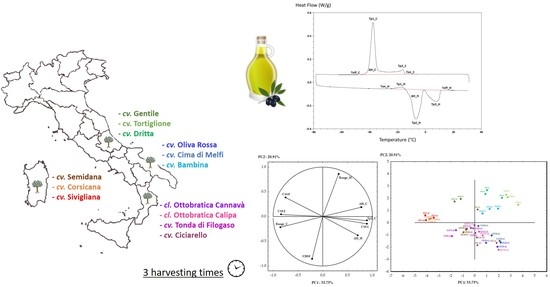
2.1. Plant Material
2.2. Fatty Acids Composition
2.3. Thermal Analysis
2.4. Viscosity Measurement
2.5. Chlorophyll Content
2.6. Color
2.7. Statistical Analysis
3. Results
3.1. Fatty Acid Composition
3.2. Thermal Analysis
3.3. Viscosity
3.4. Chlorophyll Content
3.5. Color
3.6. PCA
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean diet pyramid: A cultural model for healthy eating. Am. J. Clin. Nutr. 1995, 61, 1402S–1406S. [Google Scholar] [CrossRef]
- Escrich, E.; Moral, R.; Solanas, M. Olive oil, an essential component of the Mediterranean diet, and breast cancer. Public Health Nutr. 2011, 14, 2323–2332. [Google Scholar] [CrossRef] [Green Version]
- Ilarioni, L.; Proietti, P. Olive tree cultivars. In The Extra-Virgin Olive Oil Handbook; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 59–67. ISBN 9781118460412. [Google Scholar]
- Allalout, A.; Krichène, D.; Methenni, K.; Taamalli, A.; Daoud, D.; Zarrouk, M. Behavior of super-intensive spanish and greek olive cultivars grown in northern tunisia. J. Food Biochem. 2011, 35, 27–43. [Google Scholar] [CrossRef]
- Conte, P.; Squeo, G.; Difonzo, G.; Caponio, F.; Fadda, C.; Del Caro, A.; Urgeghe, P.P.; Montanari, L.; Montinaro, A.; Piga, A. Change in quality during ripening of olive fruits and related oils extracted from three minor autochthonous Sardinian cultivars. Emir. J. Food Agric. 2019, 31, 196–205. [Google Scholar] [CrossRef]
- The Council of the European Communities. Council regulation (EEC) N. 2081/92 of 14 July 1992 on the protection of geographical indications and designations of origin for agricultural products and foodstuffs. Off. J. Eur. Communities 1992, 208, 1–8. [Google Scholar]
- Miazzi, M.M.; di Rienzo, V.; Mascio, I.; Montemurro, C.; Sion, S.; Sabetta, W.; Vivaldi, G.A.; Camposeo, S.; Caponio, F.; Squeo, G.; et al. Re.Ger.O.P.: An Integrated Project for the Recovery of Ancient and Rare Olive Germplasm. Front. Plant Sci. 2020, 11, 73. [Google Scholar] [CrossRef] [Green Version]
- Muzzalupo, I. Olive Germplasm—Italian Catalogue of Olive Varieties. In Olive Germplasm—Italian Catalogue of Olive Varieties; InTech: Rijeka, Croatia, 2012; pp. 1–5. [Google Scholar]
- Rotondi, A.; Alfei, B.; Magli, M.; Pannelli, G. Influence of genetic matrix and crop year on chemical and sensory profiles of Italian monovarietal extra-virgin olive oils. J. Sci. Food Agric. 2010, 90, 2641–2648. [Google Scholar] [CrossRef]
- Rotondi, A.; Ganino, T.; Beghè, D.; Di Virgilio, N.; Morrone, L.; Fabbri, A.; Neri, L. Genetic and landscape characterization of ancient autochthonous olive trees in northern Italy. Plant Biosyst. 2018, 152, 1067–1074. [Google Scholar] [CrossRef]
- Piscopo, A.; De Bruno, A.; Zappia, A.; Ventre, C.; Poiana, M. Characterization of monovarietal olive oils obtained from mills of Calabria region (Southern Italy). Food Chem. 2016, 213, 313–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Vaio, C.; Nocerino, S.; Paduano, A.; Sacchi, R. Characterization and evaluation of olive germplasm in southern Italy. J. Sci. Food Agric. 2013, 93, 2458–2462. [Google Scholar] [CrossRef] [Green Version]
- Squeo, G.; Silletti, R.; Mangini, G.; Summo, C.; Caponio, F. The Potential of Apulian Olive Biodiversity: The Case of Oliva Rossa Virgin Olive Oil. Foods 2021, 10, 369. [Google Scholar] [CrossRef]
- Skiada, V.; Tsarouhas, P.; Varzakas, T. Comparison and discrimination of two major monocultivar extra virgin olive oils in the southern region of Peloponnese, according to specific compositional/traceability markers. Foods 2020, 9, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ou, G.; Hu, R.; Zhang, L.; Li, P.; Luo, X.; Zhang, Z. Advanced detection methods for traceability of origin and authenticity of olive oils. Anal. Methods 2015, 7, 5731–5739. [Google Scholar] [CrossRef]
- Chiavaro, E.; Rodriguez-Estrada, M.T.; Barnaba, C.; Vittadini, E.; Cerretani, L.; Bendini, A. Differential scanning calorimetry: A potential tool for discrimination of olive oil commercial categories. Anal. Chim. Acta. 2008, 625, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Vecchio Ciprioti, S.; Paciulli, M.; Chiavaro, E. Application of different thermal analysis techniques to characterize oxidized olive oils. Eur. J. Lipid Sci. Technol. 2017, 119, 1600074. [Google Scholar] [CrossRef]
- Ben Rached, M.; Paciulli, M.; Pugliese, A.; Abdallah, M.; Boujnah, D.; Zarrouk, M.; Guerfel, M.; Chiavaro, E. Effect of the soil nature on selected chemico-physical and thermal parameters of extra virgin olive oils from cv Chemlali. Ital. J. Food Sci. 2017, 29, 74–89. [Google Scholar]
- Jafari, M.; Kadivar, M.; Keramat, J. Detection of adulteration in Iranian olive oils using instrumental (GC, NMR, DSC) methods. J. Am. Oil Chem. Soc. 2009, 86, 103–110. [Google Scholar] [CrossRef]
- Chatziantoniou, S.E.; Triantafillou, D.J.; Karayannakidis, P.D.; Diamantopoulos, E. Traceability monitoring of Greek extra virgin olive oil by differential scanning calorimetry. Thermochim. Acta 2014, 576, 9–17. [Google Scholar] [CrossRef]
- Mallamace, D.; Vasi, S.; Corsaro, C.; Naccari, C.; Clodoveo, M.L.; Dugo, G.; Cicero, N. Calorimetric analysis points out the physical-chemistry of organic olive oils and reveals the geographical origin. Physica A 2017, 486, 925–932. [Google Scholar] [CrossRef]
- Maggio, R.M.; Barnaba, C.; Cerretani, L.; Paciulli, M.; Chiavaro, E. Study of the influence of triacylglycerol composition on DSC cooling curves of extra virgin olive oil by chemometric data processing. J. Therm. Anal. Calorim. 2014, 115, 2037–2044. [Google Scholar] [CrossRef]
- Commission of the European Communities. Commission regulation (EEC) No 2568/91 of 11 July of 1991 and subsequent integrations and amendments. Off. J. Eur. Communities 1991, 248, 1–83. [Google Scholar]
- Koriyama, T.; Wongso, S.; Watanabe, K.; Abe, H.J. Fatty acid compositions of oil species affect the 5 basic taste perceptions. J. Food Sci. 2002, 67, 868–873. [Google Scholar] [CrossRef]
- Paciulli, M.; Palermo, M.; Chiavaro, E.; Pellegrini, N. Chlorophylls and Colour Changes in Cooked Vegetables. In Fruit and Vegetable Phytochemicals: Chemistry and Human Health, 2nd ed.; Elhadi, M.Y., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017; Volume 2, pp. 703–719. [Google Scholar]
- Giuliani, A.; Cerretani, L.; Cichelli, A. Chlorophylls in olive and in olive oil: Chemistry and occurrences. Crit. Rev. Food Sci. Nutr. 2011, 51, 678–690. [Google Scholar] [CrossRef]
- Gandul-Rojas, B.; Cepero, M.R.L.; Mínguez-Mosquera, M.I. Use of chlorophyll and carotenoid pigment composition to determine authenticity of virgin olive oil. J. Am. Oil Chem. Soc. 2000, 77, 853–858. [Google Scholar] [CrossRef]
- Italian Ministry of Agricultural, Food and Forestry Policies. Available online: https://www.politicheagricole.it/ (accessed on 18 April 2021).
- Alamprese, C.; Grassi, S.; Tugnolo, A.; Casiraghi, E. Prediction of olive ripening degree combining image analysis and FT-NIR spectroscopy for virgin olive oil optimisation. Food Control. 2021, 123, 107755. [Google Scholar] [CrossRef]
- Piscopo, A.; Zappia, A.; De Bruno, A.; Poiana, M. Effect of the harvesting time on the quality of olive oils produced in Calabria. Eur. J. Lipid Sci. Technol. 2018, 120, 1700304. [Google Scholar] [CrossRef]
- Difonzo, G.; Pasqualone, A.; Silletti, R.; Cosmai, L.; Summo, C.; Paradiso, V.M.; Caponio, F. Use of olive leaf extract to reduce lipid oxidation of baked snacks. Food Res. Int. 2018, 108, 48–56. [Google Scholar] [CrossRef]
- Cerretani, L.; Bendini, A.; Rinaldi, M.; Paciulli, M.; Vecchio, S.; Chiavaro, E. DSC evaluation of extra virgin olive oil stability under accelerated oxidative test: Effect of fatty acid composition and phenol contents. J. Oleo Sci. 2012, 61, 303–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zago, L.; Squeo, G.; Bertoncini, E.I.; Difonzo, G.; Caponio, F. Chemical and sensory characterization of Brazilian virgin olive oils. Food Res. Int. 2019, 126, 108588. [Google Scholar] [PubMed]
- AOCS official method Cc 13i-96. Determination of Chlorophyll Pigments in Crude Vegetable Oils. In Official Methods and Recommended Practices of the AOCS, 7th ed.; AOCS Press: Washington, DC, USA, 2017.
- Inglese, P.; Famiani, F.; Galvano, F.; Servili, M.; Esposto, S.; Urbani, S. Factors Affecting Extra-Virgin Olive Oil Composition. In Horticultural Reviews; Janik, J., Ed.; Johnwiley & Sons, Ltsd.: West Sussex, UK, 2011; Volume 38, pp. 83–148. [Google Scholar]
- Rondanini, D.P.; Castro, D.N.; Searles, P.S.; Rousseaux, M.C. Contrasting patterns of fatty acid composition and oil accumulation during fruit growth in several olive varieties and locations in a non-Mediterranean region. Eur. J. Agron. 2014, 52, 237–246. [Google Scholar] [CrossRef]
- Rondanini, D.P.; Castro, D.N.; Searles, P.S.; Rousseaux, M.C. Fatty acid profiles of varietal virgin olive oils (Olea europaea L.) From mature orchards in warm arid valleys of Northwestern Argentina (La Rioja). Grasas Aceites. 2011, 62, 399–409. [Google Scholar]
- Chiavaro, E.; Vittadini, E.; Rodriguez-Estrada, M.T.; Cerretani, L.; Bendini, A. Monovarietal extra virgin olive oils. Correlation between thermal properties and chemical composition: Heating thermograms. J. Agric. Food Chem. 2008, 56, 496–501. [Google Scholar] [CrossRef]
- Chiavaro, E.; Vittadini, E.; Rodriguez-Estrada, M.T.; Cerretani, L.; Bonoli, M.; Bendini, A.; Lercker, G. Monovarietal extra virgin olive oils: Correlation between thermal properties and chemical composition. J. Agric. Food Chem. 2007, 55, 10779–10786. [Google Scholar] [CrossRef]
- Bayés-García, L.; Calvet, T.; Cuevas-Diarte, M.A.; Ueno, S. From trioleoyl glycerol to extra virgin olive oil through multicomponent triacylglycerol mixtures: Crystallization and polymorphic transformation examined with differential scanning calorimetry and X-ray diffration techniques. Food Res. Int. 2017, 99, 476–484. [Google Scholar]
- Caponio, F.; Chiavaro, E.; Paradiso, V.M.; Paciulli, M.; Summo, C.; Cerretani, L.; Gomes, T. Chemical and thermal evaluation of olive oil refining at different oxidative levels. Eur. J. Lipid Sci. Technol. 2013, 115, 1146–1154. [Google Scholar] [CrossRef]
- Gila, A.; Jiménez, A.; Beltrán, G.; Romero, A. Correlation of fatty acid composition of virgin olive oil with thermal and physical properties. Eur. J. Lipid Sci. Technol. 2015, 117, 366–376. [Google Scholar] [CrossRef]
- Kim, J.; Kim, D.N.; Lee, S.H.; Yoo, S.-H.; Lee, S. Correlation of fatty acid composition of vegetable oils with rheological behaviour and oil uptake. Food Chem. 2010, 118, 398–402. [Google Scholar]
- Perito, M.A.; Sacchetti, G.; Di Mattia, C.D.; Chiodo, E.; Pittia, P.; Saguy, I.S.; Cohen, E. Buy local! Familiarity and preferences for extra virgin olive oil of Italian consumers. J. Food Prod. Mark. 2019, 25, 462–477. [Google Scholar]
- Roca, M.; Mínguez-Mosquera, M.I. Changes in chloroplast pigments of olive varieties during fruit ripening. J. Agric. Food Chem. 2001, 49, 832–839. [Google Scholar] [CrossRef]
- Piscopo, A.; Mafrica, R.; De Bruno, A.; Romeo, R.; Santacaterina, S.; Poiana, M. Characterization of Olive Oils Obtained from Minor Accessions in Calabria (Southern Italy). Foods 2021, 10, 305. [Google Scholar] [CrossRef]
- Criado, M.N.; Motilva, M.J.; Goni, M.; Romero, M.P. Comparative study of the effect of the maturation process of the olive fruit on the chlorophyll and carotenoid fractions of drupes and virgin oils from Arbequina and Farga cultivars. Food Chem. 2007, 100, 748–755. [Google Scholar] [CrossRef]





| Province | Cultivar | Maximum | Minimum | Average |
|---|---|---|---|---|
| L’Aquila | TOR, DR | 18.0 | 5.2 | 11.6 |
| Teramo | GEN | 19.9 | 8.4 | 14.2 |
| Sassari | SIV, SEM, COR | 22.2 | 11.5 | 16.9 |
| Bari | CM, OR, BAM | 21.4 | 10.5 | 16.0 |
| Reggio Calabria | OTT, OTTC, TDF, CIC | 23.1 | 15.9 | 19.5 |
| C16:0 | C16:1 | C18:0 | C18:1 | C18:2 | C18:3 | C20:0 | ||
|---|---|---|---|---|---|---|---|---|
| t1 | 14.05 ± 0.83 efA | 0.56 ± 0.03 efA | 2.33 ± 0.04 bcdeA | 75.75 ± 0.76 aB | 6.50 ± 0.08 cB | 0.57 ± 0.01 abB | 0.24 ± 0.02 dB | |
| TOR | t2 | 12.41 ± 0.20 cB | 0.54 ± 0.00 efA | 2.16 ± 0.04 deB | 77.13 ± 0.13 aA | 6.87 ± 0.04 efA | 0.63 ± 0.00 bcA | 0.27 ± 0.01 eA |
| t3 | 11.70 ± 0.41 efgB | 0.52 ± 0.04 fA | 2.23 ± 0.02 defB | 77.83 ± 0.43 abA | 6.88 ± 0.02 fA | 0.57 ± 0.01 abB | 0.27 ± 0.00 dA | |
| t1 | 15.05 ± 0.43 bcdA | 1.59 ± 0.03 abA | 1.77 ± 0.05 efA | 72.40 ± 0.34 bB | 8.25 ± 0.13 cB | 0.74 ± 0.08 aA | 0.20 ± 0.01 dAB | |
| DR | t2 | 13.64 ± 0.20 abcB | 1.75 ± 0.09 bcA | 1.72 ± 0.10 fA | 73.08 ± 0.34 bcdB | 8.83 ± 0.19 cdA | 0.74 ± 0.02 aA | 0.23 ± 0.00 fA |
| t3 | 13.19 ± 0.38 cdefB | 1.27 ± 0.07 cdB | 1.87 ± 0.02 fA | 74.84 ± 0.32 cdA | 8.07 ± 0.13 eB | 0.58 ± 0.22 abA | 0.19 ± 0.02 eB | |
| t1 | 16.12 ± 0.73 abA | 1.96 ± 0.27 aA | 1.54 ± 0.02 fB | 69.06 ± 0.63 cB | 10.46 ± 0.37 bAB | 0.63 ± 0.04 abA | 0.22 ± 0.01 dA | |
| GEN | t2 | 16.24 ± 0.30 aA | 2.25 ± 0.12 aA | 1.55 ± 0.03 fB | 67.82 ± 0.94 efB | 11.32 ± 0.69 bA | 0.61 ± 0.01 bcA | 0.20 ± 0.01 fB |
| t3 | 14.66 ± 0.41 abcB | 1.99 ± 0.09 aA | 2.13 ± 0.10 defA | 71.09 ± 0.49 fA | 9.34 ± 0.16 dB | 0.59 ± 0.02 abA | 0.21 ± 0.00 eAB | |
| t1 | 15.55 ± 0.07 abcB | 1.37 ± 0.01 bcdB | 2.49 ± 0.01 abcdB | 66.01 ± 0.07 deA | 13.74 ± 0.01 aC | 0.47 ± 0.01 bA | 0.38 ± 0.00 abcA | |
| SIV | t2 | 15.30 ± 0.01 abB | 1.36 ± 0.01 cdB | 2.77 ± 0.00 bA | 64.50 ± 0.02 gB | 15.20 ± 0.03 aA | 0.49 ± 0.01 efA | 0.38 ± 0.00 bcdA |
| t3 | 16.34 ± 0.28 aA | 1.48 ± 0.02 bA | 2.40 ± 0.12 cdeB | 64.73 ± 0.17 hB | 14.24 ± 0.01 aB | 0.48 ± 0.02 bA | 0.34 ± 0.01 cB | |
| t1 | 14.80 ± 0.15 bcdA | 0.96 ± 0.01 cdeA | 2.57 ± 0.01 abcB | 72.12 ± 0.13 bA | 8.50 ± 0.02 bcC | 0.65 ± 0.01 abB | 0.39 ± 0.01 abB | |
| SEM | t2 | 14.02 ± 0.07 abcB | 0.97 ± 0.01 deA | 2.61 ± 0.02 bcB | 71.98 ± 0.08 cdA | 9.31 ± 0.00 cB | 0.68 ± 0.00 abA | 0.43 ± 0.01 aA |
| t3 | 13.58 ± 0.13 cdeC | 0.88 ± 0.01 eB | 2.74 ± 0.01 abcA | 71.19 ± 0.12 fB | 10.54 ± 0.01 cA | 0.63 ± 0.01 abB | 0.43 ± 0.01 bA | |
| t1 | 16.69 ± 0.17 aA | 1.41 ± 0.01 bcA | 2.42 ± 0.02 abcdA | 65.17 ± 0.15 eA | 13.35 ± 0.02 aC | 0.61 ± 0.01 abA | 0.35 ± 0.01 bcA | |
| COR | t2 | 16.21 ± 0.04 aB | 1.30 ± 0.00 dB | 2.37 ± 0.00 cdeB | 65.14 ± 0.03 fgA | 14.03 ± 0.00 aB | 0.60 ± 0.01 cdA | 0.36 ± 0.00 cdA |
| t3 | 15.92 ± 0.18 abB | 1.20 ± 0.02 cdC | 2.42 ± 0.02 bcdeA | 65.40 ± 0.16 hA | 14.16 ± 0.02 aA | 0.54 ± 0.00 abB | 0.36 ± 0.01 cA | |
| t1 | 15.40 ± 0.97 abcdA | 0.90 ± 0.40 defA | 1.88 ± 0.34 defA | 72.44 ± 0.69 bB | 8.48 ± 0.51 bcA | 0.58 ± 0.02 abA | 0.31 ± 0.05 cA | |
| CM | t2 | 13.72 ± 0.71 abcAB | 0.47 ± 0.01 fA | 2.37 ± 0.03 cdeAB | 74.72 ± 0.55 abcA | 7.89 ± 0.14 deA | 0.48 ± 0.02 efB | 0.35 ± 0.01 cdA |
| t3 | 11.13 ± 1.64 gB | 0.48 ± 0.02 fA | 2.46 ± 0.02 abcdA | 76.24 ± 1.27 bcA | 8.73 ± 0.35 deA | 0.59 ± 0.02 abA | 0.38 ± 0.01 cA | |
| t1 | 11.06 ± 0.28 gA | 0.49 ± 0.01 fB | 2.78 ± 0.02 abA | 74.19 ± 0.40 abA | 10.46 ± 0.09 bA | 0.62 ± 0.26 abA | 0.41 ± 0.00 abA | |
| OR | t2 | 12.10 ± 3.12 cA | 0.47 ± 0.03 fB | 2.57 ± 0.01 bcB | 75.23 ± 2.40 abA | 8.50 ± 0.61 cdB | 0.75 ± 0.06 aA | 0.38 ± 0.03 bcA |
| t3 | 12.13 ± 1.41 efgA | 0.56 ± 0.02 fA | 2.48 ± 0.03 abcdC | 75.32 ± 1.05 cdA | 8.44 ± 0.30 deB | 0.68 ± 0.05 aA | 0.37 ± 0.01 cA | |
| t1 | 14.12 ± 0.07 defA | 1.46 ± 0.00 bA | 1.98 ± 0.01 cdefB | 74.01 ± 0.04 abA | 7.49 ± 0.03 cB | 0.60 ± 0.02 abA | 0.35 ± 0.01 bcA | |
| BAM | t2 | 13.39 ± 0.83 bcA | 1.08 ± 0.51 dA | 2.32 ± 0.06 cdeA | 73.51 ± 1.89 bcdA | 8.82 ± 0.58 cdA | 0.53 ± 0.04 deA | 0.36 ± 0.01 cdA |
| t3 | 13.50 ± 0.11 cdeA | 1.33 ± 0.01 bcA | 2.27 ± 0.00 defA | 72.72 ± 0.13 efA | 9.23 ± 0.02 dA | 0.59 ± 0.01 abA | 0.36 ± 0.01 cA | |
| t1 | 16.04 ± 0.22 abA | 1.39 ± 0.09 bcB | 2.94 ± 0.15 aA | 68.22 ± 0.38 cdC | 10.44 ± 0.18 bA | 0.53 ± 0.01 abB | 0.45 ± 0.01 aA | |
| OTT | t2 | 14.40 ± 0.13 abcB | 1.91 ± 0.04 abA | 2.07 ± 0.17 eB | 71.75 ± 0.71 dB | 8.87 ± 0.52 cdB | 0.66 ± 0.03 bcA | 0.35 ± 0.01 dB |
| t3 | 12.45 ± 0.10 defgC | 1.17 ± 0.06 dC | 2.79 ± 0.14 abcA | 74.31 ± 0.23 deA | 8.38 ± 0.18 deB | 0.52 ± 0.01 abB | 0.36 ± 0.01 cB | |
| t1 | 15.04 ± 0.01 bcdA | 2.01 ± 0.01 aA | 1.97 ± 0.15 cdefC | 72.17 ± 0.12 bC | 7.77 ± 0.11 cA | 0.69 ± 0.01 abA | 0.35 ± 0.00 bcB | |
| OTTC | t2 | 12.69 ± 0.17 bcB | 1.12 ± 0.05 dB | 2.43 ± 0.11 cdB | 76.80 ± 0.39 aB | 6.10 ± 0.28 fB | 0.45 ± 0.02 fB | 0.41 ± 0.01 abA |
| t3 | 11.46 ± 0.13 fgC | 0.80 ± 0.11 eC | 2.91 ± 0.06 aA | 78.46 ± 0.47 aA | 5.45 ± 0.30 gC | 0.47 ± 0.05 bB | 0.46 ± 0.03 abA | |
| t1 | 14.44 ± 0.16 cdefB | 1.44 ± 0.06 bB | 1.62 ± 0.56 fB | 73.98 ± 1.04 abA | 7.45 ± 0.37 cC | 0.68 ± 0.01 abA | 0.39 ± 0.01 abA | |
| TDF | t2 | 15.23 ± 0.15 abA | 1.40 ± 0.02 cdB | 3.17 ± 0.11 aA | 67.69 ± 0.76 efC | 11.62 ± 0.51 bA | 0.47 ± 0.01 efB | 0.41 ± 0.02 abA |
| t3 | 14.35 ± 0.14 bcdB | 1.89 ± 0.05 aA | 2.00 ± 0.36 efB | 71.81 ± 0.69 fB | 8.95 ± 0.48 deB | 0.65 ± 0.02 abA | 0.34 ± 0.00 cB | |
| t1 | 13.25 ± 0.26 fB | 1.16 ± 0.28 bcdA | 2.23 ± 0.27 bcdeA | 75.46 ± 2.65 aA | 6.96 ± 2.33 cB | 0.53 ± 0.01 abA | 0.42 ± 0.07 abA | |
| CIC | t2 | 14.79 ± 0.30 abcA | 1.31 ± 0.05 cdA | 2.80 ± 0.25 bA | 68.47 ± 0.68 eB | 11.73 ± 0.59 bA | 0.46 ± 0.01 efB | 0.44 ± 0.01 aA |
| t3 | 14.83 ± 0.28 abcA | 1.28 ± 0.02 cdA | 2.86 ± 0.35 abA | 68.06 ± 0.45 gB | 12.02 ± 0.90 bA | 0.46 ± 0.01 bB | 0.48 ± 0.03 aA |
| Ton_C | Toff_C | Range_C | ΔH_C | Tp1_C | Tp2_C | ||
|---|---|---|---|---|---|---|---|
| TOR | t1 | −11.08 ± 0.51 bcdA | −43.59 ± 0.49 aA | 32.51 ± 0.09 gA | 67.73 ± 1.64 aA | −35.78 ± 0.62 abA | −13.75 ± 0.17 bcA |
| t2 | −12.03 ± 0.33 eAB | −43.77 ± 0.67 aA | 31.74 ± 1.00 fA | 64.45 ± 3.10 abA | −35.09 ± 0.86 abA | −14.99 ± 0.57 cdeB | |
| t3 | −12.82 ± 0.34 efB | −43.47 ± 0.59 aA | 30.65 ± 0.82 gA | 67.43 ± 2.63 abA | −34.60 ± 0.10 abA | −15.55 ± 0.61 efB | |
| DR | t1 | −12.04 ± 0.37 deA | −46.79 ± 0.82 bA | 34.75 ± 0.44 fgA | 67.12 ± 1.96 aAB | −36.46 ± 0.21 bcA | −14.96 ± 0.67 dcA |
| t2 | −12.84 ± 0.28 fA | −47.46 ± 0.45 bcdeA | 34.62 ± 0.73 deA | 65.71 ± 1.02 abB | −36.87 ± 0.37 cdeA | −16.27 ± 0.09 efB | |
| t3 | −12.60 ± 0.41 deA | −46.34 ± 0.99 bA | 33.74 ± 1.26 fA | 70.45 ± 2.40 aA | −35.56 ± 0.93 bA | −16.90 ± 0.19 gB | |
| GEN | t1 | −11.39 ± 0.43 bcdA | −47.48 ± 1.98 bcA | 36.09 ± 1.92 efA | 64.05 ± 3.20 abcA | −40.19 ± 0.20 fB | −14.00 ± 0.48 bcA |
| t2 | −12.12 ± 0.33 efA | −49.36 ± 0.34 defA | 37.24 ± 0.60 cdA | 63.16 ± 1.53 abA | −40.62 ± 0.55 iB | −14.70 ± 0.88 cdeA | |
| t3 | −11.44 ± 0.56 cA | −48.49 ± 0.88 cdeA | 37.05 ± 0.56 cdeA | 67.04 ± 2.54 abcA | −38.98 ± 0.35 cdA | −13.83 ± 0.37 bcA | |
| SIV | t1 | −11.25 ± 0.05 bcdAB | −51.97 ± 0.20 efA | 40.72 ± 0.22 abC | 61.80 ± 1.85 cA | −42.15 ± 0.13 gA | −14.13 ± 0.44 bcA |
| t2 | −11.00 ± 0.18 bcdA | −52.70 ± 0.20 ghB | 41.70 ± 0.16 aB | 61.09 ± 2.91 bA | −43.11 ± 0.24 jB | −14.49 ± 0.53 cdeA | |
| t3 | −11.33 ± 0.12 cB | −53.53 ± 0.03 fC | 42.21 ± 0.14 aA | 61.49 ± 2.82 cA | −43.27 ± 0.10 eB | −14.93 ± 0.36 deA | |
| SEM | t1 | −10.87 ± 0.08 bcdA | −50.29 ± 0.18 deA | 39.43 ± 0.26 bcdA | 65.02 ± 0.13 abcA | −39.23 ± 0.13 efA | −15.09 ± 0.04 cdA |
| t2 | −11.42 ± 0.38 deA | −49.82 ± 0.60 efA | 38.40 ± 0.49 bcA | 63.48 ± 1.38 abA | −39.61 ± 0.16 hiB | −15.18 ± 0.32 deA | |
| t3 | −11.71 ± 0.49 cA | −49.62 ± 0.51 eA | 37.91 ± 0.92 cdA | 63.73 ± 2.89 bcA | −40.32 ± 0.11 dC | −15.40 ± 0.18 eA | |
| COR | t1 | −9.72 ± 0.06 abA | −52.43 ± 0.39 fA | 42.71 ± 0.45 aA | 65.00 ± 1.47 abcA | −42.44 ± 0.15 gA | −13.25 ± 0.28 bA |
| t2 | −10.26 ± 0.34 bB | −52.84 ± 0.89 ghA | 42.58 ± 1.23 aA | 62.96 ± 0.69 abB | −42.59 ± 0.02 jAB | −13.32 ± 0.38 bcA | |
| t3 | −10.41 ± 0.32 bB | −52.58 ± 0.90 fA | 42.20 ± 0.99 aA | 63.80 ± 0.71 bcAB | −42.70 ± 0.19 eB | −14.25 ± 0.31 bcdB | |
| CM | t1 | −13.59 ± 0.11 efB | −47.33 ± 0.21 bcA | 33.74 ± 0.31 fgA | 64.71 ± 1.02 abcA | −37.58 ± 0.03 cdB | −16.28 ± 0.14 deB |
| t2 | −12.85 ± 0.04 fA | −47.37 ± 0.59 bcdA | 34.53 ± 0.63 deA | 65.83 ± 1.20 abA | −35.79 ± 0.18 abcA | −15.12 ± 0.23 deA | |
| t3 | −13.69 ± 0.00 fB | −47.40 ± 0.39 bcA | 33.71 ± 0.39 fA | 64.51 ± 1.25 bcA | −35.51 ± 0.67 bA | −16.47 ± 0.43 fgB | |
| OR | t1 | −14.22 ± 1.90 fA | −47.11 ± 0.10 bcA | 32.89 ± 1.80 gB | 63.41 ± 0.05 abcB | −38.77 ± 0.95 deB | −17.23 ± 1.61 eA |
| t2 | −13.70 ± 0.15 gA | −47.20 ± 0.01 bcdA | 33.50 ± 0.14 efAB | 67.17 ± 0.41 aA | −37.55 ± 0.55 defB | −17.25 ± 0.38 fA | |
| t3 | −11.88 ± 0.22 cdA | −47.63 ± 0.05 bcdB | 35.75 ± 0.26 defA | 67.31 ± 1.02 abA | −34.21 ± 0.66 abA | −15.04 ± 0.27 deA | |
| BAM | t1 | −11.57 ± 0.15 cdA | −44.41 ± 0.53 aA | 32.84 ± 0.45 gB | 66.79 ± 1.66 abA | −34.83 ± 0.23 aA | −13.30 ± 0.24 bA |
| t2 | −11.05 ± 0.20 cdA | −45.61 ± 1.26 abAB | 34.56 ± 1.27 deAB | 65.47 ± 2.25 abA | −36.17 ± 0.66 bcdB | −13.42 ± 0.45 bcdA | |
| t3 | −11.36 ± 0.27 cA | −46.93 ± 0.47 bcB | 35.57 ± 0.73 efA | 66.02 ± 2.04 abcA | −37.64 ± 0.01 cC | −13.90 ± 0.27 bcA | |
| OTT | t1 | −8.31 ± 0.28 aA | −48.94 ± 0.55 dA | 40.63 ± 0.83 abA | 62.655 ± 0.63 bcA | −38.66 ± 0.61 deA | −9.69 ± 0.05 aA |
| t2 | −8.81 ± 0.10 aAB | −49.04 ± 0.27 cdefA | 40.22 ± 0.33 abA | 63.64 ± 1.00 abA | −39.30 ± 0.87 ghiA | −10.33 ± 0.26 aAB | |
| t3 | −9.10 ± 0.34 aA | −49.33 ± 0.37 deA | 40.23 ± 0.71 abA | 63.72 ± 1.66 bcA | −38.62 ± 0.25 cA | −10.63 ± 0.40 aB | |
| OTTC | t1 | −10.51 ± 0.34 bcdA | −48.52 ± 0.43 bcdA | 38.01 ± 0.33 cdeA | 64.40 ± 1.20 abcA | −37.97 ± 0.10 deA | −12.99 ± 0.13 bA |
| t2 | −10.57 ± 0.51 bcA | −50.86 ± 1.87 fgA | 40.29 ± 2.03 abA | 62.44 ± 2.86 abA | −38.47 ± 0.29 fghA | −12.67 ± 0.61 bA | |
| t3 | −11.30 ± 0.09 bcA | −50.20 ± 0.04 eA | 38.90 ± 0.05 bcA | 65.29 ± 1.68 abcA | −37.62 ± 0.87 cA | −13.65 ± 0.27 bB | |
| TDF | t1 | −9.79 ± 0.47 abcA | −50.15 ± 0.49 deA | 40.36 ± 0.42 abcB | 64.11 ± 1.18 abcA | −37.89 ± 0.58 dA | −12.81 ± 0.30 bA |
| t2 | −11.09 ± 0.17 cdB | −53.52 ± 1.63 hB | 42.43 ± 1.80 aA | 63.85 ± 1.23 abA | −37.83 ± 0.23 efgA | −13.28 ± 0.37 bcA | |
| t3 | −11.78 ± 0.17 cdB | −49.81 ± 0.24 eA | 38.03 ± 0.07 cC | 64.26 ± 0.16 bcA | −37.89 ± 0.25 cA | −14.20 ± 0.18 bcdB | |
| CIC | t1 | −10.12 ± 0.26 bcA | −47.34 ± 0.34 bcA | 37.23 ± 0.60 deA | 66.81 ± 1.33 abA | −35.23 ± 0.86 abA | −13.03 ± 0.21 bA |
| t2 | −11.13 ± 0.08 cdB | −46.90 ± 0.80 bcA | 35.77 ± 0.88 cdeA | 67.26 ± 1.73 aA | −34.55 ± 1.34 aA | −13.83 ± 0.12 bcdB | |
| t3 | −11.75 ± 0.33 cdC | −47.12 ± 1.76 bcA | 35.37 ± 2.09 efA | 67.63 ± 1.57 abA | −33.28 ± 1.38 aA | −14.63 ± 0.04 cdeC |
| Ton_H | Toff_H | Range_H | ΔH_H | Tp1_H | Tp2_H | Tp3_H | ||
|---|---|---|---|---|---|---|---|---|
| TOR | t1 | −37.29 ± 0.31 gA | 13.36 ± 0.05 bcdA | 50.65 ± 0.36 aA | 69.91 ± 0.53 abcdA | −16.06 ± 0.04 aA | −4.85 ± 0.03 abB | 8.59 ± 0.12 cdefA |
| t2 | −36.96 ± 0.15 fA | 12.94 ± 0.34 bcdAB | 49.90 ± 0.39 aAB | 66.29 ± 2.47 deA | −17.90 ± 1.55 bcAB | −4.71 ± 0.14 aB | 8.15 ± 0.05 cdB | |
| t3 | −37.02 ± 0.24 fA | 12.47 ± 0.05 cdB | 49.49 ± 0.28 aB | 70.79 ± 2.58 abcA | −19.27 ± 0.78 cdeB | −4.46 ± 0.10 aA | 7.69 ± 0.02 deC | |
| DR | t1 | −36.98 ± 0.45 gA | 13.59 ± 0.37 abcdA | 50.57 ± 0.39 aA | 68.83 ± 1.86 abcdA | −20.82 ± 0.32 fgA | −6.80 ± 0.50 efgA | 9.29 ± 0.44 bcA |
| t2 | −36.63 ± 0.34 efA | 12.77 ± 0.63 cdeA | 49.40 ± 0.97 aA | 66.70 ± 0.87 deA | −20.49 ± 1.44 fgA | −7.31 ± 0.64 deA | 8.21 ± 0.92 cdA | |
| t3 | −36.30 ± 1.09 fA | 13.00 ± 0.25 abcA | 49.30 ± 1.22 aA | 71.47 ± 3.26 abA | −20.45 ± 0.16 defA | −5.75 ± 0.87 bcdA | 8.40 ± 0.28 bcA | |
| GEN | t1 | −31.47 ± 0.32 eB | 14.10 ± 0.26 abA | 45.56 ± 0.27 bA | 66.95 ± 2.05 deB | −19.19 ± 0.12 deA | −8.07 ± 0.26 iB | 9.79 ± 0.22 abA |
| t2 | −30.58 ± 0.09 dA | 13.53 ± 0.28 abAB | 44.11 ± 0.36 bB | 68.28 ± 0.68 bcdeAB | −19.89 ± 0.35 efA | −8.28 ± 0.60 eB | 9.46 ± 0.12 abA | |
| t3 | −31.03 ± 0.28 dAB | 13.14 ± 0.26 abB | 44.17 ± 0.54 bB | 72.03 ± 2.38 abA | −19.28 ± 1.15 cdeA | −7.09 ± 0.10 eA | 8.67 ± 0.07 bB | |
| SIV | t1 | −29.86 ± 0.11 dA | 11.89 ± 0.06 fA | 41.75 ± 0.16 cA | 64.64 ± 1.96 eA | −21.35 ± 0.05 gA | −7.70 ± 0.33 hiA | 7.65 ± 0.03 gA |
| t2 | −29.27 ± 0.19 cA | 11.17 ± 0.27 gB | 40.44 ± 0.43 cB | 64.76 ± 2.09 eA | −21.77 ± 0.04 gB | −7.57 ± 0.66 eA | 7.28 ± 0.14 efB | |
| t3 | −29.57 ± 0.43 cdA | 11.67 ± 0.19 efA | 41.24 ± 0.53 cAB | 65.52 ± 2.53 cA | −21.84 ± 0.06 fB | −7.03 ± 0.21 eA | 7.24 ± 0.08 eB | |
| SEM | t1 | −28.45 ± 0.57 cA | 12.45 ± 0.09 efA | 40.90 ± 0.47 cdAB | 67.84 ± 0.88 bcdeA | −19.35 ± 0.08 deA | −5.02 ± 0.03 abA | 7.83 ± 0.01 efgA |
| t2 | −29.26 ± 0.33 cA | 12.36 ± 0.11 deA | 41.62 ± 0.41 cA | 66.63 ± 1.50 deA | −19.61 ± 0.02 defB | −5.50 ± 0.34 abAB | 7.65 ± 0.09 deB | |
| t3 | −29.00 ± 0.24 bcA | 11.54 ± 0.17 fB | 40.54 ± 0.08 cB | 67.41 ± 3.18 bcA | −19.80 ± 0.04 cdefC | −5.65 ± 0.15 bcB | 7.23 ± 0.08 eC | |
| COR | t1 | −28.66 ± 0.18 cA | 12.34 ± 0.30 efA | 41.00 ± 0.48 cdA | 66.42 ± 0.38 deA | −20.85 ± 0.08 fgA | −6.42 ± 0.10 defA | 8.36 ± 0.17 defgA |
| t2 | −29.11 ± 0.36 cA | 12.02 ± 0.28 efA | 41.12 ± 0.64 cA | 66.08 ± 0.42 deA | −21.10 ± 0.01 fgB | −7.30 ± 0.08 deB | 7.93 ± 0.08 deB | |
| t3 | −28.90 ± 0.42 bcA | 12.23 ± 0.34 deA | 41.13 ± 0.56 cA | 65.10 ± 0.45 cB | −21.13 ± 0.10 efB | −6.61 ± 0.12 deA | 7.68 ± 0.15 deB | |
| CM | t1 | −26.46 ± 0.59 aA | 12.45 ± 0.18 efA | 38.91 ± 0.77 fA | 70.83 ± 0.60 abA | −17.46 ± 0.54 bA | −6.76 ± 0.14 efgB | 7.74 ± 0.04 fgA |
| t2 | −26.48 ± 0.56 aA | 11.47 ± 0.25 fgB | 37.95 ± 0.30 dB | 71.78 ± 1.67 abcA | −16.18 ± 0.10 aB | −5.55 ± 0.13 abA | 6.61 ± 0.24 fB | |
| t3 | −27.08 ± 0.45 aA | 10.74 ± 0.04 gC | 37.82 ± 0.40 eB | 71.47 ± 1.69 abA | −16.38 ± 0.28 abA | −5.43 ± 0.04 bA | 5.99 ± 0.01 fC | |
| OR | t1 | −26.40 ± 0.06 aAB | 10.62 ± 0.94 gB | 37.01 ± 1.00 gAB | 70.74 ± 0.01 abcC | −19.03 ± 1.32 cdeA | −7.20 ± 0.65 ghB | 6.03 ± 1.34 hA |
| t2 | −26.08 ± 0.16 aA | 10.01 ± 0.06 hB | 36.09 ± 0.10 eB | 75.57 ± 0.62 aA | −17.48 ± 0.38 abcA | −6.57 ± 0.33 cdB | 5.23 ± 0.11 gA | |
| t3 | −26.91 ± 0.62 aB | 11.18 ± 0.28 fgA | 38.09 ± 0.91 deA | 73.43 ± 1.85 aB | −18.01 ± 2.68 bcdA | −4.92 ± 0.57 abA | 6.34 ± 0.06 fA | |
| BAM | t1 | −35.25 ± 0.34 fA | 14.25 ± 0.24 aA | 49.50 ± 0.57 aA | 69.91 ± 0.78 abcdA | −19.57 ± 0.76 efA | −5.93 ± 0.11 cdA | 10.61 ± 0.05 aA |
| t2 | −35.52 ± 0.98 eA | 13.74 ± 0.00 aAB | 49.26 ± 0.98 aA | 67.15 ± 1.85 cdeA | −20.72 ± 0.11 fgA | −6.19 ± 0.14 bcA | 9.72 ± 0.11 aB | |
| t3 | −34.26 ± 1.78 eA | 13.29 ± 0.61 abB | 47.55 ± 2.39 aA | 67.87 ± 0.42 abcA | −18.80 ± 1.80 bcdeA | −6.50 ± 0.52 cdeA | 8.73 ± 0.66 bC | |
| OTT | t1 | −27.82 ± 0.04 bcA | 13.51 ± 0.33 abcdA | 41.33 ± 0.37 cdA | 67.23 ± 0.07 cdeA | −18.23 ± 0.37 bcdA | −5.56 ± 0.33 bcA | 8.98 ± 0.01 bcdA |
| t2 | −27.37 ± 0.22 abA | 12.89 ± 0.28 bcdB | 40.26 ± 0.23 cAB | 69.14 ± 1.76 bcdeA | −18.66 ± 0.38 cdeA | −6.28 ± 0.26 bcB | 8.21 ± 0.22 cdB | |
| t3 | −27.09 ± 0.79 aA | 12.69 ± 0.29 bcdB | 39.78 ± 1.08 cdeB | 67.25 ± 1.51 bcA | −18.38 ± 0.01 bcdA | −6.54 ± 0.22 cdeB | 7.98 ± 0.25 cdB | |
| OTTC | t1 | −27.85 ± 0.10 bcAB | 13.67 ± 0.10 abcA | 41.52 ± 0.20 cdA | 68.39 ± 0.99 abcdA | −17.84 ± 0.24 bcA | −7.03 ± 0.08 fghA | 9.48 ± 0.10 bcA |
| t2 | −28.05 ± 0.09 bcB | 13.40 ± 0.34 abcA | 41.45 ± 0.43 cA | 66.52 ± 3.10 deA | −17.90 ± 0.44 bcA | −7.71 ± 0.37 eB | 8.79 ± 0.30 bcB | |
| t3 | −27.71 ± 0.21 abA | 13.35 ± 0.28 aA | 41.06 ± 0.49 cA | 71.42 ± 2.34 abA | −19.56 ± 1.75 cdefA | −8.16 ± 0.35 fB | 9.32 ± 0.33 aAB | |
| TDF | t1 | −27.32 ± 0.43 abA | 12.99 ± 0.26 cdeA | 40.30 ± 0.64 deA | 68.17 ± 0.62 bcdeA | −18.28 ± 0.61 bcdA | −6.16 ± 0.41 cdeA | 8.72 ± 0.10 cdeA |
| t2 | −28.02 ± 0.64 bcA | 12.61 ± 0.29 deAB | 40.62 ± 0.35 cA | 69.69 ± 1.46 bcdA | −18.17 ± 0.13 bcdA | −7.36 ± 0.01 deB | 7.57 ± 0.25 deB | |
| t3 | −27.83 ± 0.74 abcA | 12.26 ± 0.05 deB | 40.08 ± 0.79 cdA | 69.45 ± 0.69 abcA | −17.78 ± 0.15 abcA | −6.60 ± 0.12 cdeA | 7.27 ± 0.13 eB | |
| CIC | t1 | −26.54 ± 0.67 aA | 12.89 ± 0.15 deA | 39.42 ± 0.52 efA | 71.80 ± 2.06 aA | −15.70 ± 0.74 aA | −4.75 ± 0.08 aA | 8.10 ± 0.00 defgA |
| t2 | −28.35 ± 1.27 bcB | 12.52 ± 0.39 deAB | 40.87 ± 1.66 cA | 72.70 ± 2.15 abA | −16.47 ± 0.78 abA | −5.31 ± 0.04 abB | 7.93 ± 0.20 deA | |
| t3 | −27.32 ± 0.83 abAB | 12.38 ± 0.19 cdB | 39.69 ± 0.64 cdeA | 73.35 ± 1.83 aA | −15.54 ± 0.53 aA | −4.38 ± 0.46 aA | 7.37 ± 0.01 eB |
| L | a* | b* | ||
|---|---|---|---|---|
| t1 | 53.67 ± 0.58 Bc | −7.33 ± 0.58 Acd | 53.00 ± 3.46 Aab | |
| TOR | t2 | 55.00 ± 0.00 Acd | −7.00 ± 0.00 Ade | 46.00 ± 2.65 ABb |
| t3 | 55.33 ± 0.58 Ac | −7.00 ± 0.00 Ad | 42.33 ± 3.51 Bb | |
| t1 | 56.33 ± 0.58 Aa | −6.00 ± 0.00 Aa | 25.33 ± 3.21 Bd | |
| DR | t2 | 56.67 ± 0.58 Aab | −6.00 ± 0.00 Acd | 25.00 ± 0.00 Bc |
| t3 | 56.33 ± 0.58 Abc | −6.67 ± 0.58 Acd | 36.00 ± 5.20 Abc | |
| t1 | 55.67 ± 0.58 Aab | −7.00 ± 0.00 Abc | 43.33 ± 8.14 Aabc | |
| GEN | t2 | 55.00 ± 1.00 Acd | −7.67 ± 0.58 Ae | 43.67 ± 4.16 Ab |
| t3 | 56.00 ± 0.00 Abc | −7.33 ± 0.58 Ad | 44.33 ± 5.51 Ab | |
| t1 | 55.33 ± 0.58 Bab | −8.00 ± 0.00 Cd | 46.00 ± 0.00 Aabc | |
| SIV | t2 | 58.00 ± 0.00 Aa | −6.00 ± 0.58 Acd | 26.67 ± 0.58 Cc |
| t3 | 57.67 ± 0.58 Aa | −7.00 ± 0.58 Bd | 30.33 ± 0.58 Bc | |
| t1 | 46.33 ± 0.58 Be | −8.00 ± 0.58 Bd | 50.67 ± 0.58 Cabc | |
| SEM | t2 | 50.67 ± 0.58 Ag | −7.67 ± 0.58 ABe | 53.67 ± 0.58 Ba |
| t3 | 51.00 ± 0.00 Ae | −7.00 ± 0.00 Ad | 55.00 ± 0.00 Aa | |
| t1 | 55.00 ± 0.00 Babc | −8.00 ± 0.00 Bd | 56.00 ± 0.00 Aa | |
| COR | t2 | 56.00 ± 0.00 Abc | −8.00 ± 0.00 Be | 54.00 ± 0.00 Ba |
| t3 | 56.50 ± 0.50 Aabc | −7.00 ± 0.00 Ad | 44.00 ± 1.00 Cb | |
| t1 | 51.67 ± 0.58 Bd | −7.33 ± 0.58 Acd | 55.33 ± 1.15 Ba | |
| CM | t2 | 56.00 ± 0.00 Abc | −7.00 ± 0.00 Ade | 58.67 ± 0.58 Aa |
| t3 | 56.00 ± 0.00 Abc | −7.00 ± 0.00 Ad | 59.00 ± 0.00 Aa | |
| t1 | 47.00 ± 0.00 Ce | −9.00 ± 0.00 Be | 51.00 ± 0.00 Cabc | |
| OR | t2 | 52.67 ± 0.58 Af | −7.67 ± 0.58 Ae | 56.00 ± 0.00 Aa |
| t3 | 49.67 ± 0.58 Bf | −7.00 ± 0.00 Ad | 53.67 ± 0.58 Ba | |
| t1 | 47.33 ± 0.58 Ce | −9.00 ± 0.00 Be | 51.33 ± 0.58 Cabc | |
| BAM | t2 | 56.00 ± 0.00 Abc | −7.00 ± 0.00 Ade | 58.67 ± 0.58 Aa |
| t3 | 54.00 ± 0.00 Bd | −7.33 ± 0.58 Ad | 56.33 ± 0.58 Ba | |
| t1 | 55.00 ± 0.00 Babc | −6.33 ± 0.58 Bab | 39.67 ± 2.52 Abc | |
| OTT | t2 | 54.33 ± 0.58 Bde | −4.33 ± 0.58 Aab | 20.67 ± 2.52 Bcd |
| t3 | 56.50 ± 0.50 Aabc | −4.00 ± 0.00 Aab | 18.50 ± 2.50 Bd | |
| t1 | 55.00 ± 0.00 Babc | −6.33 ± 0.58 Bab | 38.67 ± 10.79 Acd | |
| OTTC | t2 | 55.00 ± 0.00 Bcd | −5.00 ± 0.00 Abc | 26.33 ± 3.21 ABc |
| t3 | 56.00 ± 0.00 Abc | −4.50 ± 0.50 Ab | 17.50 ± 0.50 Bd | |
| t1 | 54.67 ± 0.58 ABbc | −7.00 ± 0.00 Bbc | 43.00 ± 3.00 Aabc | |
| TDF | t2 | 53.33 ± 0.58 Bef | −3.33 ± 0.58 Aa | 15.67 ± 1.15 Bd |
| t3 | 55.67 ± 0.58 Abc | −3.33 ± 0.58 Aa | 15.67 ± 0.58 Bd | |
| t1 | 54.33 ± 1.15 Bbc | −7.00 ± 0.00 Bbc | 48.67 ± 7.77 Aabc | |
| CIC | t2 | 55.33 ± 0.58 ABbcd | −5.67 ± 0.58 Ac | 25.33 ± 4.62 Bc |
| t3 | 56.67 ± 0.58 Aab | −5.67 ± 0.58 Ac | 30.3 ± 35.13 Bc |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paciulli, M.; Difonzo, G.; Conte, P.; Flamminii, F.; Piscopo, A.; Chiavaro, E. Physical and Thermal Evaluation of Olive Oils from Minor Italian Cultivars. Foods 2021, 10, 1004. https://doi.org/10.3390/foods10051004
Paciulli M, Difonzo G, Conte P, Flamminii F, Piscopo A, Chiavaro E. Physical and Thermal Evaluation of Olive Oils from Minor Italian Cultivars. Foods. 2021; 10(5):1004. https://doi.org/10.3390/foods10051004
Chicago/Turabian StylePaciulli, Maria, Graziana Difonzo, Paola Conte, Federica Flamminii, Amalia Piscopo, and Emma Chiavaro. 2021. "Physical and Thermal Evaluation of Olive Oils from Minor Italian Cultivars" Foods 10, no. 5: 1004. https://doi.org/10.3390/foods10051004