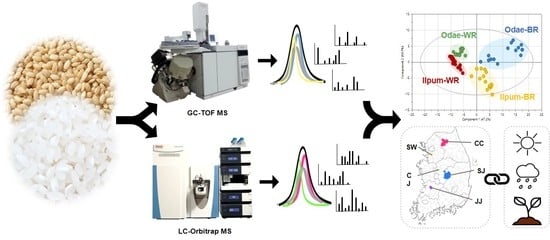
Unique Metabolic Profiles of Korean Rice According to Polishing Degree, Variety, and Geo-Environmental Factors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Metabolite Extraction
2.3. Gas Chromatography Time-of-Flight Mass Spectrometry Analysis
2.4. Liquid Chromatography-Orbitrap Mass Spectrometric Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Integrative Metabolic Profiles of Rice Seeds Based on GC-TOF MS and LC-Orbitrap MS
3.2. Unique Metabolic Signatures According to Variety Type (Odae vs. Ilpum)
3.3. Metabolic Differences Associated with Geoenvironmental Factors (Cultivation Region)
3.4. Odae Cultivar Characteristics Related to Cultivation Region
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization. Crops/Regions/World list/Production Quantity (pick lists), Rice (paddy), 2014. In Corporate Statistical Database (FAOSTAT); FAO: Rome, Italy, 2017. [Google Scholar]
- Lim, D.K.; Mo, C.; Lee, J.H.; Long, N.P.; Dong, Z.; Li, J.; Lim, J.; Kwon, S.W. The integration of multi-platform MS-based metabolomics and multivariate analysis for the geographical origin discrimination of Oryza sativa L. J. Food Drug Anal. 2018, 26, 769–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiehn, O. Metabolomics—The link between genotypes and phenotypes. In Functional Genomics; Springer: Berlin/Heidelberg, Germany, 2002; pp. 155–171. [Google Scholar]
- Lee, B.-J.; Zhou, Y.; Lee, J.S.; Shin, B.K.; Seo, J.-A.; Lee, D.; Kim, Y.-S.; Choi, H.-K. Discrimination and prediction of the origin of Chinese and Korean soybeans using Fourier transform infrared spectrometry (FT-IR) with multivariate statistical analysis. PLoS ONE 2018, 13, e0196315. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.M.; Park, S.J.; Lee, J.-E.; Lee, B.M.; Shin, B.K.; Kang, D.J.; Choi, H.-K.; Kim, Y.-S.; Lee, D.Y. Highly geographical specificity of metabolomic traits among Korean domestic soybeans (Glycine max). Food Res. Int. 2019, 120, 12–18. [Google Scholar] [CrossRef]
- Cho, H.-J.; Heo, K.; Umemoto, T.; Wang, M.-H. Physiological Properties of Two Japonica Rice (Oryza sativa L.) Cultivars: Odae and Ilpum. J. Appl. Biol. Chem 2007, 50, 127–131. [Google Scholar]
- Huo, Y.; Kamal, G.M.; Wang, J.; Liu, H.; Zhang, G.; Hu, Z.; Anwar, F.; Du, H. 1H NMR-based metabolomics for discrimination of rice from different geographical origins of China. J. Cereal Sci. 2017, 76, 243–252. [Google Scholar] [CrossRef]
- Hu, C.; Shi, J.; Quan, S.; Cui, B.; Kleessen, S.; Nikoloski, Z.; Tohge, T.; Alexander, D.; Guo, L.; Lin, H. Metabolic variation between japonica and indica rice cultivars as revealed by non-targeted metabolomics. Sci. Rep. 2014, 4, 5067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranjitha, H.P.; Gowda, R.; Nethra, N.; Amruta, N.; Kandikattu, H.K. Biochemical and metabolomics on rice cultivars. Rice Sci. 2019, 26, 189–194. [Google Scholar] [CrossRef]
- Zarei, I.; Luna, E.; Leach, J.E.; McClung, A.; Vilchez, S.; Koita, O.; Ryan, E.P. Comparative rice bran metabolomics across diverse cultivars and functional rice gene–bran metabolite relationships. Metabolites 2018, 8, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiehn, O.; Wohlgemuth, G.; Scholz, M.; Kind, T.; Lee, D.Y.; Lu, Y.; Moon, S.; Nikolau, B. Quality control for plant metabolomics: Reporting MSI-compliant studies. Plant J. 2008, 53, 691–704. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.M.; Lee, E.M.; Kang, D.J.; Seo, J.-A.; Choi, H.-K.; Kim, Y.-S.; Lee, D.Y. Discovery study of integrative metabolic profiles of sesame seeds cultivated in different countries. LWT 2020, 129, 109454. [Google Scholar] [CrossRef]
- Skogerson, K.; Wohlgemuth, G.; Barupal, D.K.; Fiehn, O. The volatile compound BinBase mass spectral database. BMC Bioinform. 2011, 12, 321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barupal, D.K.; Fiehn, O. Chemical Similarity Enrichment Analysis (ChemRICH) as alternative to biochemical pathway mapping for metabolomic datasets. Sci. Rep. 2017, 7, 14567. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Price, J.; Arze, C.; Ananthakrishnan, A.N.; Schirmer, M.; Avila-Pacheco, J.; Poon, T.W.; Andrews, E.; Ajami, N.J.; Bonham, K.S.; Brislawn, C.J. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 2019, 569, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for comprehensive and integrative metabolomics data analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Wang, C.; Xu, J.; Wang, S. Cafestol and kahweol: A review on their bioactivities and pharmacological properties. Int. J. Mol. Sci. 2019, 20, 4238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleh, A.S.; Wang, P.; Wang, N.; Yang, L.; Xiao, Z. Brown rice versus white rice: Nutritional quality, potential health benefits, development of food products, and preservation technologies. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1070–1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Chan, L.; Zhou, S. Trigonelline: A plant alkaloid with therapeutic potential for diabetes and central nervous system disease. Curr. Med. Chem. 2012, 19, 3523–3531. [Google Scholar] [CrossRef] [PubMed]
- Blasco, H.; Błaszczyński, J.; Billaut, J.-C.; Nadal-Desbarats, L.; Pradat, P.-F.; Devos, D.; Moreau, C.; Andres, C.R.; Emond, P.; Corcia, P. Comparative analysis of targeted metabolomics: Dominance-based rough set approach versus orthogonal partial least square-discriminant analysis. J. Biomed. Inform. 2015, 53, 291–299. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, Y.; Lee, B.M.; Lee, E.M.; Kim, C.-H.; Seo, J.-A.; Choi, H.-K.; Kim, Y.-S.; Lee, D.Y. Unique Metabolic Profiles of Korean Rice According to Polishing Degree, Variety, and Geo-Environmental Factors. Foods 2021, 10, 711. https://doi.org/10.3390/foods10040711
Kang Y, Lee BM, Lee EM, Kim C-H, Seo J-A, Choi H-K, Kim Y-S, Lee DY. Unique Metabolic Profiles of Korean Rice According to Polishing Degree, Variety, and Geo-Environmental Factors. Foods. 2021; 10(4):711. https://doi.org/10.3390/foods10040711
Chicago/Turabian StyleKang, Yujin, Bo Mi Lee, Eun Mi Lee, Chang-Ho Kim, Jeong-Ah Seo, Hyung-Kyoon Choi, Young-Suk Kim, and Do Yup Lee. 2021. "Unique Metabolic Profiles of Korean Rice According to Polishing Degree, Variety, and Geo-Environmental Factors" Foods 10, no. 4: 711. https://doi.org/10.3390/foods10040711