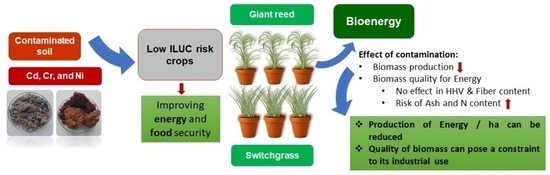
3.2. Effects of Cadmium, Chromium, and Nickel on the Biomass Productivity of Giant Read and Switchgrass
The yield performance of switchgrass grown in Cd and Ni contaminated soils, in two consecutive years, is represented in
Figure 1. Results showed that the productivity of switchgrass biomass increased from the first to the second growing cycle. This increase can be explained by the development of the root system that needs to expand in the first growing cycle, reducing the energy of the plant to produce aerial biomass [
35]. Regarding the contaminated trials, the biomass productivity was inversely proportional to the concentration of heavy metals. This decrease was noticed for both Cd and Ni contamination levels in the aerial biomass in the first year. In the second harvest, the yields in Cd and Ni contaminated soils with low level of metal contamination were not significantly affected, but the higher contamination levels caused a reduction in biomass productivity, either in the aerial and in the belowground biomass, and this reduction was significant in the Ni
220 soils.
In contrast to the behavior of switchgrass in Ni- and Cd- contaminated soils, Cr contamination, either the low or the high concentration (Cr300, Cr600, 300 and 600 mg Cr kg−1 dry matter), inhibited the growth of switchgrass. In the first year of the experiments, the seeds germinated but the toxicity of the soils did not allow the development of the seedlings. In the second year, sowing was repeated, and the same problem occurred again with the seedlings.
Figure 2 shows the aerial productivity of giant reed in Cd-, Ni-, and Cr-contaminated soils, in the first two growing cycles. In the first year, the yield was not affected by the heavy metals at their lowest concentration except in the case of chromium. As the concentration of Cr, Cr, and Ni increases, a significant decrease in biomass yield was noticed. Lowest yields were obtained in Ni
220 and Cr
600 soils. The yield of control and Ni contamination trials in the second year did not significantly differ from the yield in the first year. Contrarily, the productivity in Cd- and Cr-contaminated soils lowered from the first to the second year, for both levels of contamination. This behavior was opposite to what was seen by Fernando and collaborators in Cr-, Pb-, and Zn-contaminated soils [
68]. In the mentioned study, from the first to the second year, the yields increased, on average, by 150%. This pattern is typical from perennial crops, showing that higher energy is used by the plant in the first year to establish its belowground biomass [
33,
35], as it was seen with switchgrass. In the second harvest, all the metals–Cd, Cr and Ni–caused a reduction in the yields, which was only not significant in the case of the low contamination level of Ni. Like the first year results, as the concentration of Cd and Ni increases, a significant decrease in biomass yield was obtained. In the case of Cr contaminated soils, the yields between low and high level were similar. For the second harvest, lowest yields were observed in Cd
8, Ni
220, Cr
300 and Cr
600 soils.
The belowground productivity of giant reed is presented in
Figure 3. Results show that the contamination did not interfere with belowground biomass productivity, although some reduction was observed in Cd
8 (significant), Ni
220, and Cr
300 soils (not significant).
A comparison between giant reed yields and switchgrass yields, for the same type of soils, showed that both crops, in terms of aerial biomass, presented similar results in control, Cd-, and Ni-contaminated soils (p > 0.05). However, in Cr-contaminated soils, seeds of switchgrass did not germinate and no aerial biomass was made viable, in contrast with the development of giant reed (p < 0.05). When comparing the belowground biomass production, it was observed that giant reed presented always significantly higher yields to switchgrass for control and Cd-, Cr-, and Ni-contaminated pots at both contamination levels.
The tolerance index (TI) indicates the influence of the contaminant on the plant’s growth, and data obtained for switchgrass and giant reed (second year of experiments) are depicted in
Table 3.
Results of TI can help to classify the degree of tolerance of a plant to a metal/type of contamination. The TI can be organized in levels, such as the following ones, high tolerance, TI > 0.75; moderate tolerance: 0.50 < TI < 0.75; low tolerance: 0.25 < TI < 0.50; critical tolerance: TI < 0.25.
Concerning switchgrass, results indicate that, for Ni- and Cd-contaminated soils, only the exposure of the plant to the higher contamination levels resulted in a reduction in biomass production. This reduction was in the range of 50–60% in the aboveground fraction and in the range of 20–40% in the belowground fraction, and was higher (but not significantly) in Ni220-contaminated pots. Therefore, for the low contamination levels, switchgrass showed high tolerance to Cd and Ni; for the high contamination level, switchgrass showed low tolerance to Ni and moderate tolerance to Cd, regarding the production of aboveground biomass, and moderate tolerance to Ni and high tolerance to Cd in terms of the belowground biomass production. Concerning the effect of Cr contamination, results indicate that the crop presented a critical tolerance to this metal once no development of the seedlings was observed.
Arora and collaborators [
45] also observed that higher levels of Cd in the soil negatively affected switchgrass productivity. Studying concentrations of 2 to 10 mg.kg
−1 of Cd contamination in pots for 20 weeks, it was observed that the rise in Cd concentration reduces switchgrass’ yield (almost 65% reduction at the highest contamination level). The work of Arora et al. also showed that plant-associated microbes, such as Azospirillum and arbuscular mycorrhizae fungi, can increase the tolerance of switchgrass when it is exposed to Cd contamination [
45]. Chen et al. [
69] used a higher range of Cd concentrations (0-60 mg.kg
−1), leading to a drastic reduction in grass productivity (63% losses at the higher contamination level). In this study, the analysis of the soil’s fractions indicated that despite the Cd concentration in soil being high, the fraction mobilized was low, and therefore the plant’s survival was possible at 60 mg.kg
−1. The toxicity of Cd to switchgrass was also evaluated in a hydroponic experiment using different concentrations of Cd in solution [
70]. In the study, although the seeds’ germination occurred without showing any impact from the contamination, the plants’ development was better in Cd concentrations lower than 20 mg.L
−1. To date, experiments of switchgrass in Ni-contaminated soils were not found, but the behavior was similar to what was observed with Cd, although the patterns of toxicity of Cd and Ni in the plants are different [
67]. The total loss observed in the Cr-contaminated pots can be justified by the partial contamination with Cr (VI) [
71]. Indeed, regarding the oxidation state of the metal, the hexavalent Cr has a higher mobility in the soil, affecting the biomass growth and justifying the losses observed. Li et al. [
46] also tested a similar range of Cr contamination (131 to 600 mg.kg
−1) using Cr (VI) during three months. Results obtained showed that despite the development of switchgrass in the lower contamination levels, when the concentration was 600 mg.kg
−1, aerial biomass productivity losses were around 70%. Belowground biomass also suffered a reduction of around 50%. However, no information was added into the study as to the amount of bioavailable Cr so that a better comparison could be carried out with the results presented in
Table 3. Moreover, the higher organic matter of the soil (7.39%) can also explain the higher tolerance of switchgrass to Cr reported by Li and collaborators [
46] in their work, compared to the critical tolerance presented in
Table 3, once organic matter can trap the Cr in the soil and reduce its toxicity [
72].
Concerning giant reed, in terms of the aboveground biomass production, results indicate that, for low contamination levels, the plant showed a high tolerance to Ni, moderate tolerance to Cd, and low tolerance to Cr, and for the high contamination level showed a low tolerance to Cd and Cr and moderate tolerance to Ni. In terms of giant reed belowground biomass, the plant exhibited high tolerance to all the metals and levels, except to the high level of Cd, where the tolerance exhibited was moderate.
Results observed for Ni are aligned with the results presented by Papazoglou et al. [
43] that grew giant reed in soils irrigated with Ni-contaminated water in a two year experiment. However, results observed for Cd are different from the results also presented by Papazoglou et al. [
43] in soils irrigated with Cd-contaminated water during the same two-year experiment. Using a solution containing the metals in varying concentrations (0, 5, 50, and 100 mg.L
−1 of Ni and Cd in solution) to irrigate the pots, the total content of the metals in the soil was, at the end of the first year, 13.5 mg Cd kg
−1 and 68.5 mg Ni kg
−1, and 973.8 mg Cd kg
−1 and 2543.3 mg Ni kg
−1 at the end of the second year. It was observed that the biomass yield suffered no significant changes for both heavy metals. However, the authors found that the heavy metals remained in the topsoil, reducing the plant’s absorption. Atma et al. [
41] also studied giant reed exposed to Ni contamination. For 35 days, the authors irrigated pots containing giant reed shoots with a solution containing 10, 50, and 100 mg Ni L
−1. It was notice that the high-level nickel irrigation affected the productivity of giant reed, as observed in the present study. Sabeen et al. [
73] designed a pot experiment contaminating soils with a solution containing different levels of a Cd salt solution: 0, 50, 100, 250, 500, 750, and 1000 µg.L
–1. The study did not find significant differences in the plants’ productivity but noticed a reduction in the tillers’ number as the Cd level increased. Shaheen et al. [
74] conducted a 30-day hydroponic experiment to test Cd’s influence in giant reed growth, at 0, 25, 50, 75, and 100 mg Cd L
–1. The experiment confirmed the harmful impact of Cd in giant reed productivity, showing that the highest concentration of Cd contamination caused a decrease in biomass productivity of around 20%. However, it is difficult to make comparisons to these studies once there is no information on the amount of bioavailable Cd in the soil.
In terms of Cr contamination, Barbosa et al. [
40] conducted a similar study with giant reed, testing 300 and 600 mg.kg
−1 total Cr during two growing cycles; the bioavailable fractions were similar to the ones presented in the current study. However, Barbosa et al. [
40] observed a reduction of only 30–40%, and in the present study, the biomass yield reduction was of 70%. As observed for switchgrass, the higher loss observed in this study, when compared with the study of Barbosa et al. [
40], can be justified by the partial contamination of the soil with Cr (VI) [
71].
According to Kabata-Pendias [
67], there is no evidence as to the role of Cr and Ni in a plant’s metabolism. Cr can affect the plant in different forms; it usually accumulates in the roots binding to cell walls. Its toxicity depends on its oxidation state, and in fact small concentrations of Cr in plants (1 or 2 mg.kg
−1) can be harmful towards the plants growth [
67]. Chromium can affect the plant in different ways, reducing or even inhibiting the seed’s germination and the growth of roots, stems, and leaves [
75]. The photosynthetic apparatus can be damaged, as can the belowground organs, which causes retarded growth of the plants [
67]. Nickel can be found in different oxidation states, however, the most toxic is Ni (II). Nickel is usually taken up by the roots and transported to the leaves and stems, where it can be stored [
76]. Despite some hyper-tolerant and hyperaccumulator plants, like Berkheya coddi, that can reach Ni levels of 18,000 mg.kg
−1 in its biomass, other Ni-sensitive plants, such as oats, can be affected by concentrations from 24–308 mg.kg
−1 of metal in their biomass [
67]. Nickel can lead to several hazardous effects to the plant (related with the plant’s morphology, physiology, and biochemistry) [
77]. In some cereals, excess Ni in the soil can be noticed by some plant characteristics, such as interveinal chlorosis affecting the new leaves, grey-green leaves affecting the aboveground biomass, and, additionally, brown and stunted roots in the belowground biomass [
67]. Regarding Cd, although some enzymes depend on this metal to have regular activity, high concentrations of Cd in the soil causes root damage and growth retardation, interfering with protein synthesis, nutrient absorption, and photosynthesis. The absorption of Cd can be carried out passively by roots or metabolically. For sensitive plants, Cd levels of around 5 mg.kg
−1 of Cd in biomass are already toxic, while concentrations around 20 mg.kg
−1 can be critical for the crop’s growth and development [
67].
The belowground apparatus showed higher tolerance than the aerial fraction of the plants, either with switchgrass and giant reed. This behavior could be associated with the plants defense mechanisms, making it possible to accumulate the metals in the roots vacuoles [
78], reducing the harmful effects of these metals, either in the belowground or in the aerial fraction. The development of tissue scarification and secondary sheath bundles, which can quench the metals, are mechanisms that can contribute to increase the tolerance of the plants [
79]. Still, limited information is known regarding the interactions between the rhizomes and roots of these perennials and the growing medium, and more studies linked with them might provide hints on how these crops can become more tolerant to soils contaminated with heavy metals. The ratio of aboveground/belowground biomass is informative and shows a reduction due to contamination. The reduction in the aboveground biomass in the contaminated pots is responsible for this decrease, but in the Ni
110 and Cr
600 pots in the giant reed essay, and in the Cd
4 and Ni
110 pots in the switchgrass essay, the reduction is also due to the increase in the belowground productivity. It could indicate a response of the crop to increase the plants’ tolerance capacity [
67] once the augmentation of the root apparatus leads to maintaining the aboveground biomass productivity. The ratio of leaves/stems in the second growth cycle of the giant reed essay did not suffer any significant changes due to the increase in Cd, Cr, and Ni contamination in the soils. This reflects the tolerance of this crop to the metals once the photosynthetic apparatus associated with leaves remained balanced with the stem’s biomass.
The decrease in giant reed’s productivity from the first to the second year regarding Cd and Cr contamination indicates that the accumulation and storage of the absorbed metals during first growth cycle could have damaged the belowground organs, reducing its capacity to regrowth normally in the second growing season. The situation was different in Ni trials. Since this heavy metal is usually translocated to aboveground biomass [
76], the remaining Ni in the belowground organs did not affect the regrowth on the second growing cycle.
In this way, it is possible to observe that Cr trials were the most affected by the heavy metal, suppressing switchgrass growth and reducing giant reed productivity by 70%. For Ni and Cd trials, it was observed that when the heavy metal’s concentration increases, the yield of both giant reed and switchgrass decreases. Regarding Cr contaminate trials, giant reed showed to be less sensitive to the contaminant’s presence than switchgrass in terms of biomass production. For Cd and Ni in both contamination levels, the aerial biomass productivity was similar for both crops. On the other hand, the root system of giant reed presented a higher yield in all studied trials.
3.3. Biomass Composition
Table 4 presents the switchgrass’s aerial part composition after the second growing cycle while
Table 5 shows giant reed’s biomass composition. Although the essay was conducted in pots, the differences among the biomass composition can indicate the effect of the contamination on the biomass quality for energy.
Concerning switchgrass, in terms of HHV and ash content, contamination did not affect the values. In Cd4 pots, the plants even presented a lower ash content compared with the control, and this reduction can be linked with the higher biomass production obtained, which may have induced a factor of dilution in the ash content. Despite the values of K, Ca, Mg, and Na remaining the same or lower, as observed in Cd4 pots, the N and P content increased in the contaminated soils. In the case of P, this increment was noticed with significance in the Ni110 fields. In addition, the biomass exposure to Cd increased the nitrogen levels in the plant aerials’ part by around 25% and 55% for Cd4 and Cd8, respectively, evidencing that the increase in Cd in the soil leads to an increase in N levels in the biomass. This relation was less sensitive for nickel, leading to a slight increase for the lower level, Ni110, but to an increase of 20% in the higher level, Ni220.
Regarding the results obtained with giant reed, it was observed that Cd and Cr contamination caused an increase in the ash content of giant reed leaves, but not Ni. In terms of stems, an increment on the ash content was observed in the Cr300 pots. In general, leaves showed to have a higher ash content compared to stems, and a higher amount of ash content contributed to a reduction in the HHV of the leaves compared with the stems. However, the contaminants did not affect the HHV in the leaves nor the stems. In terms of nitrogen, leaf content was not affected by the contamination, but the content in stems increased significantly due to the contamination. Phosphorus content also increased in the leaves due to Cd4, Cr600, and Ni220 contamination, and in the stems due to Cd4 contamination. The N and P contents in leaves were also, in average, higher than the stems’ concentration. In terms of the K and Ca content, a trend to a higher concentration in the contaminated pots was noticed, either in the leaves and/or in the stems. Magnesium content was not affected by the contamination, and the sodium content decreased due to the contamination in both leaves and stems. Leaves also presented higher Mg and Na than stems, but the K and Na content did not show a clear pattern between both fractions.
Biomass composition, e.g., ash and HHV, is essential in determining its potential utilization in thermochemical processes. The ash content and the HHV of switchgrass measured by Hu et al. [
80] showed that the conversion of switchgrass in biofuels and bioenergy could be an option. In general, neither giant reed nor switchgrass suffered any alterations in HHV when compared with the literature (in the range 16.4–21.9 MJ.kg
−1 for switchgrass [
81,
82] and in the range 17.2 and 19.0 MJ.kg
−1 for giant reed [
83,
84]. Understanding that switchgrass ash content and giant reed stems ash content were not affected by contamination (except in the Cr
300 pots of giant reed) is a promising result, and shows that for the tested contaminations, there will be no further load in ash residue when using contaminated biomass in thermochemical purposes. However, the increment of ash in the leaves due to contamination can make the use of those fractions not feasible. Ash values obtained for switchgrass and giant reed are also aligned with the ones presented in literature (for giant reed stems, in the range 4.8–7.4% [
33,
85], and 3.9–8.2% for switchgrass [
86,
87]), which is interesting, considering that the biomass obtained in this study is harvested from pots (so, presenting lower growth) and those expressed in literature are from field studies.
The increase in nitrogen levels witnessed in switchgrass and giant reed stems due to the heavy metals may represent an obstacle for the utilization of contaminated biomass in thermochemical processes. The gases generated in these processes are directly related to human and environmental problems [
88], and an increment in the emissions due to contamination may represent a limitation for their use in pyrolysis, gasification, and combustion plants [
89,
90]. However, despite the increase in switchgrass and giant reed stems’ N content, their biomass can be used in pyrolysis and combustion processes since the maximum N content in these processes should be 2.5% (pyrolysis) and up to 3% (domestic stoves or pellet burners for heat) or up to 15% (fixed bed combustion) [
89,
90]. For gasification, however, some processes–e.g., bubbling fluidized beds, dual fluidized beds–limit the N content of the feedstock to 1% [
89,
90], which will limit the use of giant reed stems harvested from contaminated soils (with the exception of giant reed from Cr
300 pots) and the switchgrass collected from Cd
8 pots. Processes such as circulating fluidized beds for CHP (combined heat and power, gas engine) and circulating fluidized beds for syngas production have a higher limit of 2% in N content, and those processes can be applied to the biomass harvested from contaminated soils that exceed the limit of 1% N. Giant reed N content observed in this experiment is in agreement with literature that reports values in the range from 0.3 to 1.5% [
83,
91,
92]. Only biomass stems from Cd pots showed a higher value than the range presented. This relation between Cd and N may be a consequence of the strong synergic interaction between these two elements, which is due to the formation of very stable complexes between proteins and Cd. In fact, Cd has high electronegativity values and can bond easily with sulfur present in proteins [
67]. Therefore, the presence of Cd in the soil, can stimulate the uptake and mobilization of N in the plant. For switchgrass, the values observed in this experiment are also in line with what is observed in literature, with values ranging from 0.35 to 0.88% [
86,
87]. Interestingly, the same relation between Cd and N was also observed with switchgrass in Cd-contaminated pots, where switchgrass biomass presented a higher N content than the values presented in literature and in control pots.
The contents in terms of P, K, Ca, Mg, Na, and other elements are also important to evaluate in the potential of the biomass to be used in thermochemical processes. Indeed, they affect fly ash emissions, deposit formation, and ash handling/utilization/disposal. The values for these elements varied among the different biomasses. For switchgrass, according to the work of Monti et al. [
93], the amount of those elements in terms of g.kg
−1 are in the following ranges, 0.25–0.77 (P), 1.50–3.56 (K), 1.10–8.20 (Ca), 1.02–2.71 (Mg), and 0.32–0.87 (Na). Compared to these results, the biomass that we collected from the pot essays presented, for K, Ca, Mg, and Na, higher values, and for P, similar values. For giant reed, the same work of Monti et al. [
93] reported the following contents of those elements in stems: 0.32 (P), 5.61 (K), 0.97 (Ca), 1.03 (Mg) and 0.13 (Na), in g.kg
−1. As mentioned for switchgrass, the contents reported in the stems of giant reed harvested from the current pot essay showed consistently higher values than those reported by Monti et al. in their study [
93]. The higher concentration of elements reported in the current work compared to the literature may be derived from the fact that the biomass was harvested from pots and not from field. Therefore, the biomass production is lower, and those macro-elements may be more concentrated. The differences observed may have also resulted from the soil type, the stage of growth, type of plant tissue, environment, cultural practices, etc. [
86,
93].
Considering the elements K and Na, a high amount in the biomass is linked to the damage of the pieces of equipment such as pipes and furnaces due to corrosion processes. Potassium and sodium also remain in the ash, decreasing its melt point and provoking its volatilization [
94]. This can damage the combustion chambers (through sintering or slag formation), provoking a decrease in its lifetime and compromising the availability of the plant [
95]. The results obtained in the study provide an indication that contamination did not affect the K content of switchgrass and giant reed stems (except for stems of giant reed obtained in Cr
600 pots). Regarding giant reed leaves, the trend of an increase in K content with contamination that was significant in Cd pots (all levels of contamination) and Cr
300 pots was observed. In terms of Na content, the contamination reduced the amount in giant reed stems and leaves; in switchgrass, the contents were similar to control.
Table 6 presents the alkali index calculated for both crops in control and contaminated pots.
To determine the propensity of a fuel to slagging and fouling, Tortosa Masiá and collaborators [
63] presented an alkali index (AI) that relates the amount of Na and K in the biomass per unit of energy with the probability of slagging and fouling formation through the thermochemical conversion of biomass. According to the authors, the AI can be classified in terms of indication for slagging and fouling: 0.17 < AI < 0.34—probable; AI > 0.34, slagging and fouling is virtually certain to occur. According to these, the values of the alkali index presented in
Table 6 indicate that biomass from pots, either giant reed and switchgrass, have a high probability to cause slagging and fouling once results are higher than 0.34–even the biomass from control pots. These results are in line with what is considered a problem when perennial grasses are exploited through thermochemical processes, especially due to the high potassium content present in those biomasses, which is significantly higher than what is observed in woody biomass. Vassilev et al. [
96] indicate a mean of 10.75 of K
2O (% weight to total ash) in wood and woody biomass and a mean of 26.65 of K
2O (% weight to total ash) in herbaceous and agricultural biomass. Both the occurrence of slagging and fouling are a challenge to thermochemical conversion of biomass since the ash particles melt and accumulate in the walls of furnaces and pipes, reducing the temperature inside the furnace and the oxidation of the fuel, increasing the emission of CO [
97,
98]. Both crops showed a lower fuel quality when compared to coal or pine chips that have AI values of 0.04 and 0.17, respectively [
63]. However, they have a similar AI index to olive residues (1.14) [
63]. This indicates that in order to proceed with the thermochemical valorization of these biomasses, some treatment must be applied, specially to reduce the fouling formation trend (linked with K and Na contents). Comparing the data obtained for control and contaminated pots, it is interesting not to report differences in AI for switchgrass. In the case of giant reed stems, AI increased due to contamination, but this increment was more problematic in Cr
600 pots.
The fiber content of the samples can be observed in
Table 7. The analysis of giant reed was only made for the stems and not for the leaves, considering that the stems will be the fraction to be valorized.
The results of fiber content indicate that for both giant reed and switchgrass, the hemicellulose content is the highest fraction, followed by cellulose and lignin. This trend in the fiber composition of biomass was extensively reported in literature for both giant reed and switchgrass. For giant reed, the literature reports cellulose content from 29–44%, hemicellulose from 13–36%, and lignin from 11–34% [
83,
84,
99,
100,
101]. For switchgrass, the literature reports cellulose content from 32–40%, hemicellulose from 19–32%, and lignin from 7–23% [
70,
71,
72,
73,
74,
75,
76,
77,
78,
79,
80,
81,
82,
102,
103]. The results from the current study are aligned with the data presented, but the cellulose content obtained in both crops was somehow lower than the values reported, and hemicellulose content reported in switchgrass was similar or higher than the values reported by literature. This difference can be attributed to the fact that the biomass of the current study was obtained in pots, and data from literature is of biomass harvested from fields. In pots, the growth of the biomass is limited by the size of the pots, and the processes of lignification and cellulose formation are delayed, which causes a higher production of hemicelluloses and lesser of cellulose. In giant reed, the cellulose and lignin content are higher than in switchgrass, however the hemicellulose content is lower. What is interesting to report is the fact that contaminated soils did not disturb the fiber content of switchgrass and giant reed stems (
p < 0.05), which represents an opportunity for the use of this biomass. The high value of fiber content indicates prospects for the valorization of those biomasses in biorefinery processes. All three fractions presented appealing values for their separation and transformation into bioproducts.
3.4. Heavy Metal Concentration in Switchgrass and Giant Reed
Table 8 presents the heavy metals content for the studied crops (giant reed and switchgrass) after the second growing cycle. Results indicate that, except for Ni in the giant reed leaves, the increment in heavy metal concentration increases the amount of element in each fraction of each crop. In terms of the distribution between aboveground biomass and belowground biomass, a higher Cd and Ni concentration was observed in the belowground fraction of switchgrass, but in terms of Cr (in control plants), a higher content was observed in the aboveground fraction.
In the case of giant read, a trend was observed in which the roots/rhizomes and leaves presented a higher heavy metal concentration (although in Cr-contaminated pots, the content of Cr in leaves is significantly lower than in rhizomes). Stems are the fraction of the plant that presented the lowest content of heavy metals (in Cd, differences to leaves and rhizomes are not significant). Between species, Cd concentration was higher in the aboveground fractions of giant reed and in the belowground fraction of switchgrass. In terms of Cr, the comparison can only be made with control plants, and results indicate that similar values can be found for giant reed and switchgrass. In terms of Ni, giant reed leaves present a higher concentration than switchgrass aboveground material, but the concentration of Ni in the stems of giant reed is similar to the content observed for aboveground switchgrass. The Ni concentration in the belowground of both crops is similar to the control and Ni110 level of contamination. However, at higher levels of contamination, giant reed presents a higher content of Ni.
The results presented followed the same pattern of other data presented in the literature. The levels of Cd in switchgrass biomass were studied by Reed et al. [
104] for different concentrations and pH, despite the studied concentrations being much higher than the ones used in this work (to a maximum of 200 mg Cd.kg
−1). An increase in Cd accumulation was observed led by the increase in the content in Cd in the soil when decreasing soil pH. The increment in Cd in the above- and belowground biomass of switchgrass, with the increment of Cd in the soil, was also reported in the current study. In the study of Reed et al., the accumulated Cd in roots (to a maximum of 900 mg.kg
−1) was also considerably higher than in the aerial part of the biomass for all treatments (reaching a maximum of 270 mg.kg
−1) [
104]. The same pattern was observed in the current study, where the belowground biomass reached 10.6 mg.kg
−1 and the aerial biomass reached only 1.33 mg.kg
−1. The study of Reed et al. showed much higher Cd content in the aerial and belowground biomasses due to the higher Cd content tested in the soil and also due to the extremely low pH of the soil (4.1)–very acidic, and thus promoting the mobilization of Cd in the soil [
104].
The concentration of Cd in giant reed was also a study theme for Sabeen et al. [
73]. The authors observed that Cd content increased in all fractions of the plant with the increment of Cd in soil, as it was observed in the current study. In the current study, no significant differences can be observed among fractions of the plant in terms of Cd concentration, particularly in the higher level of contamination; the same was observed in the work of Sabeen et al. [
73]. The concentration of Cd reached a maximum of 220 mg.kg
−1 in the belowground biomass, 190 mg.kg
−1 in the stems, and 140 mg.kg
−1 in the leaves [
73]. Again, the work of Sabeen et al. [
73] presented a much higher content in Cd in all fractions of giant reed than what was reported in the current study, but this is because the concentration of Cd in the soil medium was also much higher (to a maximum of 300 mg.kg
−1), when in the current work the maximum concentration tested was 8 mg.kg
−1. Other differences between the current study and the studies of Reed et al. [
104] and Sabeen et al. [
73] are that those studies were carried out over a shorter period, 45–60 days and 21 days, respectively, and the current study analyzed the biomass after the second harvest.
Ni accumulation in giant reed was also studied by Atma et al. [
41], who observed that the increase in the concentration of this heavy metal in the soil increased the heavy metal in the plant, especially in the roots [
41]–similar to the results obtained in the presented study. Indeed, the concentration of Ni in giant reed roots when exposed to a watered solution containing 100 mg.L
−1 of Ni reached a maximum of 80 mg.kg
−1, although the difference to the content when the crop was watered with a 10 mg Ni.L
~1 solution was not significant. In the current study, rhizomes of giant reed reached a maximum of 134 mg.kg
−1 when the soil was contaminated with 220 mg.kg
−1. In the study by Atma et al. [
41] on giant reed watered with Ni solutions and fertilized with NPK, it was reported that Ni content in the leaves (34–80 mg.kg
−1) and in the stems (20–60 mg.kg
−1) did not show a significant difference between 10 and 100 mg.L
−1 of Ni. In the current study, the Ni content in the leaves did not change significantly with contamination (77–96 mg.kg
−1), and the value was similar to the study of Atma et al. However, in the case of stems, it showed an incremental increase with the Ni increment in soil (to a maximum of 15 mg.kg
−1, when giant reed was exposed to 220 mg.kg
−1), and the content was much lower than what was observed in the Atma et al. study; again, the Atma et al. study was performed in a much shorter period of time–only 36 days. To our knowledge, no studies were performed with Ni and switchgrass, and therefore the current study is pioneering the demonstration of how this crop behaves when facing this type of contamination.
Concerning the study of the effect of Cr contamination in the switchgrass and giant reed contents in Cr, the comparison to other studies can only be made with data obtained for giant reed, since no production of switchgrass was obtained in the Cr-contaminated pots.
In a similar study using the same Cr concentrations (Cr
300 and Cr
600), giant reed Cr content increased in all structures, especially in the belowground ones, with the increment in Cr in the soil [
40]. This pattern was also observed in the current study. However, the maximum content observed in the present work in the belowground fraction (744 mg.kg
−1) and in leaves (92 mg.kg
−1) and stems (16 mg.kg
−1) is by far highest than in the study by Barbosa and collaborators (only a maximum of 34 mg.kg
−1 for rhizomes and 13 mg.kg
−1 for the above ground fraction) [
40]. The difference can be attributed to the salts of Cr used to artificially contaminate the soil–namely the application of Cr (VI), which is more mobile in the soil and therefore more prone to be absorbed by the belowground organs of the plant.
Cadmium, chromium, and nickel are usually minor components of the ash. However, when using biomass harvested from contaminated soils, the values present in the biomass can significantly increase and represent a major component of the ash. Moreover, cadmium is an easily volatile element and can be disengaged from the biomass into the released gases reacting there in thermochemical processes [
95]. These issues may cause further obstacles for its use, and knowledge of how it interferes with thermochemical processes must be safeguarded.
3.5. Phytoremediation Indexes
The phytoremediation indexes of giant reed and switchgrass are presented in
Table 9 and
Table 10, respectively.
Plants’ phytoremediation potential are associated with their capability to remediate contaminated soils. This process occurs through the absorption and accumulation of the contaminants in their organs [
40], stimulating the remediation of pollutants through the release of enzymes and exudates, or stimulating microorganisms in the soil–roots interface [
40,
105]. When analyzing phytoextraction, several points must be taken into consideration: (a) the influence of the contaminant in the plant’s growth that TI indicates, (b) the capability of the plant to absorb and store the pollutants when exposed to a higher than usual amount, shown by the mAI, (c) the capability of the plant to accumulate the contaminants, presented by mBCF and mBAF, and (d) the potential of the plant to transfer the accumulated contaminants from the belowground to the aboveground fraction of the crop, indicated by the TF and mTF.
The modified accumulation index (mAI) shows the plant’s potential to extract and accumulate heavy metals when exposed to higher concentrations than usual. Thus, mAI indicates that giant reed’s ability to accumulate Cd is higher than for the other studied heavy metals. The same was observed for switchgrass, and switchgrass on average also presented a higher mAI than giant reed, a fact that is interesting when planning a phytoremediation action.
The indication of which part of the plant is used to store the heavy metals is described by the mBCF. It can be observed that giant reed accumulates Cr and Cd mainly in the belowground part, while Ni is accumulated both in the belowground structure and leaves, in similar ratios. Switchgrass accumulation of Ni and Cd also occurs primarily in the belowground biomass. On average, giant reed presented a higher mBCF for Ni and Cd than switchgrass, probably due to lower productivity and higher metal content.
The capability of the plant to phytoremediate the soil is indicated by mBAF. Results suggest that, for both giant reed and switchgrass, the remediation is mainly promoted by the belowground biomass, indicating a trend for these crops to promote the phytostabilization of heavy metals. In terms of the belowground fraction, indexes were higher for giant reed than for switchgrass. Concerning the aboveground fractions, the leaves of giant reed also show higher indexes than stems of giant reed and aboveground fractions of switchgrass, for Ni and Cd.
The values of TF and mTF translate the crops’ potential to relocate the contaminants to the aboveground biomass. A TF value higher than 1 indicate that the contamination is stored mainly in the aboveground part of the plant, while the mTF combines the TF with the production of biomass. The highest accumulation of the contaminants in the aerial part is interesting from the phytoextraction point of view. Once the contaminants are in the aerial part, the biomass harvest also removes the soil pollutants. Giant reed TF results indicate that this crop had difficulty in moving Cr and Cd to the aerial parts, making this plant more suitable for Cr and Cd phytostabilization. However, Ni has moved to the aerial parts with great ease. Therefore, the plant can translocate a significant amount of the contamination to the aerial part, enabling this contaminant to be harvested together with the crop. However, when combined with the biomass yield, translated by mTF, Ni presented less advantages to phytoextraction. Switchgrass was shown to not be a very suitable crop for phytoextraction processes regarding Ni and Cd contamination, having a very low TF and mTF, which indicate that the plant transfers these contaminants to the aboveground part at a low rate. Having a low mTF can be interesting, not from the phytoextraction potential, but from the potential of using the aerial biomass for energy purposes.