Experimental Study on the Characteristics of Activated Coal Gangue and Coal Gangue-Based Geopolymer
Abstract
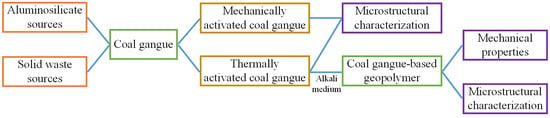
:1. Introduction
2. Materials and Methods
2.1. Coal Gangue Sample
2.2. Methods and Processes
2.2.1. Activation of As-Received Coal Gangue
2.2.2. Preparation of Alkali Medium
2.2.3. Preparation of CGGP
2.3. Sample Characterization
3. Results and Discussion
3.1. Effect of Mechanical Activation on Coal Gangue Structure
3.2. Effect of Thermal Activation on the Coal Gangue Structure
3.3. Analysis of Macro- and Micro-Properties of CGGP
4. Conclusions and Future Prospects
- (1)
- Mechanical activation and thermal activation are two effective methods for changing the reactivity of coal gangue, whereby both can destroy the stable kaolinite structure and improve the activity of coal gangue. Both FTIR and XRD spectra showed the removal of inner surface hydroxyl and inner hydroxyl, a decrease of Si–O–AlVI structure, and an increase of AlIV–O structure in the activated coal gangue.
- (2)
- The activation effect and microstructure change of coal gangue were different under different activation methods. With the increase in mechanical activation time, the crystal structure of kaolinite in coal gangue decreased and disappeared completely after 20 h grinding. Thermal activation can completely remove the hydroxyl in the kaolinite structure and form the active metakaolinite structure in coal gangue. However, when the activation temperature was too high (900 °C), the new structure in thermally activated coal gangue was destroyed, which was disadvantageous to the further improvement of the coal gangue activity.
- (3)
- The structure of activated coal gangue can be destroyed by the alkaline medium and reconstituted to form CGGP. The UCS results of CGGP prepared by different thermal activations of coal gangue were different. The UCS result of 800 °C-CGGP was the largest, which was higher than those recorded with 700 °C-CGGP and 900 °C-CGGP, respectively. In this paper, results showed that the optimized thermal activation temperature for coal gangue is about 800 °C.
- (4)
- The reason for the lower compressive strength of CGGP was that the active metakaolinite structures were destroyed in thermally activated coal gangue, which led to the decrease of the coal gangue activity and appeared to be a disadvantage for the geopolymerization. FTIR and XRD spectra showed the formation of new structures in CGGP, while the new structures in CGGP with low compressive strengths were relatively lower.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Querol, X.; Zhuang, X.; Font, O.; Izquierdo, M.; Alastuey, A.; Castro, I.; Van Drooge, B.L.; Moreno, T.; Grimalt, J.O.; Elvira, J.; et al. Influence of soil cover on reducing the environmental impact of spontaneous coal combustion in coal waste gobs: A review and new experimental data. Int. J. Coal Geol. 2011, 85, 2–22. [Google Scholar] [CrossRef]
- Song, L.; Liu, S.; Li, W. Quantitative Inversion of Fixed Carbon Content in Coal Gangue by Thermal Infrared Spectral Data. Energies 2019, 12, 1659. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.X.; Ju, Y.; Zhang, Q.; Ju, F.; Xiao, X.; Zhang, W.Q.; Zhou, N.; Li, M. Low ecological environment damage technology and method in coal mine. J. Min. Strata Contr. Eng. 2019, 1, 1–13. [Google Scholar]
- Huang, Y.-L.; Zhang, J.; Yin, W.; Sun, Q. Analysis of Overlying Strata Movement and Behaviors in Caving and Solid Backfilling Mixed Coal Mining. Energies 2017, 10, 1057. [Google Scholar] [CrossRef] [Green Version]
- Querol, X.; Izquierdo, M.; Monfort, E.; Alvarez, E.; Font, O.; Moreno, T.; Alastuey, A.; Zhuang, X.; Lu, W.; Wang, Y. Environmental characterization of burnt coal gangue banks at Yangquan, Shanxi Province, China. Int. J. Coal Geol. 2008, 75, 93–104. [Google Scholar] [CrossRef]
- Li, J.; Wang, J. Comprehensive utilization and environmental risks of coal gangue: A review. J. Clean. Prod. 2019, 239, 117946. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, J.; Wu, Z.; Chen, Y. Overview of Solid Backfilling Technology Based on Coal-Waste Underground Separation in China. Sustainability 2019, 11, 2118. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.; Spearing, A.J.S.; Ju, F.; Jessu, K.; Wang, Z.; Ning, P. Control Effects of Five Common Solid Waste Backfilling Materials on In Situ Strata of Gob. Energies 2019, 12, 154. [Google Scholar] [CrossRef] [Green Version]
- Davidovits, J. Geopolymers. J. Therm. Anal. Calorim. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers Ceramic-Like Inorganic Polymers. J. Ceram. Sci. Technol. 2017, 8, 335–350. [Google Scholar]
- McLellan, B.C.; Williams, R.; Lay, J.; Van Riessen, A.; Corder, G. Costs and carbon emissions for geopolymer pastes in comparison to ordinary portland cement. J. Clean. Prod. 2011, 19, 1080–1090. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Deng, X.; Liu, J.; Hui, D. Mechanical properties and microstructures of hypergolic and calcined coal gangue based geopolymer recycled concrete. Constr. Build. Mater. 2019, 221, 691–708. [Google Scholar] [CrossRef]
- Hajjaji, W.; Andrejkovicova, S.; Zanelli, C.; Alshaaer, M.; Dondi, M.; Labrincha, J.; Rocha, F. Composition and technological properties of geopolymers based on metakaolin and red mud. Mater. Des. 2013, 52, 648–654. [Google Scholar] [CrossRef]
- Zhang, H.; Kodur, V.; Wu, B.; Cao, L.; Qi, S.L. Comparative Thermal and Mechanical Performance of Geopolymers derived from Metakaolin and Fly Ash. J. Mater. Civ. Eng. 2016, 28, 04015092. [Google Scholar] [CrossRef]
- Part, W.K.; Ramli, M.; Cheah, C.B. An overview on the influence of various factors on the properties of geopolymer concrete derived from industrial by-products. Constr. Build. Mater. 2015, 77, 370–395. [Google Scholar] [CrossRef]
- He, J.; Zhang, J.; Yu, Y.; Zhang, G. The strength and microstructure of two geopolymers derived from metakaolin and red mud-fly ash admixture: A comparative study. Constr. Build. Mater. 2012, 30, 80–91. [Google Scholar] [CrossRef]
- Zhuang, X.Y.; Chen, L.; Komarneni, S.; Zhou, C.; Tong, D.S.; Yang, H.M.; Yu, W.H.; Wang, H. Fly ash-based geopolymer: Clean production, properties and applications. J. Clean. Prod. 2016, 125, 253–267. [Google Scholar] [CrossRef]
- Mohamad, J.M.; Rassoul, A.; Alborz, H. Preparation and application of alkali-activated materials based on waste glass and coal gangue: A review. Constr. Build. Mater. 2019, 221, 84–98. [Google Scholar]
- Guo, Y.; Yan, K.; Cui, L.; Cheng, F. Improved extraction of alumina from coal gangue by surface mechanically grinding modification. Powder Technol. 2016, 302, 33–41. [Google Scholar] [CrossRef]
- Li, Y.; Yao, Y.; Liu, X.; Sun, H.; Ni, W. Improvement on pozzolanic reactivity of coal gangue by integrated thermal and chemical activation. Fuel 2013, 109, 527–533. [Google Scholar] [CrossRef]
- Zhang, C.S.; Fang, L.M. Hardening mechanisms of alkali activated burned gangue cementitious material. Mater. Sci. Technol. 2004, 12, 597–601. [Google Scholar]
- Cheng, Y.; Ma, H.; Hongyu, C.; Jiaxin, W.; Jing, S.; Zonghui, L.; Mingkai, Y. Preparation and characterization of coal gangue geopolymers. Constr. Build. Mater. 2018, 187, 318–326. [Google Scholar] [CrossRef]
- Zhou, M.; Xu, M.; Li, Z.W.; Sun, Q.W. Preparation and basic properties of spontaneous combustion gangue-slag-fly ash gepolyer. Bull. Chin. Ceram. Soc. 2013, 32, 1826–1831. [Google Scholar]
- Zhou, M.; Wang, C.Z.; Li, Z.W.; Chang, J.; Liu, Q.; Zu, X.J. Ligand optimization of spontaneous combustion coal gangue geopolymer based on the orthogonal and response surface design. Bull. Chin. Ceram. Soc. 2013, 32, 1258–1263, 1268. [Google Scholar]
- Huang, G.; Ji, Y.; Li, J.; Hou, Z.; Dong, Z. Improving strength of calcinated coal gangue geopolymer mortars via increasing calcium content. Constr. Build. Mater. 2018, 166, 760–768. [Google Scholar] [CrossRef]
- Xu, Z.; Zou, X.; Yang, Z. Preparation and Properties of Sludge and Coal Gangue Composite Polymer. Asian J. Chem. 2014, 26, 1751–1753. [Google Scholar] [CrossRef]
- Xu, Z.F.; Zou, X.T.; Chen, J. Preparation of thermal activation sludge and coal gangue polymer. Integ. Ferroelectr. 2015, 160, 1–9. [Google Scholar]
- Geng, J.; Zhou, M.; Li, Y.; Chen, Y.; Han, Y.; Wan, S.; Zhou, X.; Hou, H. Comparison of red mud and coal gangue blended geopolymers synthesized through thermal activation and mechanical grinding preactivation. Constr. Build. Mater. 2017, 153, 185–192. [Google Scholar] [CrossRef]
- Geng, J.; Zhou, M.; Zhang, T.; Wang, W.; Wang, T.; Zhou, X.; Wang, X.; Hou, H. Preparation of blended geopolymer from red mud and coal gangue with mechanical co-grinding preactivation. Mater. Struct. 2016, 50, 109. [Google Scholar] [CrossRef]
- Koshy, N.; Dondrob, K.; Hu, L.; Wen, Q.; Meegoda, J.N. Synthesis and characterization of geopolymers derived from coal gangue, fly ash and red mud. Constr. Build. Mater. 2019, 206, 287–296. [Google Scholar] [CrossRef]
- Sun, Q.; Tian, S.; Sun, Q.; Li, B.; Cai, C.; Xia, Y.; Wei, X.; Mu, Q. Preparation and microstructure of fly ash geopolymer paste backfill material. J. Clean. Prod. 2019, 225, 376–390. [Google Scholar] [CrossRef]
- Sun, Q.; Li, B.; Tian, S.; Cai, C.; Xia, Y. Creep properties of geopolymer cemented coal gangue-fly ash backfill under dynamic disturbance. Constr. Build. Mater. 2018, 191, 644–654. [Google Scholar] [CrossRef]
- Sun, Q.; Cai, C.; Zhang, S.; Tian, S.; Li, B.; Xia, Y.; Sun, Q. Study of localized deformation in geopolymer cemented coal gangue-fly ash backfill based on the digital speckle correlation method. Constr. Build. Mater. 2019, 215, 321–331. [Google Scholar] [CrossRef]
- Reza, A.; Seyed, A.H.; Mojtaba, S.; Abbas, A.S. Updating the neural network sediment load models using different sensitivity analysis methods: A regional application. J. Hydroinform 2020, in press. [Google Scholar]
- Andrea, S. Sensitivity Analysis for Importances Assessment. Risk. Anal. 2002, 22, 579–590. [Google Scholar]
- Sobol, I. Sensitivity analysis for non-linear mathematical models. Math. Model. Comput. 1993, 1, 407–414. [Google Scholar]
- Hu, P.; Yang, H. Insight into the physicochemical aspects of kaolins with different morphologies. Appl. Clay Sci. 2013, 74, 58–65. [Google Scholar] [CrossRef]
- Frost, R.L. Hydroxy deformation in kaolin. Clays Clay Miner. 1998, 46, 280–289. [Google Scholar] [CrossRef]
- Ptáček, P.; Frajkorová, F.; Šoukal, F.; Opravil, T. Kinetics and mechanism of three stages of thermal transformation of kaolinite to metakaolinite. Powder Technol. 2014, 264, 439–445. [Google Scholar] [CrossRef]
- Frost, R.L. Combination Bands in the Infrared Spectroscopy of Kaolins—A Drift Spectroscopic Study. Clays Clay Miner. 1998, 46, 466–477. [Google Scholar] [CrossRef]
- Konan, L.K.; Peyratout, C.; Smith, A.; Bonnet, J.-P.; Rossignol, S.; Oyetola, S. Comparison of surface properties between kaolin and metakaolin in concentrated lime solutions. J. Colloid Interface Sci. 2009, 339, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Hamzaoui, R.; Muslim, F.; Guessasma, S.; Bennabi, A.; Guillin, J. Structural and thermal behavior of proclay kaolinite using high energy ball milling process. Powder Technol. 2015, 271, 228–237. [Google Scholar] [CrossRef]
- Castellano, M.; Turturro, A.; Riani, P.; Montanari, T.; Finocchio, E.; Ramis, G.; Busca, G. Bulk and surface properties of commercial kaolins. Appl. Clay Sci. 2010, 48, 446–454. [Google Scholar] [CrossRef]
- Zhu, B.Z.; Sun, Y.L.; Xie, C.W. Spectroscopy research on the Guizhou Xingyi gangue of different calcined temperatures. J. China Coal Soc. 2008, 9, 1049–1052. [Google Scholar]
- Vizcayno, C.; De Gutiérrez, R.M.; Castello, R.; Rodriguez, E.D.; Guerrero, C. Pozzolan obtained by mechanochemical and thermal treatments of kaolin. Appl. Clay Sci. 2010, 49, 405–413. [Google Scholar] [CrossRef]
- Shahri, A.A.; Larsson, S.; Johansson, F. Updated relations for the uniaxial compressive strength of marlstones based on P-wave velocity and point load index test. Innov. Infrastruct. Solut. 2016, 1, 17. [Google Scholar] [CrossRef] [Green Version]
- Asheghi, R.; Shahri, A.A.; Zak, M.K. Prediction of Uniaxial Compressive Strength of Different Quarried Rocks Using Metaheuristic Algorithm. Arab. J. Sci. Eng. 2019, 44, 8645–8659. [Google Scholar] [CrossRef]
- Chen, S.; Du, Z.; Zhang, Z.; Yin, D.; Feng, F.; Ma, J. Effects of red mud additions on gangue-cemented paste backfill properties. Powder Technol. 2020, 367, 833–840. [Google Scholar] [CrossRef]
- Jiang, H.; Fall, M.; Cui, L. Freezing behaviour of cemented paste backfill material in column experiments. Constr. Build. Mater. 2017, 147, 837–846. [Google Scholar] [CrossRef]
- Li, H.; Sun, H.-H. Microstructure and cementitious properties of calcined clay-containing gangue. Int. J. Miner. Met. Mater. 2009, 16, 482–486. [Google Scholar] [CrossRef]









| Chemical Composition | Loss on Ignition | |||||||
|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | K2O | TiO2 | MgO | Na2O | |
| 45.26 | 21.62 | 2.839 | 2.69 | 1.65 | 0.72 | 0.608 | 0.415 | 23.4 |
| Proximate Analysis | Elemental Analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Ash Content | Volatile | Fixed Carbon | Moisture | Carbon | Oxygen | Hydrogen | Nitrogen | Sulfur |
| 77.76 | 12.46 | 8.87 | 0.91 | 12.42 | 7.12 | 1.27 | 0.26 | 0.26 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Dong, C.; Huang, P.; Sun, Q.; Li, M.; Chai, J. Experimental Study on the Characteristics of Activated Coal Gangue and Coal Gangue-Based Geopolymer. Energies 2020, 13, 2504. https://doi.org/10.3390/en13102504
Zhang W, Dong C, Huang P, Sun Q, Li M, Chai J. Experimental Study on the Characteristics of Activated Coal Gangue and Coal Gangue-Based Geopolymer. Energies. 2020; 13(10):2504. https://doi.org/10.3390/en13102504
Chicago/Turabian StyleZhang, Weiqing, Chaowei Dong, Peng Huang, Qiang Sun, Meng Li, and Jun Chai. 2020. "Experimental Study on the Characteristics of Activated Coal Gangue and Coal Gangue-Based Geopolymer" Energies 13, no. 10: 2504. https://doi.org/10.3390/en13102504