Outdoor Cultivation of the Microalga Chlorella vulgaris in a New Photobioreactor Configuration: The Effect of Ultraviolet and Visible Radiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culture Medium and Microalgae
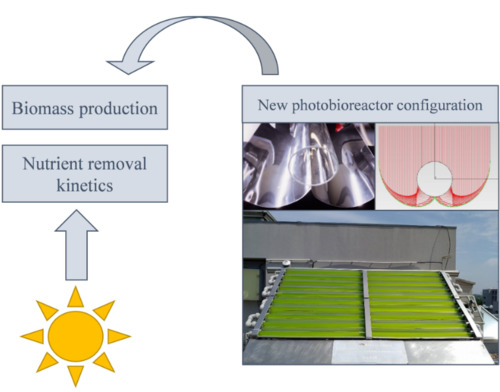
2.2. Experimental Setup
2.3. Methods of Analysis
2.4. Kinetic Models
3. Results and Discussion
3.1. Biomass Production
3.2. Nutrient Uptake
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CCAP | Culture Collection of Algae and Protozoa |
| CPC | compound parabolic collector |
| OD440 | optical density at 440 nm |
| OECD | Organization for Economic Co-operation and Development |
| PBR | photobioreactor |
| PSII | photosystem II |
| UP | uncovered period |
| UV | ultraviolet |
| UVA | ultraviolet A |
| UVB | ultraviolet B |
| VIS | visible |
Nomenclature
| average ultraviolet irradiance | |
| average visible solar irradiance in the exponential growth phase | |
| average visible solar irradiance | |
| Ar | area of the illuminated surface |
| IUV | ultraviolet irradiance |
| IVIS | visible irradiance |
| IVIS, range | range of visible irradiance |
| k | pseudo-first-order kinetic constant |
| kS,t | pseudo-first-order kinetic constant as function of accumulated energy due to visible irradiance |
| kS,t | pseudo-first-order kinetic constant as function of time |
| Px,av | average biomass productivities |
| QVIS | accumulated energy due to visible irradiance |
| r0 | initial removal rate |
| R2 | coefficient of determination |
| RRav | average nutrients removal rate |
| S | nutrients concentration |
| S2R | residual variance |
| t | time |
| t0 | initial time |
| tc | cultivation period |
| V | volume of the photobioreactor |
| X | biomass concentration in dry weight |
| X0 | biomass concentration at the initial time |
| YX/S | specific biomass yields based on nutrient consumption |
| Δti | time interval |
| δμ | standard error |
| μQ | specific growth rate as function of accumulated energy due to visible irradiance |
| μt | specific growth rate as function of time |
References
- Pereira, S.E.L.; Goncalves, A.L.; Moreira, F.C.; Silva, T.F.C.V.; Vilar, V.J.P.; Pires, J.C.M. Nitrogen removal from landfill leachate by microalgae. Int. J. Mol. Sci. 2016, 17, 1629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pires, J.C.M.; Alvim-Ferraz, M.C.M.; Martins, F.G.; Simoes, M. Carbon dioxide capture from flue gases using microalgae: Engineering aspects and biorefinery concept. Renew. Sust. Energy Rev. 2012, 16, 3043–3053. [Google Scholar] [CrossRef]
- Silva, N.F.P.; Goncalves, A.L.; Moreira, F.C.; Silva, T.F.C.V.; Martins, F.G.; Alvim-Ferraz, M.C.M.; Boaventura, R.A.R.; Vilar, V.J.P.; Pires, J.C.M. Towards sustainable microalgal biomass production by phycoremediation of a synthetic wastewater: A kinetic study. Algal Res. 2015, 11, 350–358. [Google Scholar] [CrossRef] [Green Version]
- Posten, C. Design principles of photo-bioreactors for cultivation of microalgae. Eng. Life Sci. 2009, 9, 165–177. [Google Scholar] [CrossRef]
- Slegers, P.M.; Wijffels, R.H.; van Straten, G.; van Boxtel, A.J.B. Design scenarios for flat panel photobioreactors. Appl. Energy 2011, 88, 3342–3353. [Google Scholar] [CrossRef]
- Brunelle, T.; Dumas, P.; Souty, F.; Dorin, B.; Nadaud, F. Evaluating the impact of rising fertilizer prices on crop yields. Agric. Econ. 2015, 46, 653–666. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, M.; Lu, Q.; Wu, X.; Ma, Y.; Cheng, Y.; Addy, M.; Liu, Y.; Ruan, R. Balancing carbon/nitrogen ratio to improve nutrients removal and algal biomass production in piggery and brewery wastewaters. Bioresour. Technol. 2018, 249, 479–486. [Google Scholar] [CrossRef]
- Cai, T.; Park, S.Y.; Li, Y.B. Nutrient recovery from wastewater streams by microalgae: Status and prospects. Renew. Sust. Energy Rev. 2013, 19, 360–369. [Google Scholar] [CrossRef]
- Posadas, E.; Alcántara, C.; García-Encina, P.A.; Gouveia, L.; Guieysse, B.; Norvill, Z.; Acién, F.G.; Markou, G.; Congestri, R.; Koreiviene, J.; et al. 3—microalgae cultivation in wastewater. In Microalgae-based Biofuels and Bioproducts; Gonzalez-Fernandez, C., Muñoz, R., Eds.; Woodhead Publishing: Lincolnshire, IL, USA, 2017; pp. 67–91. [Google Scholar]
- Muñoz, R.; Guieysse, B. Algal–bacterial processes for the treatment of hazardous contaminants: A review. Water Res. 2006, 40, 2799–2815. [Google Scholar] [CrossRef] [PubMed]
- Pires, J.; Alvim-Ferraz, M.; Martins, F.; Simoes, M. Wastewater treatment to enhance the economic viability of microalgae culture. Environ. Sci. Pollut. Res. 2013, 20, 5096–5105. [Google Scholar] [CrossRef]
- Goncalves, A.L.; Pires, J.C.M.; Simoes, M. A review on the use of microalgal consortia for wastewater treatment. Algal Res. 2017, 24, 403–415. [Google Scholar] [CrossRef]
- Lopez-Serna, R.; Garcia, D.; Bolado, S.; Jimenez, J.J.; Lai, F.Y.; Golovko, O.; Gago-Ferrero, P.; Ahrens, L.; Wiberg, K.; Munoz, R. Photobioreactors based on microalgae-bacteria and purple phototrophic bacteria consortia: A promising technology to reduce the load of veterinary drugs from piggery wastewater. Sci. Total Environ. 2019, 692, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Rattanapoltee, P.; Kaewkannetra, P. Cultivation of microalga, chlorella vulgaris under different auto–hetero–mixo trophic growths as a raw material during biodiesel production and cost evaluation. Energy 2014, 78, 4–8. [Google Scholar] [CrossRef]
- Evans, L.; Hennige, S.J.; Willoughby, N.; Adeloye, A.J.; Skroblin, M.; Gutierrez, T. Effect of organic carbon enrichment on the treatment efficiency of primary settled wastewater by chlorella vulgaris. Algal Res. 2017, 24, 368–377. [Google Scholar] [CrossRef]
- Garcia, D.; Posadas, E.; Grajeda, C.; Blanco, S.; Martinez-Paramo, S.; Acien, G.; Garcia-Encina, P.; Bolado, S.; Munoz, R. Comparative evaluation of piggery wastewater treatment in algal-bacterial photobioreactors under indoor and outdoor conditions. Bioresour. Technol. 2017, 245, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.L.; Pires, J.C.M.; Simoes, M. Wastewater polishing by consortia of chlorella vulgaris and activated sludge native bacteria. J. Clean Prod. 2016, 133, 348–357. [Google Scholar] [CrossRef]
- Filali, R.; Tebbani, S.; Dumur, D.; Isambert, A.; Pareau, D.; Lopes, F. Growth modeling of the green microalga chlorella vulgaris in an air-lift photobioreactor. IFAC Proc. Vol. 2011, 44, 10603–10608. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Phooi, W.B.A.; Lim, Z.J.; Tong, Y.W. Control of co2 input conditions during outdoor culture of chlorella vulgaris in bubble column photobioreactors. Bioresour. Technol. 2015, 186, 238–245. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T. Cultivation of chlorella vulgaris in a pilot-scale sequential-baffled column photobioreactor for biomass and biodiesel production. Energy Convers. Manag. 2014, 88, 399–410. [Google Scholar] [CrossRef]
- Bosma, R.; de Vree, J.H.; Slegers, P.M.; Janssen, M.; Wijffels, R.H.; Barbosa, M.J. Design and construction of the microalgal pilot facility algaeparc. Algal Res. 2014, 6, 160–169. [Google Scholar] [CrossRef]
- OCDE. Test no. 201: Freshwater Alga and Cyanobacteria, Growth Inhibition Test, Ocde Guidelines for the Testing of Chemicals; OCDE Publishing: Paris, France, 2011. [Google Scholar]
- Blanco, J.; Malato, S.; Fernandez, P.; Vidal, A.; Morales, A.; Trincado, P.; Oliveira, J.C.; Minero, C.; Musci, M.; Casalle, C.; et al. Compound parabolic concentrator technology development to commercial solar detoxification applications. Sol. Energy 1999, 67, 317–330. [Google Scholar] [CrossRef]
- Gomes, A.I.; Silva, T.F.C.V.; Duarte, M.A.; Boaventura, R.A.R.; Vilar, V.J.P. Cost-effective solar collector to promote photo-fenton reactions: A case study on the treatment of urban mature leachate. J. Clean Prod. 2018, 199, 369–382. [Google Scholar] [CrossRef]
- Gonçalves, A.L.; Pires, J.C.M.; Simoes, M. The effects of light and temperature on microalgal growth and nutrient removal: An experimental and mathematical approach. Rsc Adv. 2016, 6, 22896–22907. [Google Scholar] [CrossRef] [Green Version]
- Myers, J.A.; Curtis, B.S.; Curtis, W.R. Improving accuracy of cell and chromophore concentration measurements using optical density. Bmc Biophys. 2013, 6, 4. [Google Scholar] [CrossRef] [Green Version]
- Bunker, P.R.; Mills, I.M.; Jensen, P. The planck constant and its units. J. Quant. Spectrosc. Radiat. Transf. 2019, 237, 106594. [Google Scholar] [CrossRef]
- ASTM. Standard Tables for Reference Solar Spectral Irradiances: Direct Normal and Hemispherical on 37° Tilted Surface; ASTM: West Conshohocken, PA, USA, 2003. [Google Scholar]
- Wang, M.; Kuo-Dahab, W.C.; Dolan, S.; Park, C. Kinetics of nutrient removal and expression of extracellular polymeric substances of the microalgae, chlorella sp. And micractinium sp., in wastewater treatment. Bioresour. Technol. 2014, 154, 131–137. [Google Scholar] [CrossRef]
- Jiang, H.B.; Qiu, B.S. Inhibition of photosynthesis by uv-b exposure and its repair in the bloom-forming cyanobacterium microcystis aeruginosa. J. Appl. Phycol. 2011, 23, 691–696. [Google Scholar] [CrossRef]
- Beardall, J.; Heraud, P.; Roberts, S.; Shelly, K.; Stojkovic, S. Effects of uv-b radiation on inorganic carbon acquisition by the marine microalga dunaliella tertiolecta (chlorophyceae). Phycologia 2002, 41, 268–272. [Google Scholar] [CrossRef]
- Babu, G.S.; Joshi, P.C.; Viswanathan, P.N. Uvb-induced reduction in biomass and overall productivity of cyanobacteria. Biochem. Biophs. Res. Commun. 1998, 244, 138–142. [Google Scholar] [CrossRef]
- Wong, C.Y.; Teoh, M.L.; Phang, S.-M.; Chu, W.-L. Effects of ultraviolet radiation (uvr) on the tropical microalgae chlorella vulgaris. Malays. J. Sci. 2011, 30, 3–15. [Google Scholar]
- Åkerström, A.M.; Mortensen, L.M.; Rusten, B.; Gislerød, H.R. Biomass production and removal of ammonium and phosphate by chlorella sp. In sludge liquor at natural light and different levels of temperature control. SpringerPlus 2016, 5, 676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juneja, A. Model Predictive Control for Optimum Algal Growth; Oregon State University: Corvallis, OR, USA, 2015. [Google Scholar]
- Ruiz, J.; Álvarez, P.; Arbib, Z.; Garrido, C.; Barragán, J.; Perales, J.A. Effect of nitrogen and phosphorus concentration on their removal kinetic in treated urban wastewater by chlorella vulgaris. Int. J. Phytoremediation 2011, 13, 884–896. [Google Scholar] [CrossRef] [PubMed]







| Assay | PBR | UP (h) | Xi (g·L−1) | [N-NO3-]i (mg N·L−1) | [P-PO43-]i (mg P·L−1) | (W·m−2) | (W·m−2) | tc (h) |
|---|---|---|---|---|---|---|---|---|
| I | 1 | 0 | 0.19 | 22.2 | 6.7 | 98 | 7 | 81 |
| 2 | 15 | 0.20 | 20.8 | 8.0 | 162 | 11 | ||
| II | 1 | 22 | 0.33 | 16.5 | 6.2 | 221 | 15 | 44 |
| 2 | 0 | 0.31 | 16.9 | 6.1 | 82 | 6 | ||
| III | 1 | 0 | 0.33 | 26.9 | 8.4 | 101 | 6 | 52 |
| 2 | 0 | 0.33 | 27.9 | 8.6 | 101 | 6 | ||
| IV | 1 | 0 | 0.31 | 38.7 | 12.3 | 143 | 9 | 69 |
| 2 | 0 | 0.31 | 38.7 | 12.2 | 143 | 9 |
| Assay | PBR | PX,av (g·L−1·h−1) | µt (h−1) | R2t | S2R (g2·L−2) | µQ (L·kJ−1) | R2Q | S2R (g2·L−2) |
|---|---|---|---|---|---|---|---|---|
| I | 1 | (2.3 ± 0.6) × 10−3 | (1.2 ± 0.1) × 10−2 | 0.967 | 3.1 × 10−4 | (7.4 ± 0.4) × 10−4 | 0.983 | 4.1 × 10−5 |
| 2 | (2.9 ± 0.8) × 10−3 | (1.6 ± 0.1) × 10−2 | 0.981 | 4.1 × 10−4 | (9.7 ± 0.6) × 10−4 | 0.979 | 1.1 × 10−4 | |
| II | 1 | (3 ± 1) × 10−3 | (1.1 ± 0.3) × 10−2 | 1.000 | 9.9 × 10−7 | (4.8 ± 0.4) × 10−4 | 0.996 | 4.2 × 10−6 |
| 2 | (4 ± 2) × 10−3 | (1.5 ± 0.7) × 10−2 | 0.999 | 7.5 × 10−6 | (7 ± 1) × 10−4 | 0.964 | 1.2 × 10−4 | |
| III | 1 | (4 ± 1) × 10−3 | (1.4 ± 0.3) × 10−2 | 0.996 | 3.7 × 10−5 | (8 ± 4) × 10−4 | 0.962 | 1.6 × 10−4 |
| 2 | (4 ± 1) × 10−3 | (1.2 ± 0.2) × 10−2 | 0.988 | 1.5 × 10−4 | (7 ± 2) × 10−4 | 0.956 | 1.8 × 10−4 | |
| IV | 1 | (5 ± 1) × 10−3 | (2.0 ± 0.6) × 10−2 | 0.992 | 1.3 × 10−4 | (7 ± 1) × 10−4 | 0.999 | 5.9 × 10−6 |
| 2 | (5 ± 1) × 10−3 | (1.8 ± 0.7) × 10−2 | 0.987 | 1.9 × 10−4 | (6 ± 2) × 10−4 | 0.998 | 1.7 × 10−5 |
| Assay | PBR | Kinetic Parameters | [N-NO3−]f (mg N L−1) | RRav (mg N L−1 h-1) | YX/N (g/(g N)) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time | Energy | |||||||||||
| kN,t (h−1) | R2 | S2R (mg2 L−2) | r0,N,t (mg N L−1 h−1) | kN,Q (L·kJ−1) | R2 | S2R (mg2 L−2) | r0,N,Q (mg N kJ−1) | |||||
| I | 1 | (1.0 ± 0.2) × 10−2 | 0.97 | 4.0 × 10−1 | 0.21 ± 0.04 | (5.2 ± 0.7) × 10−4 | 0.97 | 3.6 × 10−1 | (1.1 ± 0.2) × 10−2 | 12.1 | 0.14 ± 0.03 | (1.7 ± 0.5) × 10 |
| 2 | (1.2 ± 0.1) × 10−2 | 0.99 | 8.8 × 10−2 | 0.24 ± 0.02 | (4 ± 1) × 10−4 | 0.90 | 1.5 | (8 ± 2) × 10−3 | 8.8 | 0.15 ± 0.01 | (1.9 ± 0.6) × 10 | |
| II | 1 | (1.5 ± 0.9) × 10−2 | 0.96 | 3.1 × 10−1 | 0.2 ± 0.1 | (2.4 ± 0.5) × 10−4 | 0.99 | 6.7 × 10−2 | (3.9 ± 0.9) × 10−3 | 9.8 | 0.15 ± 0.06 | (2 ± 1) × 10 |
| 2 | (2 ± 1) × 10−2 | 0.96 | 5.4 × 10−1 | 0.4 ± 0.2 | (1.2 ± 0.2) × 10−3 | 1.00 | 6.6 × 10−2 | (2.0 ± 0.3) × 10−2 | 8.3 | 0.2 ± 0.1 | (2 ± 1) × 10 | |
| III | 1 | (2.5 ± 0.9) × 10−2 | 0.97 | 1.4 | 0.7 ± 0.2 | (1.4 ± 0.4) × 10−3 | 0.96 | 2.0 | (4 ± 1) × 10−2 | 9.1 | 0.43 ± 0.09 | 9 ± 3 |
| 2 | (3 ± 1) × 10−2 | 0.96 | 2.4 | 0.9 ± 0.4 | (1.7 ± 0.7) × 10−3 | 0.92 | 5.0 | (5 ± 2) × 10−2 | 7.2 | 0.51 ± 0.05 | 8 ± 2 | |
| IV | 1 | (1.8 ± 0.3) × 10−2 | 0.94 | 6.8 | 0.7 ± 0.2 | (7 ± 2) × 10−4 | 0.91 | 9.0 | (2.7 ± 0.8) × 10−2 | 14.0 | 0.47 ± 0.09 | (1.1 ± 0.3) × 10 |
| 2 | (1.4 ± 0.2) × 10−2 | 0.98 | 1.1 | 0.54 ± 0.09 | (5 ± 1) × 10−4 | 0.96 | 2.6 | (2.1 ± 0.4) × 10−2 | 17.3 | 0.37 ± 0.05 | (1.4 ± 0.3) × 10 | |
| Assay | PBR | Kinetic Parameters | [P-PO43−]f (mg P L−1) | RRav (mg P L−1 h−1) | YX/P (g/(g P)) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time | Energy | |||||||||||
| kP,t (h−1) | R2 | S2R (mg2 L−2) | r0,P,t (mg P L−1 h−1) | kP,Q (L·kJ−1) | R2 | S2R (mg2 L−2) | r0,P,Q (mg P kJ−1) | |||||
| I | 1 | (1.8 ± 0.4) × 10−3 | 0.98 | 2.5 × 10−3 | 0.012 ± 0.004 | (1.0 ± 0.2) × 10−4 | 0.97 | 2.7 × 10−3 | (6 ± 2) × 10−4 | 5.7 | (1.1 ± 0.2) × 10−2 | (2.1 ± 0.6) × 102 |
| 2 | (6.1 ± 0.9) × 10−3 | 0.98 | 1.5 × 10−2 | 0.05 ± 0.01 | (3.3 ± 0.2) × 10−4 | 1.00 | 1.2 × 10−3 | (2.6 ± 0.5) × 10−3 | 5.2 | (3.6 ± 0.5) × 10−2 | (8 ± 2) × 10 | |
| II | 1 | (9 ± 4) × 10−3 | 0.98 | 1.1 × 10−2 | 0.05 ± 0.03 | (1.3 ± 0.5) × 10−4 | 0.97 | 1.5 × 10−2 | (8 ± 4) × 10−4 | 4.6 | (4 ± 2) × 10−2 | (8 ± 5) × 10 |
| 2 | (8 ± 3) × 10−3 | 0.99 | 4.3 × 10−3 | 0.05 ± 0.03 | (4 ± 1) × 10−4 | 0.96 | 1.9 × 10−2 | (2.4 ± 0.9) × 10−3 | 4.8 | (3 ± 2) × 10−2 | (1.1 ± 0.7) × 102 | |
| III | 1 | (2.0 ± 0.3) × 10−2 | 0.99 | 2.9 × 10−2 | 0.16 ± 0.04 | (8.9 ± 0.5) × 10−4 | 1.00 | 3.2 × 10−3 | (7 ± 2) × 10−3 | 3.3 | (9 ± 3) × 10−2 | (4 ± 2) × 10 |
| 2 | (2.0 ± 0.1) × 10−2 | 1.00 | 6.7 × 10−3 | 0.17 ± 0.04 | (9.3 ± 0.9) × 10−4 | 1.00 | 1.2 × 10−2 | (8 ± 2) × 10−3 | 3.4 | (1.0 ± 0.2) × 10−1 | (4 ± 1) × 10 | |
| IV | 1 | (6.1 ± 0.7) × 10−3 | 0.99 | 1.9 × 10−2 | 0.07 ± 0.01 | (2.4 ± 0.2) × 10−4 | 0.99 | 1.9 × 10−2 | (2.9 ± 0.4) × 10−3 | 8.6 | (6.1 ± 0.5) × 10−2 | (9 ± 2) × 10 |
| 2 | (1.0 ± 0.1) × 10−2 | 0.99 | 5.0 × 10−2 | 0.12 ± 0.02 | (4.0 ± 0.3) × 10−4 | 0.99 | 2.2 × 10−2 | (4.9 ± 0.6) × 10−3 | 5.5 | (9 ± 2) × 10−2 | (6 ± 2) × 10 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, A.P.; Santos, F.M.; Silva, T.F.C.V.; Vilar, V.J.P.; Pires, J.C.M. Outdoor Cultivation of the Microalga Chlorella vulgaris in a New Photobioreactor Configuration: The Effect of Ultraviolet and Visible Radiation. Energies 2020, 13, 1962. https://doi.org/10.3390/en13081962
Lopes AP, Santos FM, Silva TFCV, Vilar VJP, Pires JCM. Outdoor Cultivation of the Microalga Chlorella vulgaris in a New Photobioreactor Configuration: The Effect of Ultraviolet and Visible Radiation. Energies. 2020; 13(8):1962. https://doi.org/10.3390/en13081962
Chicago/Turabian StyleLopes, Alcinda P., Francisca M. Santos, Tânia F. C. V. Silva, Vítor J. P. Vilar, and José C. M. Pires. 2020. "Outdoor Cultivation of the Microalga Chlorella vulgaris in a New Photobioreactor Configuration: The Effect of Ultraviolet and Visible Radiation" Energies 13, no. 8: 1962. https://doi.org/10.3390/en13081962