3.1. Quantitative MRI Contrast Agent Evaluation
To evaluate the efficacy of the specific agent, SBK2-Tris-(Gd-DOTA)
3, we compared this targeted agent to the scrambled-Tris-(Gd-DOTA)
3, and the non-specific conventional clinical contrast agent Optimark™. We acquired and analyzed 2D axial
T1 mapping images in GFP-positive LN-229 human glioma flank tumors heterotopically implanted in athymic (nu/nu) mice at 2–5 weeks post-implantation. Five
T1 maps of the tumor region were acquired before injection of contrast agents to serve as baseline measurements, and then at 2.5 min intervals for 62.5 min following intravenous injection of Optimark™, scrambled-Tris-(Gd-DOTA)
3, or SBK2-Tris-(Gd-DOTA)
3 agents administered at a dose of 0.2 mmol·Gd/kg. Representative fluorescence and MRI images of mice with the implanted flank tumors are shown in
Figure 1 for each contrast agent. Using optical imaging, the physical outline of the flank tumors is evident under brightfield illumination (
Figure 1) and the GFP-positive LN-229 glioma cells are also clearly visible in the fluorescence images (
Figure 1). For MR imaging, the axial
T2-weighted images (
Figure 1) are shown with the regions of interest (ROIs) indicated by dashed lines that were used for
T1 mapping. In this view, the flank tumors are clearly visible in the
T2-weighted images allowing for accurate ROI selection.
Figure 1 also shows
T1-weighted images acquired from the
T1 mapping acquisitions both at baseline (
Figure 1) and 15 min following contrast agent injection (
Figure 1), respectively. All three contrast agents showed significant contrast uptake as evidenced by the relative change in the
T1-weighted images. However, a rigorous quantitative comparison is not possible with these
T1-weighted images.
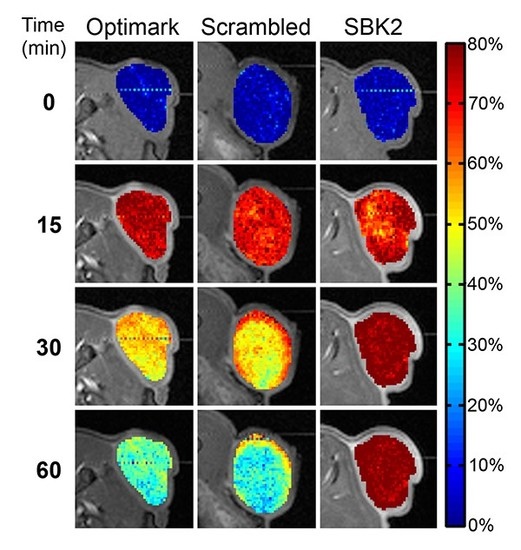
3.2. Quantitative T1 Mapping of MRI Contrast Agent Efficacy in Heterotopic Glioma Flank Tumors
To obtain a quantitative comparison of the difference in tumor enhancement over time between the three contrast agents,
T1 mapping was employed to compare the changes in
T1 relaxation times in the tumor for each contrast agent over time. Pixel-wise maps of
T1 relaxation time were normalized to the mean baseline
T1 values and are shown as heat maps overlaying the corresponding gray scale axial images for the indicated time points in
Figure 2. The color-coded scale bar indicates the normalized
T1 relaxation time from lowest (blue) to highest (red). As expected, all of the contrast agents resulted in a reduction in
T1 relaxation time within the first 15 min after agent injection. Importantly, by 30 min post-injection, both the Optimark™ and scrambled-Tris-(Gd-DOTA)
3 agents had started to clear from the tumor while the targeted SBK2-Tris-(Gd-DOTA)
3 contrast agent was retained within the tumor as evidenced by the limited change in
T1 relaxation time relative to that observed at 15 min post-injection (
Figure 2). At one-hour post-injection, the normalized
T1 relaxation time had returned to ~60% of the pre-contrast values for both Optimark™ and scrambled-Tris-(Gd-DOTA)
3 agents (
Figure 2).
Figure 1.
The SBK2-Tris-(Gd-DOTA)
3 molecular imaging agent and the non-specific agents all enhance LN-229 tumors. Representative brightfield image of GFP-positive LN-229 flank tumors for animals where 0.2 mmol Gd/kg of Optimark™, scrambled-Tris-(Gd-DOTA)
3, or SBK2-Tris-(Gd-DOTA)
3 was administered.
N = 6 for Optimark™,
N = 5 for scrambled-Tris-(Gd-DOTA)
3, and
N = 5 for SBK2-Tris-(Gd-DOTA)
3. GFP fluorescence image of LN-229 tumor cells for each of the three contrast agents.
T2 low-resolution images with Region of Interest (ROI), illustrated by a dashed red line, show the tumor area used for
T1 map quantification in
Figure 2,
Figure 3 and
Figure 4. Axial
T1-weighted images of LN-229 flank tumor at baseline (before injection of contrast agents) and at time of maximum contrast (15 min) following intravenous injection.
Figure 1.
The SBK2-Tris-(Gd-DOTA)
3 molecular imaging agent and the non-specific agents all enhance LN-229 tumors. Representative brightfield image of GFP-positive LN-229 flank tumors for animals where 0.2 mmol Gd/kg of Optimark™, scrambled-Tris-(Gd-DOTA)
3, or SBK2-Tris-(Gd-DOTA)
3 was administered.
N = 6 for Optimark™,
N = 5 for scrambled-Tris-(Gd-DOTA)
3, and
N = 5 for SBK2-Tris-(Gd-DOTA)
3. GFP fluorescence image of LN-229 tumor cells for each of the three contrast agents.
T2 low-resolution images with Region of Interest (ROI), illustrated by a dashed red line, show the tumor area used for
T1 map quantification in
Figure 2,
Figure 3 and
Figure 4. Axial
T1-weighted images of LN-229 flank tumor at baseline (before injection of contrast agents) and at time of maximum contrast (15 min) following intravenous injection.
Figure 2.
The specific molecular imaging agent SBK2-Tris-(Gd-DOTA)3 results in prolonged decrease in T1 relaxation time in tumors compared to non-specific agents. Normalized T1 maps of the flank tumors overlaid onto T1-weighted images at pre-contrast (0 min); 15 min post-injection; 30 min post-injection; and 60 min post-injection. The color-coded scale bar indicates normalized T1 relaxation time values with dark blue representing the lowest T1 values resulting from the T1 shortening effect of the contrast agents. Note the prolonged decrease in normalized T1 values with the SBK2-Tris-(Gd-DOTA)3 agent resulting in lower T1 map values while the T1 values of the non-specific agents have returned to about 60% of baseline.
Figure 2.
The specific molecular imaging agent SBK2-Tris-(Gd-DOTA)3 results in prolonged decrease in T1 relaxation time in tumors compared to non-specific agents. Normalized T1 maps of the flank tumors overlaid onto T1-weighted images at pre-contrast (0 min); 15 min post-injection; 30 min post-injection; and 60 min post-injection. The color-coded scale bar indicates normalized T1 relaxation time values with dark blue representing the lowest T1 values resulting from the T1 shortening effect of the contrast agents. Note the prolonged decrease in normalized T1 values with the SBK2-Tris-(Gd-DOTA)3 agent resulting in lower T1 map values while the T1 values of the non-specific agents have returned to about 60% of baseline.
To evaluate the retention of the agents in greater detail, we plotted the mean tumor normalized
T1 values for groups of mice administered each contrast agent over the entire scanning session of 62.5 min. We found that the mean tumor normalized
T1 values of all three agents showed maximal decreases in
T1 between 10 and 15 min following injection (
Figure 3). Importantly, the normalized
T1 values for the SBK2-Tris-(Gd-DOTA)
3 agent remain reduced over the entire 62.5 min scanning session (
Figure 3), while the scrambled-Tris-(Gd-DOTA)
3 and Optimark™ normalized
T1 mapping values begin to return towards baseline levels starting at approximately 20 min. Normalized
T1 values are significantly different between SBK2-Tris-(Gd-DOTA)
3 and Optimark™ from 30–62.5 min (ranges from
p < 0.001 to
p < 0.04 depending upon the time point), and between SBK2-Tris-(Gd-DOTA)
3 and scrambled-Tris-(Gd-DOTA)
3 from 17.5–62.5 min (ranges from
p < 0.002 to
p < 0.03). The normalized
T1 values for the Optimark™ and scrambled-Tris-(Gd-DOTA)
3 agents were not significantly different at any time point. The rate of contrast agent clearance from the tumor was calculated by determining the change in mean tumor normalized
T1 over time from 15 to 60 min for each agent. The recovery slopes of scrambled-Tris-(Gd-DOTA)
3 and Optimark™ were not statistically different from one another. In contrast, the slope of SBK2-Tris-(Gd-DOTA)
3 recovery differed significantly from that of both scrambled-Tris-(Gd-DOTA)
3 (
p < 0.01) and Optimark™ (
p < 0.0002) demonstrating quantitatively that SBK2-Tris-(Gd-DOTA)
3 is specifically retained in the flank tumors over time.
Figure 3.
Mean tumor normalized T1 values and slope analysis following intravenous administration of Optimark™, scrambled-Tris-(Gd-DOTA)3, or SBK2-Tris-(Gd-DOTA)3 contrast agents in cohorts of nu/nu athymic mice bearing glioma flank tumors administered at a dose of 0.2 mmol·Gd/kg. Note the sustained decrease in normalized T1 for SBK2-Tris-(Gd-DOTA)3 as well as the significant difference in slope due to agent clearance between the non-specific agents compared to SBK2-Tris-(Gd-DOTA)3, which showed the highest retention. N = 6 for Optimark™, N = 5 for scrambled-Tris-(Gd-DOTA)3, and N = 5 for SBK2-Tris-(Gd-DOTA)3. Data plotted as means ± standard error. (A) Mean tumor normalized T1 values at baseline and after agent injection measured every 2.5 min for 62.5 min. Normalized T1 values are significantly different between SBK2-Tris-(Gd-DOTA)3 and Optimark™ from 30–62.5 min (ranges from p < 0.001 to p < 0.04 depending upon the time point), and between SBK2-Tris-(Gd-DOTA)3 and scrambled-Tris-(Gd-DOTA)3 from 17.5–62.5 min (ranges from p < 0.002 to p < 0.03). Optimark™ and scrambled-Tris-(Gd-DOTA)3 were not significantly different at any time point. (B) The slopes of the lines were determined between 15 and 60 min post-injection to examine the rate of agent clearance. The slope of SBK2-Tris-(Gd-DOTA)3 recovery was significantly different than that of both Optimark™ (p < 0.0002) and scrambled-Tris-(Gd-DOTA)3 (p < 0.01). The slopes of Optimark™ and scrambled-Tris-(Gd-DOTA)3 were not significantly different from one another.
Figure 3.
Mean tumor normalized T1 values and slope analysis following intravenous administration of Optimark™, scrambled-Tris-(Gd-DOTA)3, or SBK2-Tris-(Gd-DOTA)3 contrast agents in cohorts of nu/nu athymic mice bearing glioma flank tumors administered at a dose of 0.2 mmol·Gd/kg. Note the sustained decrease in normalized T1 for SBK2-Tris-(Gd-DOTA)3 as well as the significant difference in slope due to agent clearance between the non-specific agents compared to SBK2-Tris-(Gd-DOTA)3, which showed the highest retention. N = 6 for Optimark™, N = 5 for scrambled-Tris-(Gd-DOTA)3, and N = 5 for SBK2-Tris-(Gd-DOTA)3. Data plotted as means ± standard error. (A) Mean tumor normalized T1 values at baseline and after agent injection measured every 2.5 min for 62.5 min. Normalized T1 values are significantly different between SBK2-Tris-(Gd-DOTA)3 and Optimark™ from 30–62.5 min (ranges from p < 0.001 to p < 0.04 depending upon the time point), and between SBK2-Tris-(Gd-DOTA)3 and scrambled-Tris-(Gd-DOTA)3 from 17.5–62.5 min (ranges from p < 0.002 to p < 0.03). Optimark™ and scrambled-Tris-(Gd-DOTA)3 were not significantly different at any time point. (B) The slopes of the lines were determined between 15 and 60 min post-injection to examine the rate of agent clearance. The slope of SBK2-Tris-(Gd-DOTA)3 recovery was significantly different than that of both Optimark™ (p < 0.0002) and scrambled-Tris-(Gd-DOTA)3 (p < 0.01). The slopes of Optimark™ and scrambled-Tris-(Gd-DOTA)3 were not significantly different from one another.
![Diagnostics 05 00318 g003]()
To complement the data presented in
Figure 2 and
Figure 3, maps of the percent change in
T1 values were also calculated at multiple time points for the flank tumors (
Figure 4). Baseline (pre-contrast) values are shown in
Figure 4 and are near zero, as expected. As anticipated, the percent change in
T1 at 15 min post-injection is dramatically increased for all three agents (
Figure 4).
Figure 4.
Maps of percent change in T1 relaxation time for flank tumors overlaid onto axial T1-weighted images plotted at pre-contrast (0 min); 15 min post-injection; 30 min post-injection; and 60 min post-injection. The percent change in T1 values demonstrate that the agent SBK2-Tris-(Gd-DOTA)3 is retained in the tumor for a longer period of time than either Optimark™ or scrambled-Tris-(Gd-DOTA)3 agents. The color scale indicates percent change in T1 relaxation time on a 0%–80% scale.
Figure 4.
Maps of percent change in T1 relaxation time for flank tumors overlaid onto axial T1-weighted images plotted at pre-contrast (0 min); 15 min post-injection; 30 min post-injection; and 60 min post-injection. The percent change in T1 values demonstrate that the agent SBK2-Tris-(Gd-DOTA)3 is retained in the tumor for a longer period of time than either Optimark™ or scrambled-Tris-(Gd-DOTA)3 agents. The color scale indicates percent change in T1 relaxation time on a 0%–80% scale.
By 30 min, the percent change in
T1 is showing some initial reduction for the Optimark™ and scrambled-Tris-(Gd-DOTA)
3 agents, while the percent change in
T1 for the SBK2-Tris-(Gd-DOTA)
3 remains essentially unchanged from that at 15 min (
Figure 4). By 60 min post-injection, the percent change in
T1 values for Optimark™ and scrambled-Tris-(Gd-DOTA)
3-treated animals is closer to that observed at baseline. In contrast, percent change in
T1 values in the animal administered SBK2-Tris-(Gd-DOTA)
3 remains virtually unchanged in comparison to the 15 and 30 min time points (
Figure 4).
A second group of heterotopic glioma flank tumor bearing mice with larger tumors were also administered Optimark™, scrambled-Tris-(Gd-DOTA)
3, and SBK2-Tris-(Gd-DOTA)
3 agents as shown in
Figure 5 with enlarged images. These images show the non-heterogeneous labeling of the tumors by the non-specific agents, whereas the SBK2-Tris-(Gd-DOTA)
3 agent uniformly recognizes the entire tumor. The “rim” or “edge” effect of the non-specific agents is often seen in clinical imaging [
14]. A similar result demonstrating this “rim” effect for non-specific agents was also observed in our previous studies [
8]. In contrast, the SBK2-Tris-(Gd-DOTA)
3 agent exhibits remarkable uniformity across the entire tumor consistent with binding of the PTPµ fragment within the tumors rather than in just the tumor (neo)vasculature.
Figure 5.
Maps representing the percent change in T1 values indicates that the tumors of mice at pre-contrast (0 min); 15 min post-injection; 30 min post-injection; and 60 min post-injection show SBK2-Tris-(Gd-DOTA)3 is retained for a much longer period of time than Optimark™ and scrambled-Tris-(Gd-DOTA)3 agents even in larger tumors. Note also that the T1 changes at 30 min and 60 min for the SBK2-Tris-(Gd-DOTA)3 agent are uniformly distributed throughout the tumor while the non-specific agents show rim enhancement typical of conventional agents. The color scale indicates percent change in T1 relaxation time on a 0%–80% scale.
Figure 5.
Maps representing the percent change in T1 values indicates that the tumors of mice at pre-contrast (0 min); 15 min post-injection; 30 min post-injection; and 60 min post-injection show SBK2-Tris-(Gd-DOTA)3 is retained for a much longer period of time than Optimark™ and scrambled-Tris-(Gd-DOTA)3 agents even in larger tumors. Note also that the T1 changes at 30 min and 60 min for the SBK2-Tris-(Gd-DOTA)3 agent are uniformly distributed throughout the tumor while the non-specific agents show rim enhancement typical of conventional agents. The color scale indicates percent change in T1 relaxation time on a 0%–80% scale.
A significant benefit of
T1 mapping analysis is that this quantitative imaging method allows for the calculation of contrast agent concentration in any region of interest. As shown in
Figure 6, the Gd content in tumors of animals treated with the specific SBK2-Tris-(Gd-DOTA)
3 agent remains at peak levels even an hour after treatment while clearance begins to occur before 30 min in the animals treated with the non-specific agents. As expected, the Gd concentration in control areas of muscle is much lower than that of tumor (
Figure 6B).
Figure 6.
Gadolinium concentrations in tumor and muscle of animals treated with different contrast agents. (A) Maps of gadolinium concentration overlaid onto axial T1-weighted images plotted at pre-contrast (0 min); 10 min post-injection; 15 min post-injection; 30 min post-injection; and 60 min post-injection. Consistent with T1 map values observed for the non-specific contrast agents, Gd concentrations are highest at 10 and 15 min in tumors of animals treated with Optimark™ and scrambled-Tris-(Gd-DOTA)3, and then rapidly decrease at later time points. Gd concentration in tumor of animal receiving SBK2-Tris-(Gd-DOTA)3 remains at near peak levels at 60 min indicating retention of the agent in the tumor. (B) Mean gadolinium concentrations ± SE are plotted for tumor and muscle for groups of animals treated with the indicated contrast agents at different time points. Gd concentrations in tumors of animals treated with Optimark™ and scrambled-Tris-(Gd-DOTA)3 are highest at 10 and 15 min after injection and then decline. In contrast, the Gd concentration in tumors of animals treated with SBK2-Tris-(Gd-DOTA)3 persist at approximately 0.15 mM from 10 to 60 min. Gd concentrations calculated in control muscle regions (hatched bars) are substantially lower than those in tumors.
Figure 6.
Gadolinium concentrations in tumor and muscle of animals treated with different contrast agents. (A) Maps of gadolinium concentration overlaid onto axial T1-weighted images plotted at pre-contrast (0 min); 10 min post-injection; 15 min post-injection; 30 min post-injection; and 60 min post-injection. Consistent with T1 map values observed for the non-specific contrast agents, Gd concentrations are highest at 10 and 15 min in tumors of animals treated with Optimark™ and scrambled-Tris-(Gd-DOTA)3, and then rapidly decrease at later time points. Gd concentration in tumor of animal receiving SBK2-Tris-(Gd-DOTA)3 remains at near peak levels at 60 min indicating retention of the agent in the tumor. (B) Mean gadolinium concentrations ± SE are plotted for tumor and muscle for groups of animals treated with the indicated contrast agents at different time points. Gd concentrations in tumors of animals treated with Optimark™ and scrambled-Tris-(Gd-DOTA)3 are highest at 10 and 15 min after injection and then decline. In contrast, the Gd concentration in tumors of animals treated with SBK2-Tris-(Gd-DOTA)3 persist at approximately 0.15 mM from 10 to 60 min. Gd concentrations calculated in control muscle regions (hatched bars) are substantially lower than those in tumors.
![Diagnostics 05 00318 g006]()