1. Introduction
Bent-core molecules have attracted significant attention in the field of material science due to their remarkable and unique properties. This is because they exhibit macroscopic polar order and chirality in their mesophases, although the constituent molecules are achiral [
1,
2,
3,
4,
5,
6,
7]. Although the initial application of these materials targeted the field of display application, at present researchers are looking to identify other uses, such as organic structural units for the fabrication of semiconductors or optical devices [
8].
It is well known that the mesophase behavior of liquid crystalline compounds is influenced by structural parameters such as the number and structure of the cycles, the structure, position and direction of the linking units, the presence of lateral groups and by the length of the terminal flexible chain [
9,
10,
11]. However, compared with calamitic compounds, the mesophase behavior of bent-core liquid crystals depends to a greater extent on structural variations in the molecules [
12]. According to Weissflog et al. [
13], the mesophase behavior and physical properties of bent-core compounds are more dependent on the direction of the carboxyl linkage units between aromatic rings, compared with calamitic structures.
Most bent-core liquid crystals are derived from resorcinol [
14,
15,
16] because of their easy synthesis, while molecules with reversed esteric linking groups at the central phenyl ring derived from isophthalic acid, are fewer [
17,
18] According to Nguyen et al. [
19], isophthalic acid units as the central part of bent-core compounds are less favorable for generating the optimal packing of bent-core molecules. Indeed, compounds based on isophthalic acid prevent mesophase formation in some cases, or induce high phase transition temperatures and metastable phases [
18]. Nevertheless, depending on the nature of the linking units and the number of aromatic rings, the packing of bent-shaped molecules that are more or less rigid with long terminal chains, induces optimum distribution of the electronic density through the molecules and stimulates mesomorphism.
The goal of this paper was to study the influence on the mesophase behavior of the ester unit orientation between the central benzene cycle and the two wings of symmetrically bent-core compounds derived from resorcinol and isophthalic acid, see
Figure 1. For this reason, we synthetized two classes of bent-core mesogens with seven rings into structures with the same connection units between the aromatic cycles and equal length of the terminal chains.
In 2014, we reported the mesophase behavior of 1,3-disubstituted benzene compounds (
I), derived from resorcinol containing seven aromatic rings and three different linkages between aromatic cycles: ester, imine and azo type [
16]. All the reported compounds present liquid crystalline behavior, with characteristic polar smectic textures and stable mesophase domains. Here, we synthetized the second new class of seven benzene-ring containing banana-shaped liquid crystals (
II) derived from 1,3-isophthalic acid, in order to understand the different thermal behavior, compared with the previously reported compounds. For this reason, several physical methods were used: differential scanning calorimetry, polarizing optical microscopy and thermogravimetric study, along with molecular modeling studies. Information at the molecular level were obtained by combining quantum calculation with atomistic ones. Theoretical studies were performed for single molecules, but also for molecules assembled in different ways.
2. Materials and Methods
The starting materials such as resorcinol, isophthalic acid, 4-nitrophenol, DCC, DMAP, 1-bromohexane, 1-bromoheptane, 1-bromooctane, 1-bromodecane and 1-bromooctadecane were obtained from (Sigma-Aldrich, St. Louis, MO, USA) and were used without further purification. Synthesis of 4-((4-alkyloxyphenyl)azo)-benzaldehyde derivatives was previously reported in [
16]. All intermediary compounds were purified by column chromatography with Silica gel 60 for the 4-((4-alkyloxyphenyl)azo)-benzaldehyde derivatives and Al
2O
3 (Merck, Darmstadt, Germany) for the 1,3-bis(4-aminophenyl) isophthalate. The chromatography (TLC) was performed on silica gel plates (Merck, silica gel F254, Darmstadt, Germany). All organic solvents (acetone, dichloromethane, ethyl acetate, hexane) used in the chemical synthesis, and for purification, the reaction products were purchased from Chemical Company (Iași, Romania) were dried, distilled (conventional methods) or used as bought.
Confirmation of the structure of the intermediate compounds 1,3-bis(4-nitrophenyl) isophthalate and 1,3-bis(4-aminophenyl) isophthalate was obtained by
1H-NMR and
13C-NMR spectra (
Figures S1–S4 in Supplementary Materials). The experiments were recorded using a Bruker Avance III (Bruker, Karlsruhe, Germany), 500 MHz frequency spectrometer, equipped with a 5 mm Pabbo detection probe and operating at 500 MHz for
1H nucleus and 125 MHz for
13C nucleus. Chemical shifts are reported in delta (δ) units, part per million (ppm), relative to the deuterated solvent dimethyl sulfoxide (ref. DMSO-d6: 2.50 ppm (
1H) and 39.52 ppm (
13C). The following abbreviations were used to designate chemical shift multiplicities: s = singlet, brs = broad singlet, d = doublet, dd = doublet of doublet, t = triplet. All spectra were recorded at 298 K. NMR data were processed and analyzed using BrukerTopSpin3.2. software. The chemical structure of the final compounds
IIa–
IIe was confirmed by infrared spectroscopy on solid samples and elemental analysis, due to the compounds’ insolubility. IR-ATR analysis was performed with a resolution of 1 cm
−1 in the 4000 cm
−1 to 700 cm
−1 range on a Bruker Vertex 70 spectrometer (Bruker, Karlsruhe, Germany), with atmospheric compensation for water and CO
2 gases. IR spectra consists of the co-addition of 32 interferograms, using the Blackman–Harris apodization function in the FT mathematical apparatus. Elemental analysis was carried out by means of the CHN 2400 II Perkin Elmer analyzer (PerkinElmer, Waltham, MA, USA).
The optical microscopy studies were carried out with an Olympus BX60F5 polarizing microscope (Olympus Corporation, Tokyo, Japan) equipped with a Linkam LTS 350 hotstage (Linkam Scientific Instruments Ltd., Tadworth, UK). The textures of the compounds were observed using polarized light with a cross polarizer, whereby the studied sample was prepared in a thin film sandwiched between a glass slide and coverslip.
The evaluation of the thermal stability of the synthesized materials was carried out by thermal gravimetric analysis (TGA). Thermogravimetric (TG), derived thermogravimetric (DTG) and differential thermal (DTA) curves were recorded using Mettler Toledo 851e equipment (Mettler Toledo, Greifensee, Switzerland) in an inert atmosphere with a nitrogen flow rate of 20 mL/min and a heating rate of 10 °C/min. The mass of the samples subjected to thermogravimetric analysis ranged from 2.5 to 4.7 mg.
The differential scanning calorimetry (DSC) technique was also applied to highlight the phase transitions occurring in the analyzed materials. Mettler Toledo DSC equipment was used, which allowed the recording of DSC curves in an inert atmosphere with a heating rate of 10 °C/min and a sample mass between 2.4 and 5.0 mg. Three heating steps and two cooling steps were performed in the temperature range of 25–300 °C. The TGA and DSC curves obtained were evaluated with Mettler Toledo’s STARe software.
We utilized the DMol
3 and Forcite packages in Material Studio 4.0 software to calculate the properties of the systems [
20]. Since DMol
3 minimizes the energy of the starting structure, for the calculation of the global energy minimum the torsion angles of structures
Ia and
IIa were successively changed. For the obtained structure a geometry optimization was performed by DFT calculation, using the PWC functional considering 2 × 10
−5 Ha energy convergence. Starting from these structures, methyl groups were added in order to obtain the higher isomers. All these isomers were minimized by density functional theory (DFT) through the DMol
3 module. For compounds
I and
II with 7 and 10 carbon atoms in a flexible terminal chain, different types of stacking were considered. These structures were optimized by atomistic simulations using Forcite module until 2 × 10
−4 kcal/mol energy convergence was obtained [
21]. In order to simulate the behavior under repeated heating–cooling, and to verify the stability of the supramolecular structures, an annealing procedure was applied to these stacks: 3 annealing cycles, 300–550 K for type
I structures and 300–500 K for type
II structures (considering the information from thermal analysis), 6 heating ramps per cycle, 10.000 dynamic steps per ramp, and keeping the same level of convergence, 2 × 10
−4 kcal/mol, for minimization procedures.
4. Discussion
4.1. Mesomorphic Behavior
The mesophases were assigned and characterized by polarizing microscopy. All compounds exhibit mesomorphic properties, as a result of the presence of multiple transitions on heating and cooling. However, in the case of bent-core compounds derived from isophthalic acid, the mesophases go up to high temperatures and exist in the metastable state (
Table 1).
The thermal study evidenced that the direction of ester-connecting groups strongly influences the type of mesophases and their thermal stability. Hence, in spite of the minor structural differences between the two classes of bent-core compounds, the mesophases for isophthalic derivatives were totally different compared with the mesophases of resorcinol derivatives. Moreover, the isotropization temperatures increased over 300 °C for the first three compounds of the class (IIa–c). The melting and isotropization temperatures decreased with lengthening of the alkyl flexible chains.
According to the experimental data, the stability of the mesophase domains for compounds derived from resorcinol derivatives (
Ia–
e) (
Table 2) [
16] was higher compared to compounds derived from isophthalic acid (
IIa–
e) (
Figure 2).
A relationship between the mesophase stability on heating and the odd/even number of carbon atoms in the flexible chain for compounds Ia–e was also observed, while for derivatives IIa–e, this ratio was no longer met. The greatest difference in mesophase stability was observed for compounds with 10 (if Ie is compared to IId) carbon atoms in the terminal chain, where compounds derived from resorcinol (IIe) had the highest stability.
The comparison of transition temperatures (first heating and cooling) between compounds
I and
II with 7 atoms in a flexible terminal chain is shown in
Figure 3.
It is clear that compound Ib exhibits reversible mesomorphic behavior, as evidenced by four phase transitions on heating and three on cooling, corresponding to smectic phases, which were also assigned by POM. As for compound IIb, the first crystal to crystal transition peak appears at 138 °C, followed by crystal to smectic transitions and smectic to nematic transition at 283 °C, respectively. The transition to isotropic phase was assigned by microscopy at 312 °C, when partial degradation was observed at the edge of the sample, at 30 °C/min rate.
While textures of mesophases for compounds
Ia–
e were all of smectic type, compounds
IIa–
e presented both smectic and nematic phases on heating and cooling cycles, but on shorter domains (
Figure 4). Compound
IIa (n = 6) was an exception as it showed the nematic phase only on cooling, from 305 °C to 242 °C in the form of characteristic Schlieren texture (
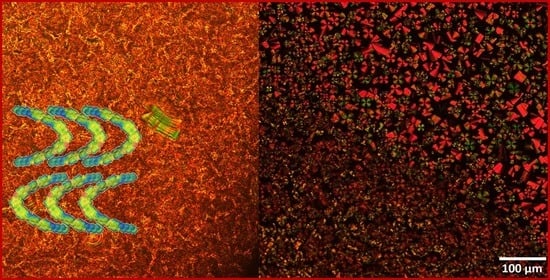
Figure 5a). In addition, it can be seen that as the number of carbon atoms on the terminal chains increased, the nematic phase on cooling was suppressed, so that the last compounds of the series (with 10 and 18 carbon atoms) showed only smectic phases.
In terms of compound
IIb, on cooling, the isotropic to nematic transition was assigned by microscopy at 304 °C, followed by a transition to the smectic phase at 263 °C and crystallization at 241 °C. The pictures taken at 279 °C on first cooling confirm the nematic phase with characteristic Schlieren and ribbon like textures (
Figure 5b) while smectic and focal conic arrangements are visible at 262 °C (
Figure 5c). Smectic phases with relatively low viscosity and spherulitic domains growing from isotropic melt were observed with polarized optical microscopy on cooling for compound
IIc (
Figure 5d) and transition to the crystal phase at 236 °C (
Figure 5e), respectively.
The compound with 10 carbon atoms on each wing,
IId, shows a different mesophase texture compared with previous ones, of an unidentified smectic type (
Figure 5f). Unidentified smectic phase texture on heating and cooling was observed in the last compound of the class,
IIe, which also features a higher viscosity compared with previous compounds with shorter flexible chains.
4.2. Thermogravimetric Study
The results showed lower thermal stability in compounds derived from isophthalic acid,
II, compared with the ones derived from resorcinol
I. In the case of derivatives,
I, thermal decomposition (DTG curves) starts at about 345 °C and shows two main stages and the decomposition rate is maximum at 361 °C (T
peak1) and 437 °C (T
peak2) (
Figure 6a). Thermal decomposition in an inert atmosphere starts for compound
II at temperatures above 300 °C and also shows two main stages, while the decomposition rate is maximum at 351 °C (T
peak1) and 437 °C (T
peak2) (
Figure 6b).
The first stage of decomposition in both classes of compounds is a strongly exothermic process (DTA curves,
Figure 6c,d) which takes place in a narrow temperature range of approximately 80 °C and can be attributed to the cleavage of –N=N– bonds. This process probably continues with the cleavage of aromatic units and the terminal chain [
22]. The TG curves shown in
Figure 6e,f indicate that the percentage mass losses in the second step increase with increasing terminal chain length. The highest mass loss in this stage was identified for the sample containing 18 carbon atoms in the terminal chain (
IIe). For this sample we also found the lowest percentage of residue at 900 °C, i.e., 30%. For the other isophthalic derivatives, the percentage of residue recorded at the end of the thermogravimetric analysis was about 42%. In the case of bent-core resorcinol derivatives, the amount of residue varied between 31 and 39%.
It was found that by changing the way the two wings are connected to the central unit, the thermal stability was influenced to a quite large extent, but this did not influence the degradation mechanism. Thermal decomposition of class compounds II starts at temperatures about 45 °C lower than class compounds I. In the case of both classes of compounds, two main degradation steps were evident, with the same thermogravimetric curve shape and similar thermal characteristics.
4.3. Molecular Modeling
In order to explore the potential energy surface of compounds
Ia and
IIa, the most important torsion angles (see
Figure 7) were modified one by one with a step of 60° and energetically minimized.
The angles in the flexible terminal chains were not taken into account, considering they are only important for the mesophase stability. After the complete scanning process, the structures were subjected to ab initio energy minimization. Following this, changes in torsion occurred (
Table 3), with the most significant change being observed for compound
Ia.
Quantum mechanics allows the determination of the electron density map (
Figure 8), which shows the distribution of electrons around nuclei.
Even if the central angle after minimization was 120°, it is obvious that the wings in the structures derived from resorcinol are “closer”, while they are “wider” in the isophthalic derivatives. This is expected to influence the ordering mode in the self-assembled structures along with the packing density of the mesogens [
23]. The total dipole moment for all
Ia–
e and
IIa–
e structures was also calculated, noting the odd-even effect that alkyl groups induce (
Table 4).
Next, we examined the macroscale behavior of the purposed structures [
1,
21]. Considering the characteristic B phases that bent-core compounds form, we looked to see, only from a theoretical point of view, what the preferred molecular arrangements of compounds
I and
II would be. Three types of multi-level organization have been considered for structures with 7 and 10 carbon atoms in terminal alkyl groups: a columnar arrangement (assigned to the B1 phase) and ferro/antiferroelectric arrangements, respectively (
Figure 9).
After minimization of the packed structures, it was found that type
Ib and
Ie mesogens prefer smectic antiferroelectric ordering (
Table 5—energy before heat treatment) over ferroelectric or columnar smectic structures. By increasing the number of carbon atoms from 7 to 10, all systems show greater stability, which can probably be attributed to van der Waals interactions. By comparing the energies of the structures of mesogens with the same number of atoms, i.e.,
Ib with
IIb and
Ie with
IId, it can be seen that derivatives from isophthalic acid have lower energies than resorcinol equivalent compounds, for all the proposed ordering types. This is in line with the trend in the experimental data, i.e., for type
II compounds neither polar smectic nor columnar ordering were observed. Additionally, bent-core compounds derived from isophthalic acid (
II structures) have lower moment dipole values than compounds derived from resorcinol (
I structures). This, along with the steric hindrance observed in molecular simulations, may indicate that type II structures cannot form polar smectic mesophases.
After heat treatment, it was found that all structures with a columnar packing order lose this property, rather, they adopt an isotropic type structure after each heating–cooling cycle (
Figure 10). As smectic structures, whether ferroelectric or antiferroelectric, they evolve but without losing ordering in the layers.
Hence, the theoretical calculations indicated that columnar-type ordering is not favorable for all of the considered compounds, while a smectic order is favorable for bent-core resorcinol derivatives.