Bio-Surfactant Assisted Aqueous Exfoliation of High-Quality Few-Layered Graphene
Abstract
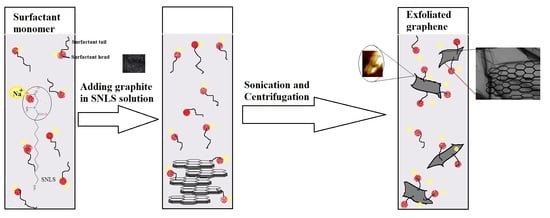
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Graphene Concentration as a Function of Sonication Time (Cg vs. t)
3.2. Graphene Concentration as a Function of Surfactant Concentration (Cg vs. Cs)
3.3. Graphene Concentration as a Function of Centrifugation Frequency (Cg vs. Cf)
3.4. XRD Analysis
3.5. Characterization of Graphene Using TEM and AFM
3.6. Raman Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nguyen, B.H.; Nguyen, V.H. Promising applications of graphene and graphene-based nanostructures. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 23002. [Google Scholar] [CrossRef] [Green Version]
- Radich, J.G.; McGinn, P.J.; Kamat, P.V. Graphene-based composites for electrochemical energy storage. Electrochem. Soc. Interface 2011, 20, 63–66. [Google Scholar] [CrossRef] [Green Version]
- Justino, C.I.L.; Gomes, A.R.; Freitas, A.C.; Duarte, A.C. Graphene-based sensors and biosensors T.A.P. Rocha-Santos. TrAC Trends Anal. Chem. 2017, 91, 53–66. [Google Scholar] [CrossRef]
- Liu, C.H.; Chang, Y.; Norris, T.B.; Zhong, Z. Graphene photodetectors with ultra-broadband and high responsivity at room temperature. Nat. Nanotech. 2014, 9, 273–278. [Google Scholar] [CrossRef]
- Tong, Y.; Bohmb, S.; Song, M. Graphene-based materials and their composites as coatings. Austin. J. Nanomed. Nanotechnol. 2013, 1, 1003. [Google Scholar]
- Liu, J.; Cui, L.; Losic, D. Graphene, and graphene oxide as new nanocarriers for drug delivery applications. Acta Biomater. 2013, 9, 9243–9257. [Google Scholar] [CrossRef]
- Shen, H.; Zhang, L.; Liu, M.; Zhang, Z. Biomedical applications of graphene. Theranostics 2012, 2, 283. [Google Scholar] [CrossRef] [Green Version]
- Ismail, Z.; Abdullah, A.H.; Abidin, A.S.Z.; Yusoh, K. Production of functional graphene by kitchen mixer: Mechanism and metric development for in situ measurement of sheet size. J. Nanostruct. Chem. 2017, 7, 231–242. [Google Scholar] [CrossRef]
- Güler, O.; Güler, S.H.; Selen, V.; Albayrak, M.G.; Evin, E. Production of graphene layer by liquid-phase exfoliation with low sonication power and sonication time from synthesized expanded graphite. Fuller. Nanotub. Carbon Nanostruct. 2015, 24, 123–127. [Google Scholar] [CrossRef]
- Xu, J.; Dang, D.K.; Tran, V.T.; Liu, X.; Chung, J.S.; Hur, S.H.; Choi, W.M.; Kim, E.J.; Kohl, P.A. Liquid-phase exfoliation of graphene in organic solvents with addition of naphthalene. J. Colloid Interface Sci. 2014, 418, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.W.; Nong, W.J. Direct exfoliation of graphene in organic solvents with addition of “NaOH”. Chem. Commun. 2011, 47, 6888–6890. [Google Scholar]
- Wencheng, D.; Jie, L.; Peipei, S.; Yinyan, Z.; Xiaoqing, O. Organic salt-assisted liquid-phase exfoliation of graphite to produce high-quality graphene. J. Chem. Phys. Lett. 2013, 198, 568–569. [Google Scholar]
- Deshmukh, K.; Khatake, S.M.; Joshi, G.M. Surface properties of graphene oxide reinforced polyvinylchloride nanocomposites. J. Polym. Res. 2013, 20, 286. [Google Scholar] [CrossRef]
- Wang, Z.-D.; Inagaki, M. Stage Formation of Graphite Intercalation Compounds in Molten Salts. J. Mater. Chem. 1992, 2, 629–632. [Google Scholar] [CrossRef]
- Coleman, J.N. Liquid exfoliation of Defect-free graphene. Acc. Chem. Res. 2013, 46, 14–22. [Google Scholar] [CrossRef]
- Nicolosi, V.; Chhowalla, M.; Kanatzidis, M.G.; Strano, M.S.; Coleman, J.N. Liquid exfoliation of layered materials. Science 2013, 340, 1226419. [Google Scholar] [CrossRef] [Green Version]
- Creighton, J.R.; Ho, P. Introduction to chemical vapor deposition (CVD). Chem. Vap. Depos. 2001, 2, 1–22. [Google Scholar]
- Hunt, A.T.; Pohl, M. Combustion chemical vapor deposition (CCVD). In Chemical Vapor Deposition; Surface Engineering Series; Park, J.-H., Sudarshan, T.S., Eds.; ASM International: Materials Park, OH, USA, 2001; Volume 2, pp. 81–102. [Google Scholar]
- Lotya, M.; Hernandez, Y.; King, P.J.; Smith, R.J.; Nicolosi, V.; Karlsson, L.S.; Blighe, F.M.; De, S.; Wang, Z.; McGovern, I.T.; et al. Liquid phase production of graphene by exfoliation of graphite in surfactant/water solutions. J. Am. Chem. Soc. 2009, 131, 3611–3620. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formations, properties, and applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef] [Green Version]
- Hanno, I.; Centini, M.; Anselmi, C.; Bibiani, C. Green cosmetic surfactant from rice: Characterization and application. Cosmetics 2015, 2, 322–341. [Google Scholar] [CrossRef]
- Chen, D.; Li, Z.; Wan, Y.; Tu, X.; Shi, Y.; Chen, Z.; Shen, W.; Yu, C.; Tua, B.; Zhao, D. Anionic surfactant induced mesophase transformation to synthesize highly ordered large-pore mesoporous silica structures. J. Mater. Chem. 2006, 16, 1511–1519. [Google Scholar] [CrossRef]
- Ranieri, D.; Preisig, N.; Stubenrauch, C. On the Influence of Intersurfactant H-bonds on Foam Stability: A Study with Technical Grade Surfactants. Tenside Surf. Det. 2018, 55, 1. [Google Scholar] [CrossRef]
- Akter, N.; Radiman, S.; Mohamed, F.; Rahman, I.A.; Reza, M.I.H. Ternary phase and vesicle formation of a sodium Nlauroylsarcosinate hydrate/1-decanol/water system. Sci. Rep. 2011, 1, 71. [Google Scholar] [CrossRef]
- Ambühl, M.; Bangerter, F.; Luisi, P.L.; Skrobal, P.; Watzke, H.J. Configurational changes accompanying vesiculation of mixed single-chain amphiphiles. Langmuir 1993, 9, 36–38. [Google Scholar] [CrossRef]
- Ghosh, S.; Dey, J. Interaction of sodium N-lauroyl sarcosinate with N-alkyl pyridinium chloride surfactants: Spontaneous formation of pH-responsive, stable vesicles in aqueous mixtures. J. Colloid Interface Sci. 2011, 358, 208–216. [Google Scholar] [CrossRef]
- Akter, N.; Radiman, S. Effect of polyethylene glycol-2000 on amino acid surfactant-based vesicles. Colloid Polym. Sci. 2014, 292, 1619–1625. [Google Scholar] [CrossRef]
- Nuvoli, D.; Valentini, L.; Alzari, V.; Scognamillo, S.; Bon, S.B.; Piccinini, M.; Illescasd, J.; Mariani, A. High concentration few-layer graphene sheets obtained by liquid-phase exfoliation of graphite in ionic liquid. J. Mater. Chem. 2011, 21, 3428–3431. [Google Scholar] [CrossRef]
- Choi, W.S.; Lee, Y.B.; Noh, Y. Production of graphene by exfoliation of graphite in a volatile organic solvent. Nanotechnology 2011, 22, 365601. [Google Scholar] [CrossRef] [PubMed]
- Kravets, V.G.; Grigorenko, A.N.; Nair, R.P.; Blake, P.; Anisimova, S.; Novoselov, K.S.; Geim, A.K. Spectroscopic ellipsometry of graphene and an exciton-shifted van Hove peak in absorption. Phys. Rev. B 2010, 81, 155413. [Google Scholar] [CrossRef] [Green Version]
- Elliott, J.D.; Xu, Z.; Umari, P.; Jayaswal, G.; Chen, M.; Zhang, X.; Martucci, A.; Marsili, M.; Merano, M. Surface susceptibility and conductivity o MoS and WSe2 monolayers: A first-principles and ellipsometry characterization. Phys. Rev. B 2020, 101, 045414. [Google Scholar] [CrossRef] [Green Version]
- Khan, U.; O’Neill, A.; Lotya, M.; De, S.; Coleman, J.N. High-concentration solvent exfoliation of graphene. Small 2010, 6, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Cao, H.; Xue, Y.; Li, B.; Cai, W. Liquid-phase exfoliation of graphene: An overview on exfoliation media, techniques. Nanomaterials 2018, 8, 942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salavagione, H.J.; Sherwood, A.; De Bruyn, M.; Budarin, V.L.; Ellis, G.J.; Clark, J.H.; Shuttleworth, P.S. Identification of high-performance solvents for the sustainable processing of graphene. Green Chem. 2017, 19, 2550–2560. [Google Scholar] [CrossRef] [Green Version]
- Ciesielski, A.; Samorì, P. Graphene via sonication assisted liquid-phase exfoliation. Chem. Soc. Rev. 2014, 43, 381–398. [Google Scholar] [CrossRef]
- Wang, S.; Yi, M.; Liang, S.; Shen, Z.; Zhang, X.; Ma, S.S.; Wang, M.; Yi, Z.; Shen, T. The effect of surfactants and their concentration on the liquid exfoliation of graphene. RSC Adv. 2016, 6, 56705–56710. [Google Scholar] [CrossRef] [Green Version]
- Fendler, J.H. Membrane Mimetic Chemistry; Wiley-InterScience: New York, NY, USA, 1982. [Google Scholar]
- Alargova, R.G.; Ivanova, V.P.; Kralchevsky, P.A.; Mehreteab, A.; Broze, G. Growth of rod-like micelles in anionic surfactant solutions in the presence of Ca 2+ counterions. Colloids Surf. A 1998, 142, 201–218. [Google Scholar] [CrossRef]
- Shirley, S.; Sonia, V.P.; Eneida, P. Surface active drugs: Self-association and interaction with membranes and surfactant. Physiochemical and biological aspects. Biophys. Acta 2000, 1508, 210–234. [Google Scholar]
- Santos, H.M.; Lodeiro, C.; Capelo-Martinez, J.L. The power of ultrasound. In Ultrasound in Chemistry: Analytical Applications; Wiley: New York, NY, USA, 2009. [Google Scholar]
- Lotya, M.; King, P.J.; Khan, U.; De, S.; Coleman, J.N. High-concentration, surfactant-stabilized graphene dispersions. ACS Nano 2010, 4, 3155–3162. [Google Scholar] [CrossRef]
- Lee, H.; Park, J.Y. Height determination of single-layer graphene on mica at controlled humidity using atomic force microscopy. Rev. Sci. Instrum. 2019, 90, 103702. [Google Scholar] [CrossRef]
- Wu, Z.; Ren, W.; Gao, L.; Liu, B.; Jiang, C.; Cheng, H. Synthesis of high-quality graphene with a pre-determined number of layers. Carbon 2009, 47, 493–499. [Google Scholar] [CrossRef]
- Nemes-Incze, P.; Osváth, Z.; Kamarás, K.; Biró, L.P. Anomalies in thickness measurements of graphene and few-layer graphite crystals by tapping mode atomic force microscopy. Carbon 2008, 46, 1435–1442. [Google Scholar] [CrossRef] [Green Version]
- Rao, C.N.R.; Biswas, K.; Subrahmanyam, K.S.; Govindaraj, A. Graphene, the new Carbon. J. Mater. Chem. 2009, 19, 2457–2469. [Google Scholar] [CrossRef]
- Vidano, R.P.; Fishbach, D.B.; Willis, L.J.; Loehr, T.M. Observation of Raman band shifting with excitation wavelength for carbons and graphites. Solid State Commun. 1981, 39, 341–344. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [Green Version]
- Eckmann, A.; Felten, A.; Mishchenko, A.; Britnell, L.; Krupke, R.; Novoselov, K.S.; Casiraghi, C. Probing nature of defects in graphene by Raman spectroscopy. Nano Lett. 2012, 12, 3925–3930. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Kelly, P.B.; Clifford, A.J. Biological/biomedical accelerator mass spectrometry targets. 2. Physical, morphological, and structural characteristics. Anal. Chem. 2008, 80, 7661. [Google Scholar] [CrossRef] [Green Version]
- Arao, Y.; Mizuno, Y.; Araki, K.; Kubouchi, M. Mass production of high-aspect-ratio few-layer-graphene by high-speed laminar flow. Carbon 2016, 102, 330–338. [Google Scholar] [CrossRef]
- Ou, E.; Xie, Y.; Peng, C.; Song, Y.; Peng, H.; Xiong, Y.; Xu, W. High concentration and stable few-layer graphene dispersions prepared by the exfoliation of graphite in different organic solvents. RSC Adv. 2013, 3, 9490–9499. [Google Scholar] [CrossRef]
- Haar, S.; Ciesielski, A.; Clough, J.; Yang, H.; Mazzaro, R.; Richard, F.; Conti, S.; Merstorf, N.; Cecchini, M.; Morandi, V.; et al. A supramolecular strategy to leverage the liquid-phase exfoliation of graphene in the presence of surfactants. Unraveling the role of the length of fatty acids. Small 2015, 11, 1691. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.-K.; Kim, T.-S.; Park, C.; Xu, W.; Baek, K.; Bae, S.-H.; Ahn, J.-H.; Kim, K.; Choi, H.C.; Lee, T.-W. Value-added Synthesis of Graphene: Recycling Industrial carbon waste into electrodes for high-performance electronic devices. Sci. Rep. 2015, 5, 16710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unalan, I.U.; Wan, C.; Trabattoni, S.; Piergiovanni, L.; Farris, S. Polysaccharire-assisted rapid exfoliation of graphite platelets into high-quality water-dispersible graphene sheets. RSC Adv. 2015, 5, 26482–26490. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Lua, A.C. A facile method for the large-scale continuous synthesis of graphene sheets using a novel catalyst. Sci. Rep. 2013, 3, 3037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valles, C.; Drummond, C.; Saadaoui, H.; Furtado, C.A.; He, M.; Rouleau, O.; Ortolani, L.; Monthioux, M.; Penicaud, A. Solutions of negatively charged graphene sheets and ribbons. J. Am. Chem. Soc. 2008, 130, 15802–15804. [Google Scholar] [CrossRef] [PubMed]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akter, N.; Mawardi Ayob, M.T.; Radiman, S.; Khandaker, M.U.; Osman, H.; Alamri, S. Bio-Surfactant Assisted Aqueous Exfoliation of High-Quality Few-Layered Graphene. Crystals 2021, 11, 944. https://doi.org/10.3390/cryst11080944
Akter N, Mawardi Ayob MT, Radiman S, Khandaker MU, Osman H, Alamri S. Bio-Surfactant Assisted Aqueous Exfoliation of High-Quality Few-Layered Graphene. Crystals. 2021; 11(8):944. https://doi.org/10.3390/cryst11080944
Chicago/Turabian StyleAkter, Nasima, Muhammad Taqiyuddin Mawardi Ayob, Shahidan Radiman, Mayeen Uddin Khandaker, Hamid Osman, and Sultan Alamri. 2021. "Bio-Surfactant Assisted Aqueous Exfoliation of High-Quality Few-Layered Graphene" Crystals 11, no. 8: 944. https://doi.org/10.3390/cryst11080944