Chemical-Physical Characterization of a Binary Mixture of a Twist Bend Nematic Liquid Crystal with a Smectogen
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
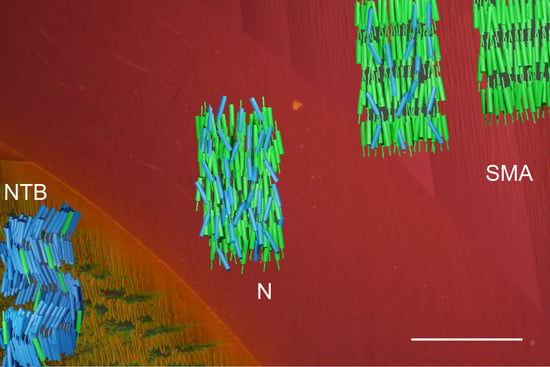
2.2. Optical Microscopy
2.3. Birefringence Measurements
2.4. X-Ray Characterization
2.5. Differential Scanning Calorimetry
2.6. Dielectric and Elastic Characterization
3. Results and Discussions
3.1. Phase Diagrams
3.2. Enthalpies of the Phase Transitions
3.3. Evolution of Birefringence
3.4. Anchoring Transition
3.5. Dielectric Permittivities
3.6. Nematic Elastic Constants
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Panov, V.P.; Nagaraj, M.; Vij, J.K.; Panarin, Y.P.; Kohlmeier, A.; Tamba, M.G.; Lewis, R.A.; Mehl, G.H. Spontaneous Periodic Deformations in Nonchiral Planar-Aligned Bimesogens with a Nematic-Nematic Transition and a Negative Elastic Constant. Phys. Rev. Lett. 2010, 105, 167801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cestari, M.; Diez-Berart, S.; Dunmur, D.A.; Ferrarini, A.; De la Fuente, M.R.; Jackson, D.J.B.; Lopez, D.O.; Luckhurst, G.R.; Perez-Jubindo, M.A.; Richardson, R.M.; et al. Phase behavior and properties of the liquid-crystal dimer 1′′,7′′-bis(4-cyanobiphenyl-4′-yl) heptane: A twist-bend nematic liquid crystal. Phys. Rev. E 2011, 84, 031704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borshch, V.; Kim, Y.-K.; Xiang, J.; Gao, M.; Jákli, A.; Panov, V.P.; Vij, J.K.; Imrie, C.T.; Tamba, M.G.; Mehl, G.H.; et al. Nematic twist-bend phase with nanoscale modulation of molecular orientation. Nat. Commun. 2013, 4, 2635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.; Porada, J.H.; Hooper, J.B.; Klittnick, A.; Shen, Y.; Tuchband, M.R.; Korblova, E.; Bedrov, D.; Walba, D.M.; Glaser, M.A.; et al. Chiral heliconical ground state of nanoscale pitch in a nematic liquid crystal of achiral molecular dimers. Proc. Natl. Acad. Sci. USA 2013, 110, 15931–15936. [Google Scholar] [CrossRef] [Green Version]
- Meyer, R.B. Structural problems in liquid crystal physics. In Les Houches Summer School in Theoretical Physics; Molecular Fluids; Balian, R., Weill, G., Eds.; Gordon and Breach: New York, NY, USA, 1976; Volume 25, pp. 316–320. ISBN 0-677-16020-8. [Google Scholar]
- Dozov, I. On the spontaneous symmetry breaking in the mesophases of achiral banana-shaped molecules. Eur. Lett. 2001, 56, 247–253. [Google Scholar] [CrossRef]
- Meyer, C.; Luckhurst, G.R.; Dozov, I. The temperature dependence of the heliconical tilt angle in the twist-bend nematic phase of the odd dimer CB7CB. J. Mater. Chem. C 2015, 3, 318–328. [Google Scholar] [CrossRef]
- Panov, V.P.; Balachandran, R.; Nagaraj, M.; Vij, J.K.; Tamba, M.G.; Kohlmeier, A.; Mehl, G.H. Microsecond linear optical response in the unusual nematic phase of achiral bimesogens. Appl. Phys. Lett. 2011, 99, 261903. [Google Scholar] [CrossRef] [Green Version]
- Meyer, C.; Luckhurst, G.R.; Dozov, I. Flexoelectrically Driven Electroclinic Effect in the Twist-Bend Nematic Phase of Achiral Molecules with Bent Shapes. Phys. Rev. Lett. 2013, 111, 067801. [Google Scholar] [CrossRef]
- Walker, R. The twist-bend phases: Structure–property relationships, chirality and hydrogen-bonding. Liq. Cryst. Today 2020, 29, 2–14. [Google Scholar] [CrossRef]
- Wang, Y.; Singh, G.; Agra-Kooijman, D.M.; Gao, M.; Bisoyi, H.K.; Xue, C.; Fisch, M.R.; Kumar, S.; Li, Q. Room temperature heliconical twist-bend nematic liquid crystal. CrystEngComm 2015, 17, 2778–2782. [Google Scholar] [CrossRef]
- Walker, R. Synthesis and Characterisation of Novel Liquid Crystalline Materials: Structure-Property Relationships, Chirality, and the Twist-Bend Nematic Phase. Ph.D. Thesis, University of Aberdeen, Aberdeen, UK, 2019. [Google Scholar]
- Paterson, D.A.; Abberley, J.P.; Harrison, W.T.; Storey, J.M.; Imrie, C.T. Cyanobiphenyl-based liquid crystal dimers and the twist-bend nematic phase. Liq. Cryst. 2017, 1–20. [Google Scholar] [CrossRef]
- Arakawa, Y.; Komatsu, K.; Ishida, Y.; Tsuji, H. Thioether-linked azobenzene-based liquid crystal dimers exhibiting the twist-bend nematic phase over a wide temperature range. Liq. Cryst. 2020, 1–12. [Google Scholar] [CrossRef]
- Arakawa, Y.; Ishida, Y.; Tsuji, H. Ether- and Thioether-Linked Naphthalene-Based Liquid-Crystal Dimers: Influence of Chalcogen Linkage and Mesogenic-Arm Symmetry on the Incidence and Stability of the Twist–Bend Nematic Phase. Chem. Eur. J. 2020, 26, 3767–3775. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, C.S.P.; Losada-Pérez, P.; Glorieux, C.; Kohlmeier, A.; Tamba, M.-G.; Mehl, G.H.; Leys, J. Nematic-nematic phase transition in the liquid crystal dimer CBC9CB and its mixtures with 5CB: A high-resolution adiabatic scanning calorimetric study. Phys. Rev. E 2011, 84, 041707. [Google Scholar] [CrossRef] [Green Version]
- Tuchband, M.R.; Shuai, M.; Graber, K.A.; Chen, D.; Radzihovsky, L.; Klittnick, A.; Foley, L.; Scarbrough, A.; Porada, J.H.; Moran, M.; et al. The twist-bend nematic phase of bent mesogenic dimer CB7CB and its mixtures. arXiv 2015, arXiv:1511.07523. [Google Scholar]
- Trbojevic, N.; Read, D.J.; Nagaraj, M. Dielectric properties of liquid crystalline dimer mixtures exhibiting the nematic and twist-bend nematic phases. Phys. Rev. E 2017, 96, 052703. [Google Scholar] [CrossRef]
- Ramou, E.; Welch, C.; Hussey, J.; Ahmed, Z.; Karahaliou, P.K.; Mehl, G.H. The induction of the N tb phase in mixtures. Liq. Cryst. 2018, 45, 1929–1935. [Google Scholar] [CrossRef]
- Trbojevic, N.; Read, D.J.; Nagaraj, M. Metastable room-temperature twist-bend nematic phases via photopolymerization. Phys. Rev. E 2019, 99, 062704. [Google Scholar] [CrossRef] [Green Version]
- Krishnamurthy, K.S.; Shankar Rao, D.S.; Kanakala, M.B.; Yelamaggad, C.V.; Madhusudana, N.V. Saddle-splay-induced periodic edge undulations in smectic- A disks immersed in a nematic medium. Phys. Rev. E 2020, 101, 032704. [Google Scholar] [CrossRef]
- Dozov, I.; Meyer, C. Analogy between the twist-bend nematic and the smectic A phases and coarse-grained description of the macroscopic NTB properties. Liq. Cryst. 2016, 1–20. [Google Scholar] [CrossRef]
- Barnes, P.J.; Douglass, A.G.; Heeks, S.K.; Luckhurst, G.R. An enhanced odd-even effect of liquid crystal dimers Orientational order in the α,ω-bis(4′-cyanobiphenyl-4-yl)alkanes. Liq. Cryst. 1993, 13, 603–613. [Google Scholar] [CrossRef]
- Mandle, R.J.; Davis, E.J.; Voll, C.-C.A.; Archbold, C.T.; Goodby, J.W.; Cowling, S.J. The relationship between molecular structure and the incidence of the NTB phase. Liq. Cryst. 2014, 1–16. [Google Scholar] [CrossRef]
- Mandle, R.J.; Cowling, S.J.; Goodby, J.W. Combined Microscopy, Calorimetry and X-ray Scattering Study of Fluorinated Dimesogens. Sci. Rep. 2017, 7, 13323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abberley, J.P.; Killah, R.; Walker, R.; Storey, J.M.D.; Imrie, C.T.; Salamończyk, M.; Zhu, C.; Gorecka, E.; Pociecha, D. Heliconical smectic phases formed by achiral molecules. Nat. Commun. 2018, 9, 228. [Google Scholar] [CrossRef] [Green Version]
- Walker, R.; Pociecha, D.; Strachan, G.J.; Storey, J.M.D.; Gorecka, E.; Imrie, C.T. Molecular curvature, specific intermolecular interactions and the twist-bend nematic phase: The synthesis and characterisation of the 1-(4-cyanobiphenyl-4′-yl)-6-(4-alkylanilinebenzylidene-4′-oxy)hexanes (CB6O. m). Soft Matter 2019, 15, 3188–3197. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.; Tuchband, M.R.; Young, A.; Shuai, M.; Scarbrough, A.; Walba, D.M.; Maclennan, J.E.; Wang, C.; Hexemer, A.; Clark, N.A. Resonant carbon K-edge soft X-ray scattering from lattice-free heliconical molecular ordering: Soft dilative elasticity of the twist-bend liquid crystal phase. Phys. Rev. Lett. 2016, 116, 147803. [Google Scholar] [CrossRef] [Green Version]
- Babakhanova, G.; Parsouzi, Z.; Paladugu, S.; Wang, H.; Nastishin, Y.A.; Shiyanovskii, S.V.; Sprunt, S.; Lavrentovich, O.D. Elastic and viscous properties of the nematic dimer CB7CB. Phys. Rev. E 2017, 96, 062704. [Google Scholar] [CrossRef] [Green Version]
- Morris, S.W.; Palffy-muhoray, P.; Balzarini, D.A. Measurements of the Bend and Splay Elastic Constants of Octyl-Cyanobiphenyl. Mol. Cryst. Liq. Cryst. 1986, 139, 263–280. [Google Scholar] [CrossRef]
- Madhusudana, N.V.; Pratibha, R. Elasticity and Orientational Order in Some Cyanobiphenyls: Part IV. Reanalysis of the Data. Mol. Cryst. Liq. Cryst. 1982, 89, 249–257. [Google Scholar] [CrossRef]
- Shamid, S.M.; Dhakal, S.; Selinger, J.V. Statistical mechanics of bend flexoelectricity and the twist-bend phase in bent-core liquid crystals. Phys. Rev. E 2013, 87, 052503. [Google Scholar] [CrossRef] [Green Version]
- Parsouzi, Z.; Shamid, S.M.; Borshch, V.; Challa, P.K.; Baldwin, A.R.; Tamba, M.G.; Welch, C.; Mehl, G.H.; Gleeson, J.T.; Jakli, A.; et al. Fluctuation Modes of a Twist-Bend Nematic Liquid Crystal. Phys. Rev. X 2016, 6, 021041. [Google Scholar] [CrossRef]
- Cukrov, G.; Mosaddeghian Golestani, Y.; Xiang, J.; Nastishin, Y.A.; Ahmed, Z.; Welch, C.; Mehl, G.H.; Lavrentovich, O.D. Comparative analysis of anisotropic material properties of uniaxial nematics formed by flexible dimers and rod-like monomers. Liq. Cryst. 2016, 1–13. [Google Scholar] [CrossRef]
- Chen, D.; Nakata, M.; Shao, R.; Tuchband, M.R.; Shuai, M.; Baumeister, U.; Weissflog, W.; Walba, D.M.; Glaser, M.A.; Maclennan, J.E.; et al. Twist-bend heliconical chiral nematic liquid crystal phase of an achiral rigid bent-core mesogen. Phys. Rev. E 2014, 89, 022506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnamurthy, K.S.; Kumar, P.; Palakurthy, N.B.; Yelamaggad, C.V.; Virga, E.G. Interfacial and morphological features of a twist-bend nematic drop. Soft Matter 2016, 12, 4967–4978. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Martinez-Miranda, L.J.; Hsiung, H.; Shen, Y.R. Orientational wetting behavior of a liquid-crystal homologous series. Phys. Rev. Lett. 1989, 62, 1860–1863. [Google Scholar] [CrossRef]
- Self, R.H.; Please, C.P.; Sluckin, T.J. Deformation of nematic liquid crystals in an electric field. Eur. J. Appl. Math. 2002, 13, 1–23. [Google Scholar] [CrossRef]
- Deuling, H.J. Deformation of Nematic Liquid Crystals in an Electric Field. Mol. Cryst. Liq. Cryst. 1972, 19, 123–131. [Google Scholar] [CrossRef]
- Gruler, H.; Scheffer, T.J.; Meier, G. Elastic Constants of Nematic Liquid Crystals. Z. Für Nat. A 1972, 27. [Google Scholar] [CrossRef]
- Bogi, A.; Faetti, S. Elastic, dielectric and optical constants of 4′-pentyl-4-cyanobiphenyl. Liq. Cryst. 2001, 28, 729–739. [Google Scholar] [CrossRef]
- A new approach to capacitive measurement of the conductivity, elastic moduli and dielectric permittivities of liquid crystals. (unpublished; manuscript in preparation).
- López, D.O.; Sebastian, N.; De la Fuente, M.R.; Martínez-García, J.C.; Salud, J.; Pérez-Jubindo, M.A.; Diez-Berart, S.; Dunmur, D.A.; Luckhurst, G.R. Disentangling molecular motions involved in the glass transition of a twist-bend nematic liquid crystal through dielectric studies. J. Chem. Phys. 2012, 137, 034502. [Google Scholar] [CrossRef]
- Salamończyk, M.; Vaupotič, N.; Pociecha, D.; Walker, R.; Storey, J.M.D.; Imrie, C.T.; Wang, C.; Zhu, C.; Gorecka, E. Multi-level chirality in liquid crystals formed by achiral molecules. Nat. Commun. 2019, 10, 1922. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, W.D.; Zou, H.; Zeng, X.; Welch, C.; Ungar, G.; Mehl, G.H. Dynamic calorimetry and XRD studies of the nematic and twist-bend nematic phase transitions in a series of dimers with increasing spacer length. Phys. Chem. Chem. Phys. 2018, 20, 25268–25274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandle, R.J.; Goodby, J.W. A twist-bend nematic to an intercalated, anticlinic, biaxial phase transition in liquid crystal bimesogens. Soft Matter 2016, 12, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathi, S.; Rao, D.S.S.; Palakurthy, N.B.; Yelamaggad, C.V.; Krishna Prasad, S. Binary System Exhibiting the Nematic to Twist-Bend Nematic Transition: Behavior of Permittivity and Elastic Constants. J. Phys. Chem. B 2016, 120, 5056–5062. [Google Scholar] [CrossRef]
- López, D.O.; Robles-Hernandez, B.; Salud, J.; Diez-Berart, S.; Jaen, X.; Dunmur, D.A.; Luckhurst, G.R. Miscibility studies of two twist-bend nematic liquid crystal dimers with different average molecular curvatures. A comparison between experimental data and predictions of a Landau mean-field theory for the NTB–N phase transition. 2016, 18, 4394. [Google Scholar] [CrossRef] [Green Version]
- Haller, I. Thermodynamic and static properties of liquid crystals. Prog. Solid State Chem. 1975, 10, 103–118. [Google Scholar] [CrossRef]
- Sebastián, N.; López, D.O.; Robles-Hernández, B.; De la Fuente, M.R.; Salud, J.; Pérez-Jubindo, M.A.; Dunmur, D.A.; Luckhurst, G.R.; Jackson, D.J.B. Dielectric, calorimetric and mesophase properties of 1′′-(2′,4-difluorobiphenyl-4′-yloxy)-9′′-(4-cyanobiphenyl-4′-yloxy) nonane: An odd liquid crystal dimer with a monotropic mesophase having the characteristics of a twist-bend nematic phase. Phys. Chem. Chem. Phys. 2014, 16, 21391–21406. [Google Scholar] [CrossRef]
- Challa, P.K.; Borshch, V.; Parri, O.; Imrie, C.T.; Sprunt, S.N.; Gleeson, J.T.; Lavrentovich, O.D.; Jákli, A. Twist-bend nematic liquid crystals in high magnetic fields. Phys. Rev. E 2014, 89, 060501. [Google Scholar] [CrossRef] [Green Version]
- Vaupotič, N.; Ali, M.; Majewski, P.W.; Gorecka, E.; Pociecha, D. Polarization Gratings Spontaneously Formed from a Helical Twist-Bend Nematic Phase. ChemPhysChem 2018, 19, 2566–2571. [Google Scholar] [CrossRef] [Green Version]
- Mandle, R.J.; Davis, E.J.; Archbold, C.T.; Cowling, S.J.; Goodby, J.W. Microscopy studies of the nematic NTB phase of 1,11-di-(1′′-cyanobiphenyl-4-yl)undecane. J. Mater. Chem. C 2014, 2, 556–566. [Google Scholar] [CrossRef]
- Salili, S.M.; Kim, C.; Sprunt, S.; Gleeson, J.T.; Parri, O.; Jákli, A. Flow properties of a twist-bend nematic liquid crystal. RSC Adv. 2014, 4, 57419–57423. [Google Scholar] [CrossRef]
- Al-Janabi, A.; Mandle, R.J.; Goodby, J.W. Isomeric trimesogens exhibiting modulated nematic mesophases. RSC Adv. 2017, 7, 47235–47242. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.Y.; Superfine, R.; Shen, Y.R. Nonlinear spectroscopic study of coadsorbed liquid-crystal and surfactant monolayers: Conformation and interaction. Phys. Rev. A 1990, 42, 3660. [Google Scholar] [CrossRef] [PubMed]
- Maier, W.; Saupe, A. Eine einfache molekulare Theorie des nematischen kristallinflüssigen Zustandes. Z. Für Nat. A 1958, 13, 564–566. [Google Scholar] [CrossRef]
- Dunmur, D.A.; Manterfield, M.R.; Miller, W.H.; Dunleavy, J.K. The Dielectric and Optical Properties of the Homologous Series of Cyano-Alkyl-Biphenyl Liquid Crystals. Mol. Cryst. Liq. Cryst. 1978, 45, 127–144. [Google Scholar] [CrossRef]
- Robles-Hernández, B.; Sebastián, N.; De la Fuente, M.R.; López, D.O.; Diez-Berart, S.; Salud, J.; Ros, M.B.; Dunmur, D.A.; Luckhurst, G.R.; Timimi, B.A. Twist, tilt, and orientational order at the nematic to twist-bend nematic phase transition of 1″,9″-bis(4-cyanobiphenyl-4′-yl) nonane: A dielectric, H 2 NMR, and calorimetric study. Phys. Rev. E 2015, 92, 062505. [Google Scholar] [CrossRef] [Green Version]
- Meyer, C.; Blanc, C.; Luckhurst, G.R.; Davidson, P.; Dozov, I. Biaxiality-driven twist-bend to splay-bend nematic phase transition induced by an electric field. Sci. Adv. 2020, 6, eabb8212. [Google Scholar] [CrossRef]
- Iadlovska, O.S.; Babakhanova, G.; Mehl, G.H.; Welch, C.; Cruickshank, E.; Strachan, G.J.; Storey, J.M.D.; Imrie, C.T.; Shiyanovskii, S.V.; Lavrentovich, O.D. Temperature dependence of bend elastic constant in oblique helicoidal cholesterics. Phys. Rev. Res. 2020, 2, 013248. [Google Scholar] [CrossRef] [Green Version]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aouini, A.; Nobili, M.; Chauveau, E.; Dieudonné-George, P.; Damême, G.; Stoenescu, D.; Dozov, I.; Blanc, C. Chemical-Physical Characterization of a Binary Mixture of a Twist Bend Nematic Liquid Crystal with a Smectogen. Crystals 2020, 10, 1110. https://doi.org/10.3390/cryst10121110
Aouini A, Nobili M, Chauveau E, Dieudonné-George P, Damême G, Stoenescu D, Dozov I, Blanc C. Chemical-Physical Characterization of a Binary Mixture of a Twist Bend Nematic Liquid Crystal with a Smectogen. Crystals. 2020; 10(12):1110. https://doi.org/10.3390/cryst10121110
Chicago/Turabian StyleAouini, Abir, Maurizio Nobili, Edouard Chauveau, Philippe Dieudonné-George, Gauthier Damême, Daniel Stoenescu, Ivan Dozov, and Christophe Blanc. 2020. "Chemical-Physical Characterization of a Binary Mixture of a Twist Bend Nematic Liquid Crystal with a Smectogen" Crystals 10, no. 12: 1110. https://doi.org/10.3390/cryst10121110