Size Effects of the Crystallite of ZSM-5 Zeolites on the Direct Catalytic Conversion of L-Lactic Acid to L, L-Lactide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methodology
2.2.1. Characterization of the ZSM-5 Zeolites
2.2.2. Synthesis of L, L-Lactide
2.2.3. Analytical Methods
3. Results and Discussion
3.1. Characterization of the ZSM-5 Zeolites with Different Crystallite Sizes
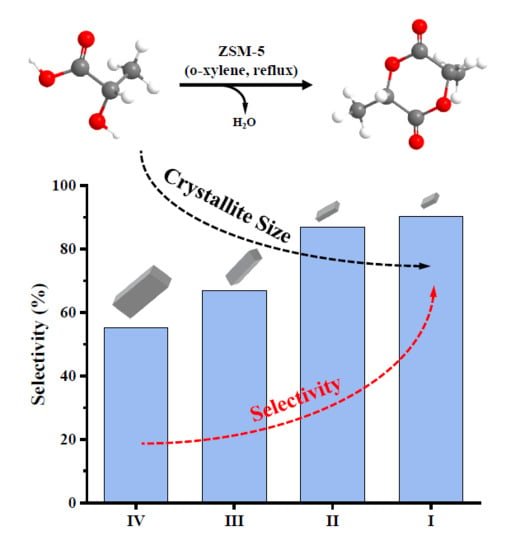
3.2. Transformation of L-Lactic Acid into L, L-Lactide
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Madhavan Nampoothiri, K.; Nair, N.R.; John, R.P. An overview of the recent developments in polylactide (PLA) research. Bioresour. Technol. 2010, 101, 8493–8501. [Google Scholar] [CrossRef] [PubMed]
- Dechy-Cabaret, O.; Martin-Vaca, B.; Bourissou, D. Controlled Ring-Opening Polymerization of Lactide and Glycolide. Chem. Rev. 2004, 104, 6147–6176. [Google Scholar] [CrossRef] [PubMed]
- Harold, E.B.; Kamlesh, K.B. Continuous catalyzed vapor phase dimeric cyclic ester process. U.S. Patent 5138074A, 11 August 1992. [Google Scholar]
- Upare, P.P.; Yoon, J.W.; Hwang, D.W.; Lee, U.H.; Hwang, Y.K.; Hong, D.-Y.; Kim, J.C.; Lee, J.H.; Kwak, S.K.; Shin, H.; et al. Design of a heterogeneous catalytic process for the continuous and direct synthesis of lactide from lactic acid. Green Chem. 2016, 18, 5978–5983. [Google Scholar] [CrossRef]
- Park, H.W.; Chang, Y.K. Economically Efficient Synthesis of Lactide Using a Solid Catalyst. Org. Process Res Dev. 2017, 21, 1980–1984. [Google Scholar] [CrossRef]
- Dusselier, M.; Van Wouwe, P.; Dewaele, A.; Jacobs, P.A.; Sels, B.F. Shape-selective zeolite catalysis for bioplastics production. Science 2015, 349, 78–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Zheng, B.; Wu, G.; Ma, F.; Liu, C. Effect of the Si/Al ratio on the performance of hierarchical ZSM-5 zeolites for methanol aromatization. RSC Adv. 2016, 6, 83581–83588. [Google Scholar] [CrossRef]
- Palcic, A.; Ordomsky, V.V.; Qin, Z.; Georgieva, V.; Valtchev, V. Tuning Zeolite Properties for a Highly Efficient Synthesis of Propylene from Methanol. Chemistry 2018, 24, 13136–13149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, W.; Chen, K.; Takahashi, A.; Li, X.; Mu, X.; Han, C.; Liu, L.; Nakamura, I.; Fujitani, T. Effects of particle size on catalytic conversion of ethanol to propylene over H-ZSM-5 catalysts—Smaller is better. Catal. Commun. 2016, 73, 27–33. [Google Scholar] [CrossRef]
- Jabbari, A.; Abbasi, A.; Zargarnezhad, H.; Riazifar, M. A study on the effect of SiO2/Al2O3 ratio on the structure and performance of nano-sized ZSM-5 in methanol to propylene conversion. React. Kinet. Mech. Catal. 2017, 121, 763–772. [Google Scholar] [CrossRef]
- Lónyi, F.; Valyon, J. On the interpretation of the NH3-TPD patterns of H-ZSM-5 and H-mordenite. Microporous Mesoporous Mater. 2001, 47, 293–301. [Google Scholar] [CrossRef]
- Bian, C.; Wang, X.; Yu, L.; Zhang, F.; Zhang, J.; Fei, Z.; Qiu, J.; Zhu, L. Generalized Methodology for Inserting Metal Heteroatoms into the Layered Zeolite Precursor RUB-36 by Interlayer Expansion. Crystals 2020, 10, 530. [Google Scholar] [CrossRef]
- Ma, F.; Li, H.L.; Jiang, J.X. Furfural Reduction via Hydrogen Transfer from Supercritical Methanol. Chem. Res. Chin. Univ. 2019, 35, 498–503. [Google Scholar] [CrossRef]
- Martin, A.; Wolf, U.; Nowak, S.; Lucke, B. Pyridine-i.r. investigations of hydrothermally treated dealuminated HZSM-5 zeolites. Zeolites 1991, 11, 85–87. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Z.; Liu, Z.; Peng, W.; Liu, Y.; Liu, C. Correlation between H-ZSM-5 crystal size and catalytic performance in the methanol-to-aromatics reaction. Chin. J. Catal. 2017, 38, 683–690. [Google Scholar] [CrossRef]
- Emeis, C.A. Determination of Integrated Molar Extinction Coefficients for Infrare Absorption Bands of Pyridine Adsorbed on Solid Acid Catalysts. J. Catal. 1993, 141, 347–354. [Google Scholar] [CrossRef]
- Espartero, J.L.; Rashkov, I.; Li, S.M.; Manolova, N.; Vert, M. NMR Analysis of Low Molecular Weight Poly(lactic acid)s. Macromolecules 1996, 29, 3535–3539. [Google Scholar] [CrossRef]
- Vu, D.T.; Kolah, A.K.; Asthana, N.S.; Peereboom, L.; Lira, C.T.; Miller, D.J. Oligomer distribution in concentrated lactic acid solutions. Fluid Phase Equilib. 2005, 236, 125–135. [Google Scholar] [CrossRef]
- Ercan, C.; Dautzenberg, F.M.; Yeh, C.Y.; Barner, H.E. Mass-Transfer Effects in Liquid-Phase Alkylation of Benzene with Zeolite Catalysts. Ind. Eng. Chem. Res. 1998, 37, 1724–1728. [Google Scholar] [CrossRef]





| Zeolite | Relative Crystallinity (%) | Si/Al Molar Ratios 1 | ABET 2 (m2/g) | Vmicro 3 (cm3/g) | External Surface Area 3 (m2/g) |
|---|---|---|---|---|---|
| I | 100.0 | 12.4 | 397.5 | 0.15 | 42.6 |
| II | 90.0 | 12.0 | 393.4 | 0.15 | 36.7 |
| III | 89.1 | 10.7 | 411.8 | 0.16 | 12.0 |
| IV | 80.0 | 9.3 | 403.7 | 0.16 | 6.7 |
| III-b.m. | 81.4 | 10.6 | 362.9 | 0.14 | 27.3 |
| IV-b.m. | 70.6 | 9.5 | 336.2 | 0.13 | 25.1 |
| Catalyst | I | II | III | IV | III-b.m. | IV-b.m. |
|---|---|---|---|---|---|---|
| B 1 (μmol/g) | 148.2 | 156.5 | 171.1 | 187.1 | 164.5 | 180.2 |
| L 2 (μmol/g) | 48.0 | 45.3 | 40.5 | 39.1 | 43.8 | 45.0 |
| Total acid (B + L) (μmol/g) | 196.2 | 201.8 | 211.6 | 226.2 | 208.3 | 225.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Q.; Li, R.; Fu, G.; Jiang, J. Size Effects of the Crystallite of ZSM-5 Zeolites on the Direct Catalytic Conversion of L-Lactic Acid to L, L-Lactide. Crystals 2020, 10, 781. https://doi.org/10.3390/cryst10090781
Huang Q, Li R, Fu G, Jiang J. Size Effects of the Crystallite of ZSM-5 Zeolites on the Direct Catalytic Conversion of L-Lactic Acid to L, L-Lactide. Crystals. 2020; 10(9):781. https://doi.org/10.3390/cryst10090781
Chicago/Turabian StyleHuang, Qintong, Rui Li, Guangying Fu, and Jiuxing Jiang. 2020. "Size Effects of the Crystallite of ZSM-5 Zeolites on the Direct Catalytic Conversion of L-Lactic Acid to L, L-Lactide" Crystals 10, no. 9: 781. https://doi.org/10.3390/cryst10090781