On the New Oxyarsenides Eu5Zn2As5O and Eu5Cd2As5O
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Single-Crystal X-ray Diffraction
2.3. Electronic Structure Calculations
3. Results and Discussion
3.1. Structure Description
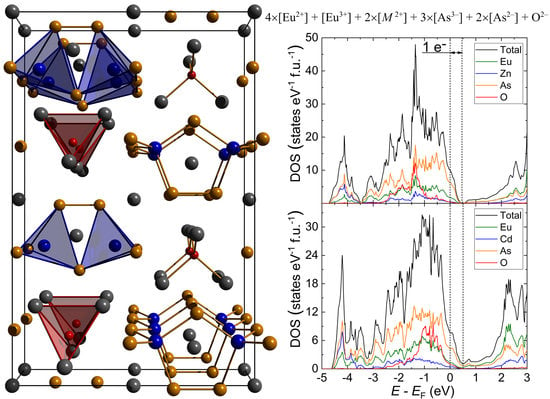
3.2. Electron Count, Electronic Structure and Possibility of Mixed-Valent State for Europium
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zintl, E. Intermetallische Verbindungen. Angew. Chem. 1939, 52, 1–6. [Google Scholar] [CrossRef]
- Schäfer, H.; Eisenman, B.; Müller, W. Zintl Phases-Transitions between Metallic and Ionic Bonding. Angew. Chem. Int. Ed. 1973, 12, 694–712. [Google Scholar]
- Kauzlarich, S.M. Chemistry, Structure and Bonding of Zintl Phases and Ions; Wiley-VCH: Weinheim, Germany, 1996. [Google Scholar]
- Ovchinnikov, A.; Bobev, S. Zintl phases with group 15 elements and the transition metals: A brief overview of pnictides with diverse and complex structures. J. Solid State Chem. 2019, 270, 346–359. [Google Scholar] [CrossRef]
- Fisher, I.R.; Bud’ko, S.L.; Song, C.; Canfield, P.C.; Ozawa, T.C.; Kauzlarich, S.M. Yb14ZnSb11: Charge Balance in Zintl Compounds as a Route to Intermediate Yb Valence. Phys. Rev. Lett. 2000, 85, 1120–1123. [Google Scholar] [CrossRef] [PubMed]
- Kauzlarich, S.M.; Brown, S.R.; Snyder, G.J. Zintl phases for thermoelectric devices. Dalton Trans. 2007, 21, 2099–2107. [Google Scholar] [CrossRef] [Green Version]
- Mandrus, D.; Sefat, A.S.; McGuire, M.A.; Sales, B.C. Materials Chemistry of BaFe2As2: A Model Platform for Unconventional Superconductivity. Chem. Mater. 2010, 22, 715–723. [Google Scholar] [CrossRef]
- Sales, B.C.; Mandrus, D.; Williams, R.K. Filled Skutterudite Antimonides: A New Class of Thermoelectric Materials. Science 1996, 272, 1325–1328. [Google Scholar] [CrossRef]
- Hu, Y.; Cerretti, G.; Wille, E.L.K.; Bux, S.K.; Kauzlarich, S.M. The remarkable crystal chemistry of the Ca14AlSb11 structure type, magnetic and thermoelectric properties. J. Solid State Chem. 2019, 271, 88–102. [Google Scholar] [CrossRef] [Green Version]
- Owens-Baird, B.; Wang, J.; Wang, S.G.; Chen, Y.S.; Lee, S.; Donadio, D.; Kovnir, K. III–V Clathrate Semiconductors with Outstanding Hole Mobility: Cs8In27Sb19 and A8Ga27Sb19 (A = Cs, Rb). J. Am. Chem. Soc. 2020, 142, 2031–2041. [Google Scholar] [CrossRef]
- Xia, S.Q.; Bobev, S. Cation-Anion Interactions as Structure Directing Factors: Structure and Bonding of Ca2CdSb2 and Yb2CdSb2. J. Am. Chem. Soc. 2007, 129, 4049–4057. [Google Scholar] [CrossRef]
- Xia, S.Q.; Bobev, S. Interplay between size and electronic effects in determining the homogeneity range of the A9Zn4+xPn9 and A9Cd4+xPn9 phases (0 ≤ x ≤ 0.5), A = Ca, Sr, Yb, Eu; Pn = Sb, Bi. J. Am. Chem. Soc. 2007, 129, 10011–10018. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.Q.; Bobev, S. Zintl phase variations through cation selection. Synthesis and structure of A21Cd4Pn18 (A = Eu, Sr, Ba; Pn = Sb, Bi). Inorg. Chem. 2008, 47, 1919–1921. [Google Scholar] [CrossRef] [PubMed]
- Saparov, B.; He, H.; Zhang, X.H.; Greene, R.; Bobev, S. Synthesis, crystallographic and theoretical studies of the new Zintl phases Ba2Cd2Pn3 (Pn = As, Sb), and the solid solutions (Ba1–xSrx)2Cd2Sb3 and Ba2Cd2(Sb1–xAsx)3. Dalton Trans. 2010, 39, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Saparov, B.; Bobev, S. Isolated ∞1[ZnPn2]4– Chains in the Zintl Phases Ba2ZnPn2 (Pn = As, Sb, Bi) - Synthesis, Structure, and Bonding. Inorg. Chem. 2010, 49, 5173–5179. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.K.; Saparov, B.; Bobev, S. Synthesis, Crystal Structures and Properties of the Zintl Phases Sr2ZnP2, Sr2ZnAs2, A2ZnSb2 and A2ZnBi2 (A = Sr and Eu). Z. Anorg. Allg. Chem. 2011, 637, 2018–2025. [Google Scholar] [CrossRef]
- He, H.; Tyson, C.; Saito, M.; Bobev, S. Synthesis and structural characterization of the ternary Zintl phases AE3Al2Pn4 and AE3Ga2Pn4 (AE = Ca, Sr, Ba, Eu; Pn = P, As). J. Solid State Chem. 2012, 188, 59–65. [Google Scholar] [CrossRef]
- Zimmer, B.I.; Jeitschko, W.; Albering, J.H.; Glaum, R.; Reehuis, M. The rare earth transition metal oxides LnFePO, LnRuPO and LnCoPO with ZrCuSiAs type structure. J. Alloys Compd. 1995, 229, 238–242. [Google Scholar] [CrossRef]
- Kamihara, Y.; Hiramatsu, H.; Hirano, M.; Kawamura, R.; Yanagi, H.; Kamiya, T.; Hosono, H. Iron-Based Layered Superconductor: LaOFeP. J. Am. Chem. Soc. 2006, 128, 10012–10013. [Google Scholar] [CrossRef]
- Ren, Z.A.; Che, G.C.; Dong, X.L.; Yang, J.; Lu, W.; Yi, W.; Shen, X.L.; Li, Z.C.; Sun, L.L.; Zhou, F.; et al. Superconductivity and phase diagram in iron-based arsenic-oxides ReFeAsO1–δ (Re = rare-earth metal) without fluorine doping. EPL 2008, 83, 17002. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.C.; Yu, J.; Ruan, B.B.; Pan, B.J.; Mu, Q.G.; Liu, T.; Zhao, K.; Chen, G.F.; Ren, Z.A. Revisiting the Electron-Doped SmFeAsO: Enhanced Superconductivity up to 58.6 K by Th and F Codoping. Chinese Phys. Lett. 2017, 34, 077401. [Google Scholar] [CrossRef] [Green Version]
- Xia, S.Q.; Bobev, S. On the existence of Ca2Bi-crystal and electronic structure of Ca4Bi2O. J. Alloys Compd. 2007, 427, 67–72. [Google Scholar] [CrossRef]
- Xia, S.Q.; Bobev, S. Dibarium tricadmium bimuthide (-I,-III) oxide, Ba2Cd3-δBi3O. Acta Crystallogr. E. 2010, 66, i81. [Google Scholar] [CrossRef] [PubMed]
- Saparov, B.; Bobev, S. Synthesis, crystal and electronic structures of the new quaternary phases A5Cd2Sb5F (A = Sr, Ba, Eu), and Ba5Cd2Sb5Ox (0.5 < x < 0.7). Dalton Trans. 2010, 39, 11335–11343. [Google Scholar] [PubMed]
- Saparov, B.; Bobev, S. Pentaeuropium dicadmium pentaantimonide oxide, Eu5Cd2Sb5O. Acta Crystallogr. E. 2011, E67, i11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darone, G.M.; Bobev, S. Ba5Cd2Sb4O2 —A New Antimonide Oxide with a Complex Structure. Crystals 2011, 1, 206–214. [Google Scholar] [CrossRef]
- Wang, Y.; Darone, G.M.; Bobev, S. The New Zintl Phases Eu21Cd4Sb18 and Eu21Mn4Sb18. J. Solid State Chem. 2016, 238, 303–310. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Yang, M.; Pan, M.Y.; Xia, S.Q.; Tao, X.T.; He, H.; Darone, G.; Bobev, S. Synthesis, Crystal and Electronic Structures, and Properties of the New Pnictide Semiconductors A2CdPn2 (A = Ca, Sr, Ba, Eu; Pn = P, As). Inorg. Chem. 2011, 50, 8020–8027. [Google Scholar] [CrossRef]
- Makongo, J.P.A.; Darone, G.M.; Xia, S.Q.; Bobev, S. Non-stoichiometric Compositions arising from synergistic electronic and size effects. Synthesis, Crystal Chemistry and Electronic Properties of the A14Cd1+xPn11 Compounds (0 ≤ x ≤ 0.3; A = Sr, Eu; Pn = As, Sb). J. Mater. Chem. 2015, 3, 10388–10400. [Google Scholar]
- Saparov, B.; Bobev, S. Undecaeuropium hexazinc dodecaarsenide. Acta Crystallogr. E. 2010, 66, i24. [Google Scholar] [CrossRef] [Green Version]
- Baranets, S.; He, H.; Bobev, S. Niobium-bearing arsenides and germanides from elemental mixtures not involving niobium: A new twist to an old problem in solid-state synthesis. Acta Crystallogr. C. 2018, 74, 623–627. [Google Scholar] [CrossRef]
- SAINT; Bruker AXS Inc.: Madison, WI, USA, 2014.
- SADABS; Bruker AXS Inc.: Madison, WI, USA, 2014.
- Sheldrick, G.M. SHELXT – Integrated space-group and crystal-structure determination. Acta Crystallogr. A. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Gelato, L.M.; Parthé, E. STRUCTURE TIDY. J. Appl. Crystallogr. 1987, 20, 139–143. [Google Scholar] [CrossRef]
- Jespen, O.; Andersen, O.K. The Stuttgart TB-LMTO-ASA Program; MPI für Festkörperforschung: Stuttgart, Germany, 1998. [Google Scholar]
- Von Barth, U.; Hedin, L. A local exchange-correlation potential for the spin polarized case: I. J. Phys. C Solid State Phys. 1972, 5, 1629–1642. [Google Scholar] [CrossRef]
- Dronskowski, R.; Bloechl, P.E. Crystal orbital Hamilton populations (COHP): Energy-resolved visualization of chemical bonding in solids based on density-functional calculations. J. Phys. Chem. 1993, 97, 9617–9624. [Google Scholar] [CrossRef]
- He, H.; Stoyko, S.S.; Mar, A.; Bobev, S. Ternary K2Zn5As4-type pictides Rb2Cd5As4 and Rb2Zn5Sb4, and the solid solution Rb2Cd5(As,Sb)4. Acta. Crystallogr. C. 2013, 69, 455–459. [Google Scholar] [CrossRef]
- Pauling, L. Atomic Radii and Interatomic Distances in Metals. J. Am. Chem. Soc. 1947, 69, 542–553. [Google Scholar] [CrossRef]
- He, H.; Tyson, C.; Bobev, S. New Compounds with [As7]3– Clusters: Synthesis and Crystal Structures of the Zintl Phases Cs2NaAs7, Cs4ZnAs14 and Cs4CdAs14. Crystals 2011, 1, 87–98. [Google Scholar] [CrossRef]
- Suen, N.-T.; Wang, Y.; Bobev, S. Synthesis, Crystal Structures, and Physical Properties of the New Zintl Phases A21Zn4Pn18 (A = Ca, Eu; Pn = As, Sb)—Versatile Arrangements of [ZnPn4] Tetrahedra. J. Solid State Chem. 2015, 227, 204–211. [Google Scholar] [CrossRef] [Green Version]
- Stoyko, S.S.; Khatun, M.; Mar, A. Ternary Arsenides A2Zn2As3 (A = Sr, Eu) and Their Stuffed Derivatives A2Ag2ZnAs3. Inorg. Chem. 2012, 51, 2621–2628. [Google Scholar] [CrossRef]
- Childs, A.B.; Baranets, S.; Bobev, S. Five new ternary indium-arsenides discovered. Synthesis and structural characterization of the Zintl phases Sr3In2As4, Ba3In2As4, Eu3In2As4, Sr5In2As6 and Eu5In2As6. J. Solid State Chem. 2019, 278, 120889. [Google Scholar] [CrossRef]
- Baranets, S.; Darone, G.M.; Bobev, S. Synthesis and structure of Sr14Zn1+xAs11 and Eu14Zn1+xAs11 (x ≤ 0.5). New members of the family of pnictides isotypic with Ca14AlSb11, exhibiting a new type of structural disorder. J. Solid State Chem. 2019, 280, 120990. [Google Scholar] [CrossRef]
- Stoyko, S.S.; Khatun, M.; Mar, A. Ternary Arsenides A2Zn5As4 (A = K, Rb): Zintl Phases Built from Stellae Quandrangulae. Inorg. Chem. 2012, 51, 9517–9521. [Google Scholar] [CrossRef] [PubMed]
- Saparov, B.; Broda, M.; Ramanujachary, K.V.; Bobev, S. New quaternary Zintl phases - Synthesis, crystal and electronic structures of KA2Cd2Sb3 (A = Ca, Sr, Ba, Eu, Yb). Polyhedron 2010, 29, 456–462. [Google Scholar] [CrossRef]
- Wang, Y.; Calvert, L.D.; Gabe, E.J.; Taylor, J.B. Europium Arsenic Oxide Eu4As2O: A Filled La2Sb Structure and its Relation to the K2NiF4 and GeTeU Types. Acta Crystallogr. B. 1977, 33, 3122–3125. [Google Scholar] [CrossRef]
- Wang, Y.; Calvert, L.D.; Smart, M.L.; Taylor, J.B. Structure of Trieuropium Triarsenide-Tantalum Oxide (1:1). Acta Crystallogr. B. 1980, 36, 131–133. [Google Scholar] [CrossRef]
- Sanders, M.B.; Krizan, J.W.; Cava, R.J. RE3Sb3Zn2O14 (RE = La, Pr, Nd, Sm, Eu, Gd): A new family of pyrochlore derivatives with rare earth ions on a 2D Kagome lattice. J. Mater. Chem. C. 2016, 4, 541–550. [Google Scholar] [CrossRef]
- Baranets, S.; Bobev, S. From the Ternary Phase Ca14Zn1+δSb11 (δ ≈ 0.4) to the Quaternary Solid Solutions Ca14–xRExZnSb11 (RE = La–Nd, Sm, Gd, x ≈ 0.9). A Tale of Electron Doping via Rare-Earth Metal Substitutions and the Concomitant Structural Transformations. Inorg. Chem. 2019, 58, 8506–8516. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, J.; Kawamura, A.; Kovnir, K.; Kauzlarich, S.M. Yb14MgSb11 and Ca14MgSb11 – New Mg-Containing Zintl Compounds and Their Structures, Bonding, and Thermoelectric Properties. Chem. Mater. 2015, 27, 343–351. [Google Scholar] [CrossRef]
- Baranets, S.; Voss, L.; Stoyko, S.; Bobev, S. Synthesis, crystal structure and physical properties of the solid solutions Ca14–xRExCdSb11 (RE = La–Nd, Sm, Gd–Yb, x ≈ 0.85 ± 0.15). J. Appl. Phys. 2019, 125, 245101. [Google Scholar] [CrossRef]
- Prakash, J.; Stoyko, S.; Voss, L.; Bobev, S. On the Extended Series of Quaternary Zintl Phases Ca13REMnSb11 (RE = La–Nd, Sm, Gd–Dy). Eur. J. Inorg. Chem. 2016, 18, 2912–2922. [Google Scholar] [CrossRef]
- Ovchinnikov, A.; Prakash, J.; Bobev, S. Crystal chemistry and magnetic properties of the solid solutions Ca14–xRExMnBi11 (RE = La–Nd, Sm, and Gd–Ho; x ≈ 0.6–0.8). Dalton Trans. 2017, 46, 16041–16049. [Google Scholar] [CrossRef] [PubMed]


| Chemical Formula | Eu5Zn2As5O | Eu5Cd2As5O |
|---|---|---|
| Formula weight/ g mol–1 | 1281.14 | 1375.2 |
| Crystal system | Orthorhombic | |
| Space group | Cmcm | |
| Z | 4 | |
| T / K | 200(2) | |
| a/ Å | 4.3457(11) | 4.4597(9) |
| b/ Å | 20.897(5) | 21.112(4) |
| c/ Å | 13.571(3) | 13.848(3) |
| V/ Å3 | 1232.5(5) | 1303.8(4) |
| ρcalc./ g cm–3 | 6.90 | 7.01 |
| μ(Mo Kα)/ cm–1 | 421.4 | 394.2 |
| Independent reflections | 1008 | 870 |
| Goodness-of-fit | 1.069 | 1.074 |
| R1 (I > 2 σ (I))1 | 0.024 | 0.045 |
| wR2 (I > 2σ(I))1 | 0.051 | 0.095 |
| R1 (all data)1 | 0.028 | 0.052 |
| wR2 (all data)1 | 0.052 | 0.098 |
| Largest peak and hole/ e-·Å–3 | 2.0; –2.6 | 3.2; –2.5 |
| Atom | Site | x | y | z | Ueq/ Å2 |
|---|---|---|---|---|---|
| Eu5Zn2As5O | |||||
| Eu1 | 8f | 0 | 0.27425(2) | 0.61838(3) | 0.0127(1) |
| Eu2 | 4c | 0 | 0.09198(3) | 1/4 | 0.0170(1) |
| Eu3 | 4c | 0 | 0.89812(3) | 1/4 | 0.0109(1) |
| Eu4 | 4a | 0 | 0 | 0 | 0.0119(1) |
| As1 | 8f | 0 | 0.14921(4) | 0.00880(5) | 0.0120(1) |
| As2 | 8f | 0 | 0.48551(4) | 0.15898(6) | 0.0116(1) |
| As3 | 4c | 0 | 0.29310(5) | 1/4 | 0.0112(2) |
| Zn | 8f | 0 | 0.36524(4) | 0.08859(7) | 0.0134(1) |
| O | 4c | 0 | 0.6503(3) | 1/4 | 0.012(1) |
| Eu5Cd2As5O | |||||
| Eu1 | 8f | 0 | 0.26895(4) | 0.61847(7) | 0.0111(3) |
| Eu2 | 4c | 0 | 0.09462(5) | 1/4 | 0.0136(3) |
| Eu3 | 4c | 0 | 0.90246(5) | 1/4 | 0.0104(3) |
| Eu4 | 4a | 0 | 0 | 0 | 0.0110(3) |
| As1 | 8f | 0 | 0.14947(8) | 0.0214(1) | 0.0102(4) |
| As2 | 8f | 0 | 0.49163(7) | 0.1603(1) | 0.0109(4) |
| As3 | 4c | 0 | 0.2942(1) | 1/4 | 0.0090(5) |
| Cd | 8f | 0 | 0.36791(5) | 0.0826(1) | 0.0112(3) |
| O | 4c | 0 | 0.6526(7) | 1/4 | 0.010b |
| Atom Pair | M = Zn | M = Cd |
|---|---|---|
| Distance / Å | Distance / Å | |
| M—As1 × 2 | 2.561(1) | 2.680(1) |
| M—As2 | 2.689(1) | 2.825(2) |
| M—As3 | 2.659(1) | 2.791(2) |
| As2—As2 | 2.471(2) | 2.483(4) |
| Eu1—As1 | 3.132(1) | 3.180(2) |
| Eu1—As1 × 2 | 3.080(1) | 3.121(1) |
| Eu1—As3 × 2 | 3.145(1) | 3.173(1) |
| Eu1—O | 2.382(5) | 2.462(10) |
| Eu2—As1 × 2 | 3.485(1) | 3.370(2) |
| Eu2—As2 × 4 | 3.346(1) | 3.353(2) |
| Eu2—O × 2 | 2.492(4) | 2.543(7) |
| Eu3—As2 × 4 | 3.096(1) | 3.171(1) |
| Eu3—As3 × 2 | 3.088(1) | 3.193(2) |
| Eu4—As1 × 2 | 3.120(1) | 3.169(2) |
| Eu4—As2 × 4 | 3.077(1) | 3.151(1) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darone, G.M.; Baranets, S.A.; Bobev, S. On the New Oxyarsenides Eu5Zn2As5O and Eu5Cd2As5O. Crystals 2020, 10, 475. https://doi.org/10.3390/cryst10060475
Darone GM, Baranets SA, Bobev S. On the New Oxyarsenides Eu5Zn2As5O and Eu5Cd2As5O. Crystals. 2020; 10(6):475. https://doi.org/10.3390/cryst10060475
Chicago/Turabian StyleDarone, Gregory M., Sviatoslav A. Baranets, and Svilen Bobev. 2020. "On the New Oxyarsenides Eu5Zn2As5O and Eu5Cd2As5O" Crystals 10, no. 6: 475. https://doi.org/10.3390/cryst10060475