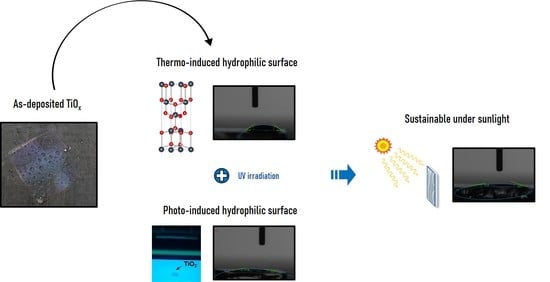
Thermoinduced and Photoinduced Sustainable Hydrophilic Surface of Sputtered-TiO2 Thin Film
Abstract
:1. Introduction
2. Experimental Section
2.1. Fabrication of TiO2 Thin Films
2.2. Formation of a Sustainable Hydrophilic Surface
2.3. Evaluation of TiO2 Thin Films
3. Result and Discussion
3.1. Generation of Light-Induced Surface Oxygen Vacancies
3.2. Photoinduced Reconstruction of Ti–OH
3.3. Influence of Annealing on Water Contact Angle
3.4. Correlation of Crystallinity and Water Contact Angle
3.5. Crystal Size and Surface Structure of TiO2 Films
3.6. Optical Properties and Sustainability of TiO2 Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.; Falaras, P.; Kontos, A.G.; Dunlop, P.; Hamilton, J.; Byrne, J.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Dionysiou, D.D.; Pillai, S.C. Self-cleaning applications of TiO2 by photo-induced hydrophilicity and photocatalysis. Appl. Catal. B Environ. 2015, 176, 396–428. [Google Scholar] [CrossRef] [Green Version]
- Ni, M.; Leung, M.K.; Leung, D.Y.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Jiang, H.; Katsumata, K.-I.; Hong, J.; Yamaguchi, A.; Nakata, K.; Terashima, C.; Matsushita, N.; Miyauchi, M.; Fujishima, A. Photocatalytic reduction of CO2 on Cu2O-loaded Zn-Cr layered double hydroxides. Appl. Catal. B Environ. 2018, 224, 783–790. [Google Scholar] [CrossRef]
- Hong, J.; Katsumata, K.-I.; Matsushita, N. High-conductivity solution-processed ZnO films realized via UV irradiation and hydrogen treatment. Acta Mater. 2016, 103, 844–849. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Parkin, I.P.; Palgrave, R.G. Self-cleaning coatings. J. Mater. Chem. 2005, 15, 1689–1695. [Google Scholar] [CrossRef]
- Takata, Y.; Hidaka, S.; Cao, J.; Nakamura, T.; Yamamoto, H.; Masuda, M.; Ito, T. Effect of surface wettability on boiling and evaporation. Energy 2005, 30, 209–220. [Google Scholar] [CrossRef]
- Fillion, R.; Riahi, A.; Edrisy, A. A review of icing prevention in photovoltaic devices by surface engineering. Renew. Sustain. Energy Rev. 2014, 32, 797–809. [Google Scholar] [CrossRef]
- Zhang, L.; Dillert, R.; Bahnemann, D.; Vormoor, M. Photo-induced hydrophilicity and self-cleaning: Models and reality. Energy Environ. Sci. 2012, 5, 7491–7507. [Google Scholar] [CrossRef]
- Nishimoto, S.; Bhushan, B. Bioinspired self-cleaning surfaces with superhydrophobicity, superoleophobicity, and superhydrophilicity. RSC Adv. 2013, 3, 671–690. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Elfanaoui, A.; Elhamri, E.; Boulkaddat, L.; Ihlal, A.; Bouabid, K.; Laanab, L.; Taleb, A.; Portier, X. Optical and structural properties of TiO2 thin films prepared by sol–gel spin coating. Int. J. Hydrogen Energy 2011, 36, 4130–4133. [Google Scholar] [CrossRef]
- Chanda, A.; Joshi, S.R.; Akshay, V.; Varma, S.; Singh, J.; Vasundhara, M.; Shukla, P. Structural and optical properties of multilayered un-doped and cobalt doped TiO2 thin films. Appl. Surf. Sci. 2021, 536, 147830. [Google Scholar] [CrossRef]
- Dundar, I.; Mere, A.; Mikli, V.; Krunks, M.; Acik, I.O. Thickness effect on photocatalytic activity of TiO2 thin films fabricated by ultrasonic spray pyrolysis. Catalysts 2020, 10, 1058. [Google Scholar] [CrossRef]
- Kayani, Z.N.; Riaz, S.; Naseem, S. Magnetic and antibacterial studies of sol-gel dip coated Ce doped TiO2 thin films: Influence of Ce contents. Ceram. Int. 2020, 46, 381–390. [Google Scholar] [CrossRef]
- Bessergenev, V.; Khmelinskii, I.; Pereira, R.; Krisuk, V.; Turgambaeva, A.; Igumenov, I. Preparation of TiO2 films by CVD method and its electrical, structural and optical properties. Vacuum 2002, 64, 275–279. [Google Scholar] [CrossRef]
- Ye, Q.; Liu, P.; Tang, Z.; Zhai, L. Hydrophilic properties of nano-TiO2 thin films deposited by RF magnetron sputtering. Vacuum 2007, 81, 627–631. [Google Scholar] [CrossRef]
- Lee, M.; Park, Y.; Kim, K.; Hong, J. Influence of sputtering conditions on the properties of aluminum-doped zinc oxide thin film fabricated using a facing target sputtering system. Thin Solid Films 2020, 703, 137980. [Google Scholar] [CrossRef]
- Shin, J.; Kim, K.; Hong, J. Zn-Al layered double hydroxide thin film fabricated by the sputtering method and aqueous solution treatment. Coatings 2020, 10, 669. [Google Scholar] [CrossRef]
- Hong, J.S.; Matsushita, N.; Kim, K.H. Investigation of the effect of oxygen gas on properties of GAZO thin films fabricated by facing targets sputtering system. Semicond. Sci. Technol. 2014, 29, 075007. [Google Scholar] [CrossRef]
- Hong, J.; Kim, K.-H. Characteristic of Al-In-Sn-ZnO Thin film prepared by FTS system with hetero targets. Trans. Electr. Electron. Mater. 2011, 12, 76–79. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Hashimoto, K.; Fujishima, A.; Chikuni, M.; Kojima, E.; Kitamura, A.; Shimohigoshi, M.; Watanabe, T. Photo-generation of highly amphiphilic TiO2 surfaces. Adv. Mater. 1998, 10, 135–138. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, X.; Tian, A.; Wang, L.; Liu, C. Electrochemical properties of Ti3+ doped Ag-Ti nanotube arrays coated with hydroxyapatite. Appl. Surf. Sci. 2018, 436, 579–584. [Google Scholar] [CrossRef]
- Wang, R.; Hashimoto, K.; Fujishima, A.; Chikuni, M.; Kojima, E.; Kitamura, A.; Shimohigoshi, M.; Watanabe, T. Light-induced amphiphilic surfaces. Nature 1997, 388, 431–432. [Google Scholar] [CrossRef]
- Sakai, N.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Enhancement of the photoinduced hydrophilic conversion rate of TiO2 film electrode surfaces by anodic polarization. J. Phys. Chem. B 2001, 105, 3023–3026. [Google Scholar] [CrossRef]
- Chi, M.; Sun, X.; Sujan, A.; Davis, Z.; Tatarchuk, B.J. A quantitative XPS examination of UV induced surface modification of TiO2 sorbents for the increased saturation capacity of sulfur heterocycles. Fuel 2019, 238, 454–461. [Google Scholar] [CrossRef]
- Lu, G.; Linsebigler, A.; Yates, J.T., Jr. Ti3+ defect sites on TiO2 (110): Production and chemical detection of active sites. J. Phys. Chem. 1994, 98, 11733–11738. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Hou, Y.; Huang, J.; Wu, L.; Fu, X. The effect of postnitridation annealing on the surface property and photocatalytic performance of N-doped TiO2 under visible light irradiation. J. Catal. 2008, 255, 59–67. [Google Scholar] [CrossRef]
- Mukherjee, S.K.; Mergel, D. Thickness dependence of the growth of magnetron-sputtered TiO2 films studied by Raman and optical transmittance spectroscopy. J. Appl. Phys. 2013, 114, 13501. [Google Scholar] [CrossRef]
- Mukherjee, S.; Nebatti, A.; Mohtascham, F.; Schipporeit, S.; Notthoff, C.; Mergel, D. Influence of thickness on the structural properties of radio-frequency and direct-current magnetron sputtered TiO2 anatase thin films. Thin Solid Films 2014, 558, 443–448. [Google Scholar] [CrossRef]
- Li, S.; Jiao, S.; Wang, D.; Gao, S.; Wang, J. The influence of sputtering power on the structural, morphological and optical properties of β-Ga2O3 thin films. J. Alloys Compd. 2018, 753, 186–191. [Google Scholar] [CrossRef]
- Nadeem, I.M.; Harrison, G.T.; Wilson, A.; Pang, C.L.; Zegenhagen, J.; Thornton, G. Bridging hydroxyls on anatase TiO2(101) by water dissociation in oxygen vacancies. J. Phys. Chem. B 2018, 122, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Scherrer, P. Determination of the size and internal structure of colloidal particles using X-rays. Nachr. Ges. Wiss. Göttingen 1918, 2, 98–100. [Google Scholar]
- Hong, J.S.; Wagata, H.; Ohashi, N.; Katsumata, K.-I.; Okada, K.; Matsushita, N. Transparent ZnO films deposited by aqueous solution process under various pH conditions. J. Electron. Mater. 2015, 44, 2657–2662. [Google Scholar] [CrossRef]
- Nakaruk, A.; Ragazzon, D.; Sorrell, C. Anatase–rutile transformation through high-temperature annealing of titania films produced by ultrasonic spray pyrolysis. Thin Solid Films 2010, 518, 3735–3742. [Google Scholar] [CrossRef]
- Michalow, K.A.; Logvinovich, D.; Weidenkaff, A.; Amberg, M.; Fortunato, G.; Heel, A.; Graule, T.; Rekas, M. Synthesis, characterization and electronic structure of nitrogen-doped TiO2 nanopowder. Catal. Today 2009, 144, 7–12. [Google Scholar] [CrossRef]
- Zakrzewska, K. Titanium Dioxide Thin Films for Gas Sensors and Photonic Applications; AGH Uczelniane Wydawnictwa Naukowo-Dydaktyczne: Krakow, Poland, 2003. [Google Scholar]
- Banerjee, S.; Pillai, S.C.; Falaras, P.; O’Shea, K.E.; Byrne, J.A.; Dionysiou, D.D. New insights into the mechanism of visible light photocatalysis. J. Phys. Chem. Lett. 2014, 5, 2543–2554. [Google Scholar] [CrossRef] [PubMed] [Green Version]












| Parameters | Conditions |
|---|---|
| Targets | Ti, 4 inches |
| Substrate | Glass microscope slides |
| Base pressure | 3 × 10−5 Torr |
| Working pressure | 2 mTorr |
| Gas flow | Ar, 10 sccm; O2, 1 sccm |
| Input power | 100 W, 150 W |
| Samples | A | B | C | D | E | F | G | H |
|---|---|---|---|---|---|---|---|---|
| Input power (W) | 100 | 150 | 150 | 150 | 100 | 150 | 150 | 150 |
| Deposition time (min) | 60 | 20 | 40 | 60 | 60 | 20 | 40 | 60 |
| Annealing (500 °C) | X | X | X | X | O | O | O | O |
| Samples | A | B | C | D | E | F | G | H |
|---|---|---|---|---|---|---|---|---|
| Water contact angle Before UV irradiation (°) | 77.5 | 75.6 | 72.9 | 78.7 | 35.5 | 34.0 | 37.4 | 35.7 |
| Water contact angle After 12 h UV irradiation (°) | 36.9 | 36.7 | 32.0 | 20.0 | 18.4 | 16.1 | 11.0 | 10.8 |
| Thickness (nm) | 90.9 | 45.5 | 91.0 | 136.4 | 90.9 | 45.5 | 91.0 | 136.4 |
| Phase (Amorphous: AP) (Anatase: AT) | AP | AP | AP | AP | AP | AP | AT | AT |
| Band gap (eV) | 3.6 | 3.6 | 3.4 | 3.3 | 3.6 | 3.6 | 3.4 | 3.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Yoon, Y.; Lee, S.; Park, T.; Kim, K.; Hong, J. Thermoinduced and Photoinduced Sustainable Hydrophilic Surface of Sputtered-TiO2 Thin Film. Coatings 2021, 11, 1360. https://doi.org/10.3390/coatings11111360
Park S, Yoon Y, Lee S, Park T, Kim K, Hong J. Thermoinduced and Photoinduced Sustainable Hydrophilic Surface of Sputtered-TiO2 Thin Film. Coatings. 2021; 11(11):1360. https://doi.org/10.3390/coatings11111360
Chicago/Turabian StylePark, Sangbin, Younghwa Yoon, Sehyun Lee, Taejun Park, Kyunghwan Kim, and Jeongsoo Hong. 2021. "Thermoinduced and Photoinduced Sustainable Hydrophilic Surface of Sputtered-TiO2 Thin Film" Coatings 11, no. 11: 1360. https://doi.org/10.3390/coatings11111360