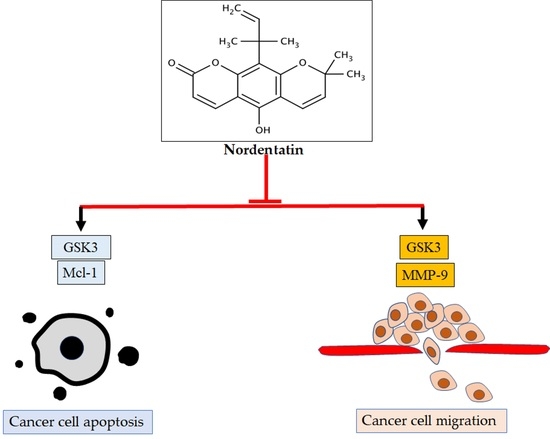
Nordentatin Inhibits Neuroblastoma Cell Proliferation and Migration through Regulation of GSK-3 Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Study of Inhibitory Activity on Cell Proliferation
2.3. Study of Cell Morphology Change by Phase-Contrast Microscopy
2.4. Study of Inhibitory Activity on Cell Migration
2.5. Cell Lysate Preparation
2.6. Study of the Molecular Mechanism of Nordentatin’s Effect on Cell Apoptosis
3. Results
3.1. Study of Inhibitory Activity on Cell Proliferation: Cell Cytotoxicity Assay
3.2. Study of Cell Morphology Change by Phase-Contrast Microscopy
3.3. Study of Inhibitory Activity on Cell Migration
3.4. Study of Molecular Mechanism of Nordentatin’s Effect on Cell Apoptosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Igney, F.H.; Krammer, P.H. Death and anti-death: Tumour resistance to apoptosis. Nat. Rev. Cancer 2002, 2, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Z.-J.; Liu, W.; Zhang, J.; Zhu, J.; Zhang, R.; Tang, J.; Xia, H. Functional polymorphisms at ERCC1/XPF genes confer neuroblastoma risk in Chinese children. EBioMedicine 2018, 30, 113–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hua, R.X.; Zhuo, Z.; Ge, L.; Zhu, J.; Yuan, L.; Chen, C.; He, J. LIN28A gene polymorphisms modify neuroblastoma susceptibility: A four-centre case-control study. J. Cell Mol. Med. 2020, 24, 1059–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, L.; Zhuo, Z.; Tang, J.; Huang, X.; Liu, J.; Wang, H.Y.; He, J. FABP4 deactivates NF-κB-IL1α pathway by ubiquitinating ATPB in tumor-associated macrophages and promotes neuroblastoma progression. Clin. Transl. Med. 2021, 11, e395. [Google Scholar] [CrossRef]
- Elmasri, H.; Ghelfi, E.; Yu, C.-W.; Traphagen, S.; Cernadas, M.; Cao, H.; Cataltepe, S. Endothelial cell-fatty acid binding protein 4 promotes angiogenesis: Role of stem cell factor/c-kit pathway. Angiogenesis 2012, 15, 457–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duda, P.; Akula, S.M.; Abrams, S.L.; Steelman, L.S.; Martelli, A.M.; Cocco, L.; McCubrey, J.A. Targeting GSK3 and Associated Signaling Pathways Involved in Cancer. Cells 2020, 9, 1110. [Google Scholar] [CrossRef]
- Kim, S.D.; Yang, S.I.; Kim, H.C.; Shin, C.Y.; Ko, K.H. Inhibition of GSK-3beta mediates expression of MMP-9 through ERK1/2 activation and translocation of NF-kappaB in rat primary astrocyte. Brain Res. 2007, 1186, 12–20. [Google Scholar] [CrossRef]
- Maurer, U.; Charvet, C.; Wagman, A.S.; Dejardin, E.; Green, D.R. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol. Cell 2006, 21, 749–760. [Google Scholar] [CrossRef]
- Govindachari, T.R.; Pai, B.R.; Subramaniam, P.S.; Muthukumaraswamy, N. Coumarins of Clausena dentata (Willd.) R. and S. Tetrahedron 1968, 24, 753–757. [Google Scholar] [CrossRef]
- Wangboonskul, J.; Pummangura, S.; Chaichantipyuth, C. Five coumarins and a carbazole alkaloids from the root bark of Clausena harmandiana. J. Nat. Prod. 1984, 47, 1058–1059. [Google Scholar] [CrossRef]
- Wangboonskul, J.; Prawan, A.; Takthaisong, P.; Sasithornwetchakun, W.; Boonyarat, C.; Yenjai, C.; Mahakunakorn, P. Analgesic, anti-inflammatory, antipyretic activities and acute toxicity of the ethanolic extract of Clausena harmandiana Pierre in animals. J. Asian Assoc. Sch. Pharm. 2012, 1, 159–169. [Google Scholar]
- Yenjai, C.; Sripontan, S.; Sriprajun, P.; Kittakoop, P.; Jintasirikul, A.; Tanticharoen, M.; Thebtaranonth, Y. Coumarins and carbazoles with antiplasmodial activity from Clausena harmandiana. Planta Med. 2000, 66, 277–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puthongking, P. Development of a New Neuroprotective Agent of Coumarins from the Root Bark of Clausena harmandiana (Pierre) ex.: Thailand Science Research and Innovation; 2011 [cited 14 May 2010]. Available online: http://elibrary.trf.or.th/project_content.asp?PJID=MRG5180175 (accessed on 30 March 2020).
- Sriphana, U.; Thongsri, Y.; Prariyachatigul, C.; Pakawatchai, C.; Yenjai, C. Clauraila E from the roots of Clausena harmandiana and antifungal activity against Pythium insidiosum. Arch Pharm Res. 2013, 36, 1078–1083. [Google Scholar] [CrossRef] [PubMed]
- King, D.; Yeomanson, D.; Bryant, H.E. PI3King the lock: Targeting the PI3K/Akt/mTOR pathway as a novel therapeutic strategy in neuroblastoma. J. Pediatr. Hematol. Oncol. 2015, 37, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhao, Y.; Luo, Q.; Zhang, Y.; Wu, K.; Wang, F. Multi-Targeted Anticancer Agents. Curr. Top. Med. Chem. 2017, 17, 3084–3098. [Google Scholar] [CrossRef]
- Mei, H.; Wang, Y.; Lin, Z.; Tong, Q. The mTOR signaling pathway in pediatric neuroblastoma. Pediatr. Hematol. Oncol. 2013, 30, 605–615. [Google Scholar] [CrossRef]
- Maris, J.M.; Hogarty, M.D.; Bagatell, R.; Cohn, S.L. Neuroblastoma. Surg. Oncol. 2007, 369, 2106–2120. [Google Scholar] [CrossRef]
- Cheung, N.K.V.; Dyer, M.A. Neuroblastoma: Developmental biology, cancer genomics and immunotherapy. Nat. Rev. Cancer 2013, 13, 397–411. [Google Scholar] [CrossRef] [Green Version]
- Maris, J.M. Recent advances in neuroblastoma. N. Engl. J. Med. 2010, 362, 2202–2211. [Google Scholar] [CrossRef] [Green Version]
- Takomthong, P.; Waiwut, P.; Yenjai, C.; Sripanidkulchai, B.; Reubroycharoen, P.; Lai, R.; Kamau, P.; Boonyarat, C. Structure–activity analysis and molecular docking studies of coumarins from Toddalia asiatica as multifunctional agents for Alzheimer’s disease. Biomedicines 2020, 8, 107. [Google Scholar] [CrossRef]
- Kumar, S.; Kumari, R.; Mishra, S. Pharmacological properties and their medicinal uses of Cinnamomum: A review. J. Pharm. Pharmacol. 2019, 71, 1735–1761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devji, T.; Reddy, C.; Woo, C.; Awale, S.; Kadota, S.; Carrico-Moniz, D. Pancreatic anticancer activity of a novel geranylgeranylated coumarin derivative. Bioorg. Med. Chem. Lett. 2011, 21, 5770–5773. [Google Scholar] [CrossRef] [PubMed]
- Nordin, N.; Fadaeinasab, M.; Mohan, S.; Hashim, N.M.; Othman, R.; Karimian, H.; Abdul Majid, N. Pulchrin A, a new natural coumarin derivative of Enicosanthellum pulchrum, induces apoptosis in ovarian cancer cells via intrinsic pathway. PLoS ONE 2016, 11, e0154023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Lu, M.; Dai, H.; Zhang, S.; Wang, H.; Wei, N. Esculetin, a coumarin derivative, exerts in vitro and in vivo antiproliferative activity against hepatocellular carcinoma by initiating a mitochondrial-dependent apoptosis pathway. Braz. J. Med. Biol. Res. 2015, 48, 245–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haghighitalab, A.; Matin, M.M.; Bahrami, A.R.; Iranshahi, M.; Saeinasab, M.; Haghighi, F. In vitro investigation of anticancer, cell-cycle-inhibitory, and apoptosis-inducing effects of diversin, a natural prenylated coumarin, on bladder carcinoma cells. Z. Nat. C. J. Biosci. 2014, 69, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Promsuwan, P.; Yenjai, C. Synthesis and cytotoxicity of coumarin derivatives and nordentatin. Asian J. Chem. 2013, 25, 3629–3632. [Google Scholar] [CrossRef]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westphal, D.; Dewson, G.; Czabotar, P.E.; Kluck, R.M. Molecular biology of Bax and Bak activation and action. Biochim. Biophys. Acta 2011, 1813, 521–531. [Google Scholar] [CrossRef] [Green Version]
- Willis, S.N.; Chen, L.; Dewson, G.; Wei, A.; Naik, E.; Fletcher, J.I.; Huang, D.C. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005, 19, 1294–1305. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, H.; Chen, M.C.; Pham, H.; Matsuo, Y.; Ishiguro, H.; Reber, H.A.; Eibl, G. Simultaneous knock-down of Bcl-xL and Mcl-1 induces apoptosis through Bax activation in pancreatic cancer cells. Biochim. Biophys. Acta 2013, 1833, 2980–2987. [Google Scholar] [CrossRef] [Green Version]
- Dufour, A.; Zucker, S.; Sampson, N.S.; Kuscu, C.; Cao, J. Role of matrix metalloproteinase-9 dimers in cell migration: Design of inhibitory peptides. J. Biol. Chem. 2010, 285, 35944–35956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Martín, V.; Jiménez-García, L.; Herranz, S.; Luque, A.; Acebo, P.; Amesty, Á.; Hortelano, S. Alpha-Hispanolol Induces Apoptosis and Suppresses Migration and Invasion of Glioblastoma Cells Likely via Downregulation of MMP-2/9 Expression and p38MAPK Attenuation. Front. Pharmacol. 2019, 10, 935. [Google Scholar] [CrossRef] [PubMed]
- Sokolosky, M.; Chappell, W.H.; Stadelman, K.; Abrams, S.L.; Davis, N.M.; Steelman, L.S.; McCubrey, J.A. Inhibition of GSK-3β activity can result in drug and hormonal resistance and alter sensitivity to targeted therapy in MCF-7 breast cancer cells. Adv. Enzym. Regul. 2014, 13, 820–833. [Google Scholar] [CrossRef] [PubMed]
- Nagini, S.; Sophia, J.; Mishra, R. Glycogen synthase kinases: Moonlighting proteins with theranostic potential in cancer. Semin. Cancer Biol. 2019, 56, 25–36. [Google Scholar] [CrossRef]
- Ding, Q.; He, X.; Hsu, J.-M.; Xia, W.; Chen, C.-T.; Li, L.-Y.; Hung, M.C. Degradation of Mcl-1 by β-TrCP mediates glycogen synthase kinase 3-induced tumor suppression and chemosensitization. Mol. Cell Biol. 2007, 27, 4006–4017. [Google Scholar] [CrossRef] [Green Version]
- Inuzuka, H.; Shaik, S.; Onoyama, I.; Gao, D.; Tseng, A.; Maser, R.S.; Hung, M.C. SCF FBW7 regulates cellular apoptosis by targeting MCL1 for ubiquitylation and destruction. Nature 2011, 471, 104–109. [Google Scholar] [CrossRef]
- Morel, C.; Carlson, S.M.; White, F.M.; Davis, R.J. Mcl-1 integrates the opposing actions of signaling pathways that mediate survival and apoptosis. Mol. Cell Biol. 2009, 29, 3845–3852. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Altman, B.J.; Coloff, J.L.; Herman, C.E.; Jacobs, S.R.; Wieman, H.L.; Rathmell, J.C. Glycogen synthase kinase 3α and 3β mediate a glucose-sensitive antiapoptotic signaling pathway to stabilize Mcl-1. Mol. Cell Biol. 2007, 27, 4328–4339. [Google Scholar] [CrossRef] [Green Version]
- Domoto, T.; Pyko, I.V.; Furuta, T.; Miyashita, K.; Uehara, M.; Shimasaki, T.; Minamoto, T. Glycogen synthase kinase-3β is a pivotal mediator of cancer invasion and resistance to therapy. Cancer Sci. 2016, 107, 1363–1372. [Google Scholar] [CrossRef]
- Kitano, A.; Shimasaki, T.; Chikano, Y.; Nakada, M.; Hirose, M.; Higashi, T.; Minamoto, T. Aberrant glycogen synthase kinase 3β is involved in pancreatic cancer cell invasion and resistance to therapy. PLoS ONE 2013, 8, e55289. [Google Scholar] [CrossRef] [Green Version]
- Cinetto, F.; Ceccato, J.; Caputo, I.; Cangiano, D.; Montini, B.; Lunardi, F.; Piazza, M.; Vianello, F. GSK-3 Inhibition Modulates Metalloproteases in a Model of Lung Inflammation and Fibrosis. Front. Mol. Biosci. 2021, 8, 633054. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; Nakamura, O.; Yamagami, Y.; Mori, M.; Horie, R.; Fukuoka, N.; Yamamoto, T. GSK-3 inhibitor inhibits cell proliferation and induces apoptosis in human osteosarcoma cells. Oncol. Rep. 2016, 35, 2348–2354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Sun, K.X.; Feng, M.X.; Sang, X.B.; Liu, B.L.; Zhao, Y. Role of glycogen synthase kinase-3beta inhibitor AZD1080 in ovarian cancer. Drug Des. Dev. Ther. 2016, 10, 1225–1232. [Google Scholar] [PubMed] [Green Version]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boonyarat, C.; Boonput, P.; Tongloh, N.; Kaewamatawong, R.; Chaiwiwatrakul, S.; Yenjai, C.; Waiwut, P. Nordentatin Inhibits Neuroblastoma Cell Proliferation and Migration through Regulation of GSK-3 Pathway. Curr. Issues Mol. Biol. 2022, 44, 1062-1074. https://doi.org/10.3390/cimb44030070
Boonyarat C, Boonput P, Tongloh N, Kaewamatawong R, Chaiwiwatrakul S, Yenjai C, Waiwut P. Nordentatin Inhibits Neuroblastoma Cell Proliferation and Migration through Regulation of GSK-3 Pathway. Current Issues in Molecular Biology. 2022; 44(3):1062-1074. https://doi.org/10.3390/cimb44030070
Chicago/Turabian StyleBoonyarat, Chantana, Panatchakorn Boonput, Nantakorn Tongloh, Rawiwun Kaewamatawong, Suchada Chaiwiwatrakul, Chavi Yenjai, and Pornthip Waiwut. 2022. "Nordentatin Inhibits Neuroblastoma Cell Proliferation and Migration through Regulation of GSK-3 Pathway" Current Issues in Molecular Biology 44, no. 3: 1062-1074. https://doi.org/10.3390/cimb44030070