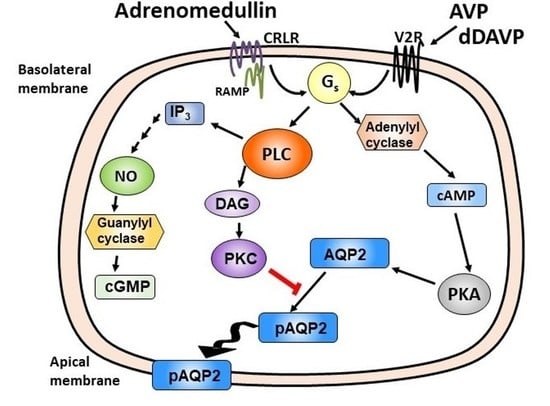
Adrenomedullin Inhibits Osmotic Water Permeability in Rat Inner Medullary Collecting Ducts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Tubule Perfusion
2.3. Tissue Incubation
2.4. Western Blot Analysis
2.5. RNA-Seq Analysis
2.6. Measurement of cAMP Level in Inner Medulla
2.7. Statistics
3. Results
3.1. ADM and Its Receptor Components (CRLR, RAMP2 and RAMP3) Are Expressed in the Inner Medulla
3.2. ADM Decreases Osmotic Water Permeability
3.3. ADM Phosphorylates AQP2
3.4. cAMP Regulates Osmotic Water Permeability Independently of ADM
3.5. ADM Increases cAMP Level
3.6. Inhibition of PLC Increases Osmotic Water Permeability
3.7. Inhibition of PLC Changes ADM-Mediated AQP2 Phosphorylation
3.8. Inhibition of PKC Increases Osmotic Water Permeability
3.9. Inhibition of PKC Alters ADM-Mediated AQP2 Phosphorylation
3.10. Inhibition of cGMP Does Not Change ADM-Mediated AQP2 Phosphorylation
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Kitamura, K.; Kangawa, K.; Kawamoto, M.; Ichiki, Y.; Nakamura, S.; Matsuo, H.; Eto, T. Adrenomedullin: A novel hypotensive peptide isolated from human pheochromocytoma. Biochem. Biophys. Res. Commun. 1993, 192, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.M.; Coppock, H.A.; Withers, D.J.; Owji, A.A.; Hay, D.L.; Choksi, T.P.; Chakravarty, P.; Legon, S.; Poyne, D.R. Adrenomedullin: Receptor and signal transduction. Biochem. Soc. Trans. 2002, 30, 432–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hay, D.L.; Conner, A.C.; Howitt, S.G.; Smith, D.M.; Poyner, D.R. The pharmacology of adrenomedullin receptors and their relationship to CGRP receptors. J. Mol. Neurosci. 2004, 22, 105–113. [Google Scholar] [CrossRef]
- Kuwasako, K.; Kitamura, K.; Nagata, S.; Hikosaka, T.; Takei, Y.; Kato, J. Shared and separate functions of the RAMP-based adrenomedullin receptors. Peptides 2011, 32, 1540–1550. [Google Scholar] [CrossRef]
- Samson, W.K. Adrenomedullin and the control of fluid and electrolyte homeostasis. Annu. Rev. Physiol. 1999, 61, 363–389. [Google Scholar] [CrossRef]
- Owada, A.; Nonoguchi, H.I.; Terada, Y.O.; Marumo, F.U.; Tomita, K. Microlocalization and effects of adrenomedullin in nephron segments and in mesangial cells of the rat. Am. J. Physiol. Renal. Physiol. 1997, 272, F691–F697. [Google Scholar] [CrossRef]
- Chini, E.N.; Chini, C.C.; Bolliger, C.; Jougasaki, M.; Grande, J.P.; Burnett, J.C., Jr.; Dousa, T.P. Cytoprotective effects of adrenomedullin in glomerular cell injury: Central role of cAMP signaling pathway. Kidney Int. 1997, 52, 917–925. [Google Scholar] [CrossRef] [Green Version]
- Jougasaki, M.; Burnett, J.C., Jr. Adrenomedullin as a renal regulator peptide. Nephrol. Dial. Transpl. 2000, 15, 293–295. [Google Scholar] [CrossRef] [Green Version]
- Jougasaki, M.; Wei, C.M.; Aarhus, L.L.; Heublein, D.M.; Sandberg, S.M.; Burnett, J.C., Jr. Renal localization and actions of adrenomedullin: A natriuretic peptide. Am. J. Physiol. 1995, 268, F657–F663. [Google Scholar] [CrossRef]
- Sato, K.; Hirata, Y.; Imai, T.; Iwashina, M.; Marumo, F. Characterization of immunoreactive adrenomedullin in human plasma and urine. Life Sci. 1995, 57, 189–194. [Google Scholar] [CrossRef]
- Jensen, B.L.; Gambaryan, S.; Schmaus, E.; Kurtz, A. Effects of dietary salt on adrenomedullin and its receptor mRNAs in rat kidney. Am. J. Physiol. 1998, 275, F55–F61. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.M.; Samson, W.K. Adrenomedullin and the integrative physiology of fluid and electrolyte balance. Microsc. Res. Tech. 2002, 57, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Ebara, T.; Miura, K.; Okumura, M.; Matsuura, T.; Kim, S.; Yukimura, T.; Iwao, H. Effect of adrenomedullin on renal hemodynamics and functions in dogs. Eur. J. Pharmacol. 1994, 263, 69–73. [Google Scholar] [CrossRef]
- Jougasaki, M.; Aarhus, L.L.; Heublein, D.M.; Sandberg, S.M.; Burnett, J.C., Jr. Role of prostaglandins and renal nerves in the renal actions of adrenomedullin. Am. J. Physiol. 1997, 272, F260–F266. [Google Scholar] [CrossRef]
- Leclerc, M.; Brunette, M.G. The paradoxical effect of adrenomedullin on Na+ transport by the renal distal tubule luminal membrane. Mol. Cell. Endocrinol. 2020, 164, 159–167. [Google Scholar] [CrossRef]
- Sands, J.M.; Nonoguchi, H.; Knepper, M.A. Vasopressin effects on urea and H2O transport in inner medullary collecting duct subsegments. Am. J. Physiol. 1987, 253, F823–F832. [Google Scholar] [CrossRef]
- Gertner, R.; Klein, J.D.; Bailey, J.L.; Kim, D.U.; Luo, X.H.; Bagnasco, S.M.; Sands, J.M. Aldosterone decreases UT-A1 urea transporter expression via the mineralocorticoid receptor. J. Am. Soc. Nephrol. 2004, 15, 558–565. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Ma, F.; Rodriguez, E.L.; Klein, J.D.; Sands, J.M. Aldosterone Decreases Vasopressin-Stimulated Water Reabsorption in Rat Inner Medullary Collecting Ducts. Cells 2020, 9, 967. [Google Scholar] [CrossRef]
- Chou, C.L.; Yip, K.P.; Michea, L.; Kador, K.; Ferraris, J.D.; Wade, J.B.; Knepper, M.A. Regulation of aquaporin-2 trafficking by vasopressin in the renal collecting duct—Roles of ryanodine-sensitive Ca2+ stores and calmodulin. J. Biol. Chem. 2000, 275, 36839–36846. [Google Scholar] [CrossRef] [Green Version]
- Knepper, M.A.; Inoue, T. Regulation of aquaporin-2 water channel trafficking by vasopressin. Curr. Opin. Cell Biol. 1997, 9, 560–564. [Google Scholar] [CrossRef]
- Hoffert, J.D.; Pisitkun, T.; Wang, G.; Shen, R.F.; Knepper, M.A. Quantitative phosphoproteomics of vasopressin-sensitive renal cells: Regulation of aquaporin-2 phosphorylation at two sites. Proc. Natl. Acad. Sci. USA 2006, 103, 7159–7164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadler, S.P. Effects of hypertonicity on ADH-stimulated water permeability in rat inner medullary collecting duct. Am. J. Physiol. 1990, 258, F266–F272. [Google Scholar] [CrossRef] [PubMed]
- Knepper, M.A.; Good, D.W.; Burg, M.B. Ammonia and bicarbonate transport by rat cortical collecting ducts perfused in vitro. Am. J. Physiol. 1985, 249, F870–F877. [Google Scholar] [CrossRef] [PubMed]
- Lankford, S.P.; Chou, C.L.; Terada, Y.O.; Wall, S.M.; Wade, J.B.; Knepper, M.A. Regulation of collecting duct water permeability independent of cAMP-mediated AVP response. Am. J. Physiol. Renal Fluid Electrolyte Physiol. 1991, 261, F554–F566. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 8th ed.; Ames, I.A., Ed.; Iowa State University Press: Iowa City, IA, USA, 1980. [Google Scholar]
- Sands, J.M.; Layton, H.E.; Fenton, R.A. Urine Concentration and Dilution. In Brenner and Rector’s The Kidney; Yu, A.S.L., Chertow, G.M., Marsden, P.A., Taal, M.W., Skorecki, K., Luyclx, V., Eds.; Elsevier: Philadelphia, PA, USA, 2020; pp. 274–302. [Google Scholar]
- Star, R.A.; Nonoguchi, H.; Balaban, R.; Knepper, M.A. Calcium and cyclic adenosine monophosphate as second messengers for vasopressin in the rat inner medullary collecting duct. J. Clin. Investig. 1988, 81, 1879–1888. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, S.; Knepper, M.A. Vasopressin activates collecting duct urea transporters and water channels by distinct physical processes. Am. J. Physiol. Renal Fluid Electrolyte Physiol. 1993, 265, F204–F213. [Google Scholar] [CrossRef]
- Hiragushi, K.; Wada, J.; Eguchi, J.; Matsuoka, T.; Yasuhara, A.; Hashimoto, I.; Yamashita, T.; Hida, K.; Nakamura, Y.; Shikata, K.; et al. The role of adrenomedullin and receptors in glomerular hyperfiltration in streptozotocin-induced diabetic rats. Kidney Int. 2004, 65, 540–550. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.W.; Chou, C.L.; Knepper, M.A. Deep Sequencing in Microdissected Renal Tubules Identifies Nephron Segment-Specific Transcriptomes. J. Am. Soc. Nephrol. 2015, 26, 2669–2677. [Google Scholar] [CrossRef]
- Bienenstock, J.; Blennerhassett, M.; Goetzl, E. Autonomic Neuroimmunology; Taylor & Francis: Oxfordshire, UK, 2003; p. 96. [Google Scholar]
- Santa Cruz Biotechnology, Inc. RAMP3 (G-1): Sc-365313. Available online: https://datasheets.scbt.com/sc-365313.pdf (accessed on 1 April 2020).
- Brown, D.; Hasler, U.; Nunes, P.; Bouley, R.; Lu, H.A. Phosphorylation events and the modulation of aquaporin 2 cell surface expression. Curr. Opin. Nephrol. Hypertens. 2010, 17, 491–498. [Google Scholar] [CrossRef] [Green Version]
- Hoffert, J.D.; Nielsen, J.; Yu, M.J.; Pisitkun, T.; Schleicher, S.M.; Nielsen, S.; Knepper, M.A. Dynamics of aquaporin-2 serine-261 phosphorylation in response to short-term vasopressin treatment in collecting duct. Am. J. Physiol. Renal Physiol. 2007, 292, F691–F700. [Google Scholar] [CrossRef]
- Baillie, G.S. Compartmentalized signalling: Spatial regulation of cAMP by the action of compartmentalized phosphodiesterases. FEBS J. 2009, 276, 1790–1799. [Google Scholar] [CrossRef] [PubMed]
- Henn, V.; Stefan, E.; Baillie, G.S.; Houslay, M.D.; Rosenthal, W.; Klussmann, E. Compartmentalized cAMP signalling regulates vasopressin-mediated water reabsorption by controlling aquaporin-2. Biochem. Soc. Trans. 2005, 33, 1316–1318. [Google Scholar] [CrossRef] [PubMed]
- Szokodi, I.; Kinnunen, P.; Tavi, P.; Weckstroöm, M.; Tóth, M.; Ruskoaho, H. Evidence for cAMP-independent mechanisms mediating the effects of adrenomedullin, a new inotropic peptide. Circulation 1998, 97, 1062–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayakawa, H.; Hirata, Y.; Kakoki, M.; Suzuki, Y.; Nishimatsu, H.; Nagata, D.; Suzuki, E.; Kikuchi, K.; Nagano, T.; Kangawa, K.; et al. Role of nitric oxide-cGMP pathway in adrenomedullin-induced vasodilation in the rat. Hypertension 1999, 33, 689–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]











| Gene ID | mRNA Expression (RPKM, n = 3) |
|---|---|
| ADM | 0.48 ± 0.13 |
| CRLR | 3.51 ± 2.17 |
| RAMP1 | No Expression |
| RAMP2 | 21.09 ± 7.01 |
| RAMP3 | 17.62 ± 2.62 |
| AQP1 (tDL marker) | 2.59 ± 0.58 |
| AQP2 (IMCD marker) | 130.8 ± 51.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, F.; Chen, G.; Rodriguez, E.L.; Klein, J.D.; Sands, J.M.; Wang, Y. Adrenomedullin Inhibits Osmotic Water Permeability in Rat Inner Medullary Collecting Ducts. Cells 2020, 9, 2533. https://doi.org/10.3390/cells9122533
Ma F, Chen G, Rodriguez EL, Klein JD, Sands JM, Wang Y. Adrenomedullin Inhibits Osmotic Water Permeability in Rat Inner Medullary Collecting Ducts. Cells. 2020; 9(12):2533. https://doi.org/10.3390/cells9122533
Chicago/Turabian StyleMa, Fuying, Guangping Chen, Eva L. Rodriguez, Janet D. Klein, Jeff M. Sands, and Yanhua Wang. 2020. "Adrenomedullin Inhibits Osmotic Water Permeability in Rat Inner Medullary Collecting Ducts" Cells 9, no. 12: 2533. https://doi.org/10.3390/cells9122533