Systemic Glycosaminoglycan Clearance by HARE/Stabilin-2 Activates Intracellular Signaling
Abstract
:1. Introduction
2. Glycosaminoglycans (GAGs)
3. Glycosaminoglycan and Proteoglycan Turnover, Biomatrix Plasticity and Recycling Receptor-Mediated Endocytosis
3.1. Systemic GAG Clearance Is also a Mechanism for Turnover of Proteoglycans (PGs) and Other Bound (Piggybacking) Proteins
3.2. Morning Stiffness, Flexibility and Biomatrix Plasticity
3.3. The Importance of Constitutive Receptor-Mediated Endocytosis and Receptor Recycling
3.4. The GAG and GAG-PG “Trash Problem”
3.5. High Capacity GAG Uptake and Degradation Is Mediated by Stab2/HARE in SECs
4. Full-Length Stab2 Mediates Macrophage Binding to and Engulfment of Apoptotic Cells and Bacteria
5. The HARE Isoform Is Created In Vivo and In Vitro by Controlled Partial Stab2 Proteolysis
5.1. HARE Is a Stab2 Protein Isoform, Not a Splice Variant
5.2. Primary and Recombinant Cells Express Distinct 2:1 Ratios of HARE:Stab2
5.3. All Stab2 Studies Unknowingly Include Data Reflecting the Ubiquitous HARE Isoform
6. All GAG Ligands Bind to HARE, the C-Terminal Stab2 Half-Receptor
6.1. Three Other GAG Ligands Were Reported between 1981 and 2003
6.2. Five Additional GAG Ligands Were Reported in 2008
6.3. Organization of GAG Binding Domains within HARE
6.4. HA Binding Is Mediated by a Link Module, also Found in Other Extracellular Proteins
6.5. Unlike TSG-6, the HARE Link Module Does Not Enable Simultaneous Binding of HA and Hep
7. Both Stab2 and HARE Can Activate Intracellular Signaling
7.1. Relatively Few Studies Have Focused on Cell Signaling Mediated by Stab2/HARE
7.2. Stab2 Mediates PS Recognition and Phagocytosis of Apoptotic Cells
7.3. Stab2/HARE Regulates Arterial-Venous Differentiation in Zebrafish
7.4. HARE Expression during Rat Liver Embryogenesis Is Biphasic
8. HARE Mediates Coordinated HA Endocytosis and ERK1/2 Phosphorylation
8.1. Free HARE Is in Complexes with Three Different Protein Kinases
8.2. HARE HA Endocytosis Activates ERK1/2 but Not JNK or p38
8.3. HARE•HA Endocytosis Stimulates Tyr Phosphorylation of HARE
9. The Stab2/HARE CD Contains Four Potential Targeting Motifs That Mediate Clathrin-Coated Pit Receptor-Mediated Endocytosis of HA and Hep
9.1. HARE Endocytosis of HA Is Mediated by Three of the Four CD Targeting Motifs
9.2. Redundant Targeting Motifs Increase Total Ligand Uptake Capacity of HARE by Enabling Parallel Coated Pit Targeting of HARE•Ligand Complexes
9.3. HARE Endocytosis of Hep Is Mediated Be a Different Subset of 3-Motifs than the 3-Motif Subset Mediating HA Uptake
10. HARE-Mediated Intracellular Signaling Is Stimulated by Endocytosis of only Some GAG Types
10.1. Only HA, Hep and DS Uptake Activate HARE-Mediated Signaling
10.2. Four Nonsignaling CS Types Block HA Uptake and Signaling
11. HARE-Mediated Activation of ERK and NF-κB during HA or Hep Internalization Requires only One of the Four Endocytic Motifs; M3
12. HA Activation of HARE Signaling Requires a Link Module N-Glycan
12.1. The HARE Link Module N-Glycans Are More Structurally Diverse and Most Are Sialylated, Unlike N-Glycans at Other HARE Glycosylation Sites
12.2. HARE Lacking an N-Glycan in the LINK Module Mediates Normal HA Binding and Endocytosis but Is Unable to Activate NF-κB and LUC Recorder Gene Expression
13. Link Modules in TSG-6, CD44 and LYVE-1 Show Conformational Changes upon Binding HA
13.1. The TSG-6 Link Module
13.2. The CD44 Link Module
13.3. The LYVE-1 Link Module
14. HA-Binding Protein Interactions with HA: Affinity Versus Avidity
14.1. There Is No Reason to Believe That the Binding Affinities between HARE and HAs of Various Size Are Much Different than for Other Proteins with a Link Module
14.2. Apparent High Affinities Are Due to Great Avidity Effects for a Link Module-Protein Interacting with One Long HA Chain
14.3. A Major HA Avidity Effect Is Due to Ultra-High HA Binding Site Concentrations Enabling Rapid Recapture of the Same Dissociated HA Chain by a Link-Module Protein
15. HARE-Mediated Signaling in Response to HA Endocytosis Is Highly Dependent on HA Size
15.1. Only Uptake of HA in the 40–400 kDa Mass Range Activates HARE Signaling.
15.2. Larger or Smaller HA or Excess Signaling HA Block the Ability of 40–400 kDa HA to Activate HARE Signal Transduction
16. A Model for HA-Size Regulation of HARE-Mediated Signaling during Endocytosis
16.1. The Central Premise
- The same HA chain could bind to two HARE ectodomains, inducing conformational changes in both that enable stable dimer formation, if the HA chain length is a suitable length to bring the two CDs together, with or without additional TMD or CD conformational changes.
- Two HARE ectodomains, and thus their CDs, come together upon binding the same appropriate-size HA chain to create a stable HARE dimer, with no conformational changes required. An appropriate connecting length HA might stabilize weak TMD•TMD or intracellular CD•CD interactions that would not otherwise be strong enough to keep the dimer together.
16.2. Why Is HARE Binding to Small HA Unable to Create Signaling-Competent HARE•HA Complexes?
16.3. Why Is HARE Binding to Large HA Unable to Create Signaling-Competent HARE•HA Complexes?
16.4. How Could Intermediate Size HA Control HARE•HA Complex Signaling Potential?
16.5. New HARE•HA Dimer Complexes Could Spontaneously Rearrange to Become More Stable
17. The Size-Dependence of HA Signal Activation Is Related to HA Link-Module Orientation in a Dimer Complex
17.1. Link Modules Could Form --HA12•HARE•[HA]•HARE•HA12-- Dimers in Two Orientations
17.2. An HA Rod-Coil Equilibrium Occurs in the 100–250 kDa Size Range
17.3. The Rod-Coil Equilibrium Might Facilitate Formation of HARE•HA Signaling Complexes
17.4. An Antiparallel, Rather than Parallel, HA Orientation for Dimeric HARE Link-Modules Likely Enables Size-Dependent Signal Activation
18. Link Module Conformational Changes upon Binding HA May Be Enhanced by a Link Module N-Glycan That Initiates a Mechanism for HA-Size Dependent Signaling
18.1. A Subset of Proteins with Link Modules Have an N-Glycosylation Site Near Their C-Terminal End
18.2. Signal Activation Is Consistent with Conformational Changes upon HA Binding That Induce Intracellular CD-CD Conformation Changes (15.1)
18.3. A Plausible Scenario
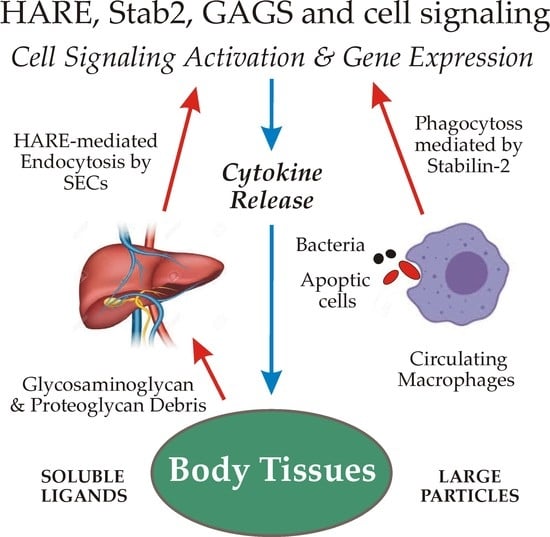
19. Two Stab2/HARE-Expressing Cell Types May Constitute a Systemic Mechanism for Monitoring Biomatrix Turnover and Health
19.1. A Tissue-Stress Sensor System
19.2. TGFβ Is Released during Stab2-Mediated Phagocytosis
19.3. Indicator Ligands
19.4. Why Might DS and Hep also Signal in a Tissue-Stress Sensor System?
19.5. Biomatrix Turnover during Normal Homeostasis Does Not Activate Signaling
19.6. The Size of Degraded HA Is a Key Indicator of Normal Versus Pathological Biomatrix Turnover
19.7. Most Normal or Pathological Physiological Processes Involve Cytokines
20. Summary
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CD | cytoplasmic domain |
| CDR | chondroitin |
| CS | chondroitin sulfate |
| DS | dermatan sulfate |
| DxS | dextran sulfate |
| E1–E4 | EGF-like repeat domains 1–4 |
| EGF | Epidermal growth factor |
| ERK | Extracelluar Regulated Kinase |
| EV | empty vector |
| F1–F7 | Fasciclin-1 domains 1–7 |
| GAG | glycosaminoglycan |
| HA | Hyaluronan, hyaluronic acid |
| HAase | hyaluronidase |
| HARE | Hyaluronic Acid Receptor for Endocytosis (the half-length 190 kDa Stab2 receptor) |
| Hep | heparin |
| JNK | c-Jun N-terminal protein kinase |
| LYVE-1 | lymphatic vessel endothelial cell HA receptor-1 |
| LUC | luciferase |
| MS | mass spectrometry |
| NF-κB | Nuclear Factor-kappaB |
| PS | phosphatidyserine |
| SR | scavenger receptor |
| SEC | sinusoidal endothelial cell |
| Stab2 | Stabilin-2 |
| TMD | transmembrane domain |
| WT | wildtype |
References
- PrabhuDas, M.R.; Baldwin, C.L.; Bollyky, P.L.; Bowdish, D.M.E.; Drickamer, K.; Febbraio, M.; Herz, J.; Kobzik, L.; Krieger, M.; Loike, J.; et al. A consensus definitive classification of scavenger receptors and their roles in health and disease. J. Immunol. 2017, 198, 3775–3789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weigel, P.H.; West, C.M.; Zhao, P.; Wells, L.; Baggenstoss, B.A.; Washburn, J. Hyaluronan synthase assembles chitin oligomers with -GlcNAc(α1→)UDP at the reducing end. Glycobiology 2015, 25, 632–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weigel, P.H.; Baggenstoss, B.A.; Washburn, J.L. Hyaluronan synthase assembles hyaluronan on a [GlcNAc(β1,4)]n-GlcNAc(α1→)UDP primer and hyaluronan retains this residual chitin oligomer as a cap at the nonreducing end. Glycobiology 2017, 27, 536–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karamanos, N.K.; Piperigkou, Z.; Theocharis, A.D.; Watanabe, H.; Franchi, M.; Baud, S.; Brézillon, S.; Götte, M.; Passi, A.; Vigetti, D.; et al. Proteoglycan chemical diversity drives multifunctional cell regulation and therapeutics. Chem. Rev. 2018, 118, 9152–9232. [Google Scholar] [CrossRef]
- Knudsen, C.B.; Knudson, W. Cartilage proteoglycans. Semin. Cell Dev. Biol. 2001, 12, 69–78. [Google Scholar] [CrossRef] [Green Version]
- DeAngelis, P.L.; Papaconstantinou, J.; Weigel, P.H. Isolation of a Streptococcus pyogenes gene locus that directs hyaluronan biosynthesis in acapsular mutants and in heterologous bacteria. J. Biol. Chem. 1993, 268, 14568–14571. [Google Scholar]
- Baggenstoss, B.A.; Weigel, P.H. SEC-MALLS analysis of hyaluronan size distributions made by membrane-bound hyaluronan synthase. Anal. Biochem. 2006, 352, 243–251. [Google Scholar] [CrossRef] [Green Version]
- Eskild, W.; Smedsrød, B.; Berg, T. Receptor mediated endocytosis of formaldehyde treated albumin, yeast invertase and chondroitin sulfate in suspensions of rat liver endothelial cells. Int. J. Biochem. 1986, 18, 647–651. [Google Scholar] [CrossRef]
- Manicourt, D.H.; Poilvache, P.; Nzeusseu, A.; van Egeren, A.; Devogelaer, J.P.; Lenz, M.E.; Thonar, E.J. Serum levels of hyaluronan, antigenic keratan sulfate, matrix metalloproteinase 3, and tissue inhibitor of metalloproteinases I change predictably in rheumatoid arthritis patients who have begun activity after a night of bed rest. Arthritis Rheum. 1999, 42, 1861–1869. [Google Scholar] [CrossRef]
- Weigel, P.H.; Yik, J.H.N. Glycans as endocytosis signals: The cases of the asialoglycoprotein and hyaluronan- chondroitin sulfate receptors. Biochim. Biophys. Acta 2002, 1572, 341–363. [Google Scholar] [CrossRef]
- Jackson, D.G. Immunological functions of hyaluronan and its receptors in the lymphatics. Immunol. Rev. 2009, 230, 216–231. [Google Scholar] [CrossRef] [PubMed]
- Laurent, T.C.; Fraser, J.R.E. Catabolism of Hyaluronan. In Degradation of Bioactive Substances: Physiology and Pathophysiology; Henriksen, J.H., Ed.; CRC Press: Boca Raton, FL, USA, 1991; pp. 249–265. [Google Scholar]
- Fraser, J.R.E.; Laurent, T.C. Turnover and metabolism of hyaluronan. In Biology of Hyaluronan; Evered, D., Whelan, J., Eds.; Wiley: Chichester, UK, 1989; Volume 143, pp. 41–59. [Google Scholar]
- Tammi, R.; Saamanen, A.M.; Maibach, H.I.; Tammi, M.I. Degradation of newly synthesized high molecular mass hyaluronan in the epidermal and dermal compartments of human skin in organ culture. J. Investig. Dermatol. 1991, 97, 126–130. [Google Scholar] [CrossRef] [Green Version]
- Raja, R.H.; McGary, C.T.; Weigel, P.H. Affinity and distribution of surface and intracellular hyaluronic acid receptors in isolated rat liver endothelial cells. J. Biol. Chem. 1988, 263, 16661–16668. [Google Scholar]
- Falkowski, M.; Schledzewski, K.; Hansen, B.; Goerdt, S. Expression of stabilin-2, a novel fasciclin-like hyaluronan receptor protein, in murine sinusoidal endothelia, avascular tissues, and at solid/liquid interfaces. Histochem. Cell Biol. 2003, 120, 361–369. [Google Scholar] [CrossRef]
- Politz, O.; Gratchev, A.; McCourt, P.A.G.; Schledzewski, K.; Guillot, P.; Johansson, S.; Svineng, G.; Franke, P.; Kannicht, C.; Kzhyshkowska, J.; et al. Stabilin-1 and -2 constitute a novel family of fasciclin-like hyaluronan receptor homologues. Biochem. J. 2002, 362, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Jung, M.Y.; Kim, H.J.; Lee, S.J.; Kim, S.Y.; Lee, B.H.; Kwon, T.H.; Park, R.W.; Kim, I.S. Rapid cell corpse clearance by stabilin-2, a membrane phosphatidylserine receptor. Cell Death Differ. 2008, 15, 192–201. [Google Scholar] [CrossRef] [Green Version]
- Jung, M.Y.; Park, S.Y.; Kim, I.S. Stabilin-2 is involved in lymphocyte adhesion to the hepatic sinusoidal endothelium via the interaction with αMβ2 integrin. J. Leukoc. Biol. 2007, 82, 1156–1165. [Google Scholar] [CrossRef]
- Kim, S.; Park, S.-Y.; Kim, S.Y.; Bae, D.-J.; Pyo, J.-H.; Hong, M.; Kim, I.S. Cross-talk between engulfment receptors Stabilin-2 and integrin αvβ5 orchestrates engulfment of phosphatidylserine-exposed erythrocytes. Mol. Cell. Biol. 2012, 32, 2698–2708. [Google Scholar] [CrossRef] [Green Version]
- Harris, E.N.; Kyosseva, S.V.; Weigel, J.A.; Weigel, P.H. Expression, processing, and glycosaminoglycan binding activity of the recombinant human 315-kDa Hyaluronic Acid Receptor for Endocytosis (HARE). J. Biol. Chem. 2007, 282, 2785–2797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.-Y.; Kim, I.-S. Stabilin receptors: Role as phosphatidylserine receptors. Biomolecules 2019, 9, 387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hare, A.K.; Harris, E.N. Tissue-specific splice variants of HARE/Stabilin-2 are expressed in bone marrow, lymph node, and spleen. Biochem. Biophys. Res. Commun. 2015, 456, 257–261. [Google Scholar] [CrossRef] [Green Version]
- Zhou, B.; McGary, C.T.; Weigel, J.A.; Saxena, A.; Weigel, P.H. Purification and molecular identification of the human hyaluronan receptor for endocytosis (HARE). Glycobiology 2003, 13, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.N.; Weigel, J.A.; Weigel, P.H. Endocytic function, glycosaminoglycan specificity, and antibody sensitivity of the recombinant human 190 kDa HA Receptor for Endocytosis (HARE). J. Biol. Chem. 2004, 279, 36201–36209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, E.N.; Weigel, P.H. The ligand-binding profile of HARE/Stabilin-2: Hyaluronan and chondroitin sulfates A, C, and D bind to overlapping sites distinct from the sites for heparin, acetylated low-density lipoprotein and dermatan sulfate. Glycobiology 2008, 18, 638–648. [Google Scholar] [CrossRef] [Green Version]
- Yannariello-Brown, J.; Zhou, B.; Ritchie, D.; Oka, J.A.; Weigel, P.H. A novel ligand blot assay detects different hyaluronan-binding proteins in rat liver hepatocytes and sinusoidal endothelial cells. Biochem. Biophys. Res. Commun. 1996, 218, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Oka, J.A.; Singh, A.; Weigel, P.H. Purification and subunit characterization of the rat liver Endocytic Hyaluronan Receptor. J. Biol. Chem. 1999, 274, 33831–33834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, B.; Weigel, J.A.; Fauss, L.; Weigel, P.H. Identification of the Hyaluronan Receptor for Endocytosis (HARE). J. Boil. Chem. 2000, 275, 37733–37741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prevo, R.; Banerji, S.; Ni, J.; Jackson, D.G. Rapid plasma membrane-endosomal trafficking of the lymph node sinus and high endothelial venule scavenger receptor/homing receptor stabilin-1 (FEEL-1/ CLEVER-1). J. Biol. Chem. 2004, 279, 52580–52592. [Google Scholar] [CrossRef] [Green Version]
- Irjala, H.; Elima, K.; Johansson, E.-L.; Merinen, M.; Kontula, K.; Alanen, K.; Grenman, R.; Salmi, M.; Jalkanen, S. The same endothelial receptor controls lymphocyte traffic both in vascular and lymphatic vessels. Eur. J. Immunol. 2003, 33, 815–824. [Google Scholar] [CrossRef]
- Lee, S.J.; Park, S.Y.; Jung, M.Y.; Bae, S.M.; Kim, I.S. Mechanism for phosphatidylserine-dependent erythrophagocytosis in mouse liver. Blood 2011, 117, 5215–5523. [Google Scholar] [CrossRef] [Green Version]
- Weigel, P.H. Discovery of the liver Hyaluronan Receptor for Endocytosis (HARE) and its progressive emergence as the multi-ligand scavenger receptor Stabilin-2. Biomolecules 2019, 9, 454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraser, J.R.F.; Laurent, T.C.; Pertoft, H.; Baxter, E. Plasma clearance, tissue distribution and metabolism of hyaluronic acid injected intravenously in the rabbit. Biochem. J. 1981, 200, 415–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smedsrod, B.; Pertoft, H.; Gustafson, S.; Laurent, T.C. Scavenger functions of the liver endothelial cell. Biochem. J. 1990, 266, 313–327. [Google Scholar] [CrossRef]
- Laurent, T.C.; Fraser, J.R.E.; Pertoft, H.; Smedsrød, B. Binding of hyaluronate and chondroitin sulphate to liver endothelial cells. Biochem. J. 1986, 234, 653–658. [Google Scholar] [CrossRef] [Green Version]
- McGary, C.T.; Raja, R.H.; Weigel, P.H. Endocytosis of hyaluronic acid by rat liver endothelial cells: Evidence for receptor recycling. Biochem. J. 1989, 257, 875–884. [Google Scholar] [CrossRef]
- Tamura, Y.; Adachi, H.; Osuga, J.; Ohashi, K.; Yahagi, N.; Sekiya, M.; Okazaki, H.; Tomita, S.; Iizuka, Y.; Shimano, H.; et al. FEEL-1 and FEEL-2 are endocytic receptors for advanced glycation end products. J. Biol. Chem. 2003, 278, 12613–12617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, E.N.; Weigel, J.A.; Weigel, P.H. The human hyaluronan receptor for endocytosis (HARE/Stabilin-2) is a systemic clearance receptor for heparin. J. Biol. Chem. 2008, 283, 17341–17350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, E.N.; Baggenstoss, B.A.; Weigel, P.H. Rat and human HARE/Stabilin-2 are clearance receptors for high and low molecular weight heparin. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G1191–G1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, B.; Weigel, J.A.; Saxena, A.; Weigel, P.H. Molecular cloning and functional expression of the rat 175-kDa Hyaluronan Receptor for Endocytosis. Mol. Biol. Cell. 2002, 13, 2853–2868. [Google Scholar] [CrossRef] [PubMed]
- Kohda, D.; Morton, C.J.; Parkar, A.A.; Hatanaka, H.; Inagaki, F.M.; Campbell, I.D.; Day, A.J. Solution structure of the Link module: A hyaluronan-binding domain involved in extracellular matrix stability and cell migration. Cell 1996, 86, 767–775. [Google Scholar] [CrossRef]
- Blundell, C.D.; Mahoney, D.J.; Almond, A.; DeAngelis, P.L.; Kahmann, J.D.; Teriete, P.; Pickford, A.; Campbell, I.D.; Day, A.J. The link module from ovulation- and inflammation-associated protein TSG-6 changes conformation on hyaluronan binding. J. Biol. Chem. 2003, 278, 49261–49270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahoney, D.J.; Mulloy, B.; Forster, M.J.; Blundell, C.D.; Fries, E.; Milner, C.M.; Day, A.J. Characterization of the interaction between tumor necrosis factor stimulated Gene-6 and heparin: Implications for the inhibition of plasmin in extracellular matrix microenvironments. J. Biol. Chem. 2005, 280, 27044–27055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamoud, N.; Tran, V.; Aimi, T.; Kakegawa, W.; Lahaie, S.; Thibault, M.P.; Pelletier, A.; Wong, G.W.; Kim, I.S.; Kania, A.; et al. Spatiotemporal regulation of the GPCR activity of BAI3 by C1qL4 and Stabilin-2 controls myoblast fusion. Nat. Commun. 2018, 9, 4470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fajardo, V.A.; Chambers, P.J.; Juracic, E.S.; Rietze, B.A.; Gamum, D.; Bellissimo, C.; Kwon, F.; Quadrilatero, J.; Tupling, R.A. Sarcolipin deletion in mdx mice impairs calcineurin signalling and worsens dystrophic pathology. Hum. Mol. Genet. 2018, 27, 4094–4102. [Google Scholar] [CrossRef]
- Leibing, T.; Géraud, C.; Augustin, I.; Boutros, M.; Augustin, H.G.; Okun, J.G.; Langhans, C.D.; Zierow, J.; Wohlfeil, S.A.; Olsavszky, V.; et al. Angiocrine Wnt signaling controls liver growth and metabolic maturation in mice. Hepatology 2018, 68, 707–722. [Google Scholar] [CrossRef] [PubMed]
- Koch, P.S.; Olsavszky, V.; Ulbrich, F.; Sticht, C.; Demory, A.; Leibing, T.; Henzler, T.; Meyer, M.; Zierow, J.; Schneider, S.; et al. Angiocrine Bmp2 signaling in murine liver controls normal iron homeostasis. Blood 2017, 29, 415–419. [Google Scholar] [CrossRef] [Green Version]
- Harris, E.N.; Cabral, F. Ligand Binding and Signaling of HARE/Stab2. Biomolecules 2019, 9, 273. [Google Scholar] [CrossRef] [Green Version]
- Park, S.Y.; Kang, K.B.; Thapa, N.; Kim, S.Y.; Lee, S.J.; Kim, I.S. Requirement of adaptor protein GULP during stabilin-2-mediated cell corpse engulfment. J. Biol. Chem. 2008, 283, 10593–10600. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.W.; Park, S.Y.; Kim, I.S. Novel function of stabilin-2 in myoblast fusion: The recognition of extracellular phosphatidylserine as a “fuse-me” signal. BMB Rep. 2016, 49, 303–304. [Google Scholar] [CrossRef] [Green Version]
- Penberthy, K.K.; Ravichandran, K.S. Apoptotic cell recognition receptors and scavenger receptors. Immunol. Rev. 2016, 269, 44–59. [Google Scholar] [CrossRef] [Green Version]
- Rost, M.S.; Sumanas, S. Hyaluronic acid receptor Stabilin-2 regulates Erk phosphorylation and arterial- venous differentiation in zebrafish. PLoS ONE 2014, 9, e88614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wein, R.O.; McGary, C.T.; Doerr, T.D.; Popat, S.R.; Howard, J.L.; Weigel, J.A.; Weigel, P.H. Hyaluronan and its receptors in mucoepidermoid carcinoma. Head Neck 2006, 28, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Duff, B.; Weigel, J.A.; Bourne, P.; Weigel, P.H.; McGary, C.T. Endothelium in hepatic cavernous hemangiomas does not express the Hyaluronan Receptor for Endocytosis. Hum. Pathol. 2002, 33, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Kyosseva, S.V.; Harris, E.N.; Weigel, P.H. The hyaluronan receptor for endocytosis mediates hyaluronan dependent signal transduction via extracellular signal-regulated kinases. J. Biol. Chem. 2008, 283, 15047–15055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulenberg, B.; Aggeler, R.; Beechem, J.M.; Capaldi, R.A.; Patton, W.F. Analysis of steady-state protein phosphorylation in mitochondria using a novel fluorescent phosphosensor dye. J. Biol. Chem. 2003, 278, 27251–27255. [Google Scholar] [CrossRef] [Green Version]
- Pandey, M.S.; Harris, E.N.; Weigel, J.A.; Weigel, P.H. The cytoplasmic domain of the hyaluronan receptor for endocytosis (HARE) contains multiple endocytic motifs targeting coated pit-mediated internalization. J. Biol. Chem. 2008, 283, 21453–21461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, M.S.; Harris, E.N.; Weigel, P.H. HARE-mediated uptake of hyaluronan and heparin are mediated by different sub-sets of three endocytic motifs. Int. J. Cell Biol. 2015, 2015, 524707. [Google Scholar] [CrossRef] [Green Version]
- Pandey, M.S.; Weigel, P.H. Hyaluronic acid receptor for endocytosis (HARE)-mediated endocytosis of hyaluronan, heparin, dermatan sulfate, and acetylated low density lipoprotein (AcLDL), but not chondroitin sulfate types A, C, D, or E, activates NF-κB-regulated gene expression. J. Biol. Chem. 2014, 289, 1756–1767. [Google Scholar] [CrossRef] [Green Version]
- Fan, F.; Wood, K.V. Bioluminescent assays for high-throughput screening. Assay Drug Dev. Technol. 2007, 5, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Pandey, M.S.; Miller, C.M.; Harris, E.N.; Weigel, P.H. Only one of four HARE/STAB2 endocytic motifs is required for ERK and NF-kβ activation in response to heparin uptake. PLoS ONE 2016, 11, e0154124. [Google Scholar] [CrossRef] [Green Version]
- Van der Geer, P.; Hunter, T.; Lindberg, R.A. Receptor protein-tyrosine kinases and their signal transduction pathways. Annu. Rev. Cell Biol. 1994, 10, 251–337. [Google Scholar] [CrossRef]
- Harris, E.N.; Parry, S.; Sutton-Smith, M.; Pandey, M.S.; Panico, M.; Morris, H.R.; Haslam, S.M.; Dell, A.; Weigel, P.H. N-Glycans on the Link domain of human HARE/Stabilin-2 are needed for HA binding to purified ectodomain, but not for cellular endocytosis of hyaluronan. Glycobiology 2010, 20, 991–1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, M.S.; Weigel, P.H. A HARE Link domain N-glycan is required for ERK and NF-κB signaling in response to the uptake of hyaluronan but not heparin, dermatan sulfate or acetylated LDL. J. Biol. Chem. 2014, 289, 21807–21817. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.; Jowitt, T.A.; Day, A.J.; Prestegard, J.H. Nuclear magnetic resonance insight into the multiple glycosaminoglycan binding modes of the Link module from human TSG-6. Biochemistry 2016, 55, 262–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blundell, C.D.; Almond, A.; Mahoney, D.J.; DeAngelis, P.L.; Campbell, I.D.; Day, A.J. Towards a structure for a TSG-6 hyaluronan complex by modeling and NMR spectroscopy: Insights into other members of the link module superfamily. J. Biol. Chem. 2005, 280, 18189–18201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teriete, P.; Banerji, S.; Noble, M.; Blundell, C.D.; Wright, A.J.; Pickford, A.R.; Lowe, E.; Mahoney, D.J.; Tammi, M.I.; Kahmann, J.D.; et al. Structure of the regulatory hyaluronan binding domain in the inflammatory leukocyte homing receptor CD44. Mol. Cell 2004, 13, 483–496. [Google Scholar] [CrossRef] [Green Version]
- Banerji, S.; Wright, A.J.; Noble, M.; Mahoney, D.J.; Campbell, I.D.; Day, A.J.; Jackson, D.G. Structures of the CD44-hyaluronan complex provide insight into a fundamental carbohydrate-protein interaction. Nat. Struct. Mol. Biol. 2007, 14, 234–239. [Google Scholar] [CrossRef]
- Takeda, M.; Ogino, S.; Umemoto, R.; Sakakura, M.; Kajiwara, M.; Sugahara, K.N.; Hayasaka, H.; Miyasaka, M.; Terasawa, H.; Shimada, I. Ligand-induced structural changes of the CD44 hyaluronan-binding domain revealed by NMR. J. Biol. Chem. 2006, 281, 40089–40095. [Google Scholar] [CrossRef] [Green Version]
- Banerji, S.; Ni, J.; Wang, S.-X.; Clasper, S.; Su, J.; Tammi, R.; Jones, M.; Jackson, D.G. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J. Cell. Biol. 1999, 144, 789–801. [Google Scholar] [CrossRef]
- Banerji, S.; Hide, B.R.; James, J.R.; Noble, M.E.; Jackson, D.G. Distinctive properties of the hyaluronan-binding domain in the lymphatic endothelial receptor Lyve-1 and their implications for receptor function. J. Biol. Chem. 2010, 285, 10724–10735. [Google Scholar] [CrossRef] [Green Version]
- Banerji, S.; Lawrance, W.; Metcalfe, C.; Briggs, D.C.; Yamauchi, A.; Dushek, O.; van der Merwe, P.A.; Day, A.J.; Jackson, D.G. Homodimerization of the Lymph Vessel Endothelial Receptor LYVE-1 through a redox-labile disulfide is critical for hyaluronan binding in lymphatic endothelium. J. Biol. Chem. 2016, 291, 25004–25018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scatchard, G. The attractions of proteins for small molecules and ions. Ann. N. Y. Acad. Sci. 1949, 61, 660–672. [Google Scholar] [CrossRef]
- Zhuo, L.; Kanamori, A.; Kannagi, R.; Itano, N.; Wu, J.; Hamaguchi, M.; Ishiguro, N.; Kimata, K. SHAP potentiates the CD44-mediated leukocyte adhesion to the hyaluronan substratum. J. Biol. Chem. 2006, 281, 20303–20314. [Google Scholar] [CrossRef] [Green Version]
- Lesley, J.; Hascall, V.C.; Tammi, M.; Hyman, R. Hyaluronan binding by cell surface CD44. J. Biol. Chem. 2000, 275, 26967–26975. [Google Scholar] [CrossRef] [PubMed]
- Stanly, T.A.; Fritzsche, M.; Banerji, S.; Shrestha, D.; Schneider, F.; Eggeling, C.; Jackson, D.G. The cortical actin network regulates avidity-dependent binding of hyaluronan by the lymphatic vessel endothelial receptor LYVE-1. J. Biol. Chem. 2020, 295, 5036–5050. [Google Scholar] [CrossRef] [Green Version]
- Lawrance, W.; Banerji, S.; Day, A.J.; Bhattacharjee, S.; Jackson, D.G. Binding of Hyaluronan to the native Lymphatic Vessel Endothelial receptor LYVE-1 is critically dependent on receptor clustering and hyaluronan organization. J. Biol. Chem. 2016, 291, 8014–8030. [Google Scholar] [CrossRef] [Green Version]
- Weigel, P.H.; Baggenstoss, B.A. What is special about 200 kDa hyaluronan that activates hyaluronan receptor signaling? Glycobiology 2017, 27, 868–877. [Google Scholar] [CrossRef] [Green Version]
- Pandey, M.S.; Baggenstoss, B.A.; Washburn, J.; Harris, E.N.; Weigel, P.H. The hyaluronan receptor for endocytosis (HARE) activates NF-κB-mediated gene expression in response to 40–400-kDa, but not smaller or larger, hyaluronans. J. Biol. Chem. 2013, 288, 14068–14079. [Google Scholar] [CrossRef] [Green Version]
- Cowman, M.K.; Lee, H.-G.; Schwertfeger, K.L.; McCarthy, J.B.; Turley, E.A. The content and size of hyaluronan in biological fluids and tissues. Front. Immunol. 2015, 6, 261. [Google Scholar] [CrossRef] [Green Version]
- Weigel, J.A.; Raymond, R.C.; McGary, C.T.; Singh, A.; Weigel, P.H. A blocking antibody to the hyaluronan (HA) receptor for endocytosis (HARE) inhibits HA clearance by perfused liver. J. Biol. Chem. 2003, 278, 9802–9812. [Google Scholar] [CrossRef] [Green Version]
- Scott, J.E. Extracellular matrix, supramolecular organisation and shape. J. Anat. 1995, 187, 259–269. [Google Scholar] [PubMed]
- Weigel, P.H.; Pandey, M.S.; Harris, E.N. A HARE/STAB2-mediated sensing system to monitor tissue biomatrix homeostasis and stress. In Structure and Function of Biomatrix: Control of Cell Function and Gene Expression; Balazs, E.A., Ed.; Matrix Biology Institute: Edgewater, NJ, USA, 2012; pp. 293–314. ISBN 978-0-9856518-1-7. [Google Scholar]
- Fadok, V.A.; Bratton, D.L.; Konowal, A.; Freed, P.W.; Westcott, J.Y.; Henson, P.M. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGFβ, PGE2, and PAF. J. Clin. Investig. 1998, 101, 890–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huynh, M.L.; Fadok, V.A.; Henson, P.M. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-β1 secretion and the resolution of inflammation. J. Clin. Investig. 2002, 109, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Kulms, D.; Schwarz, T. NF- κβ and cytokines. Vitam. Horm. 2006, 74, 283–300. [Google Scholar] [CrossRef]
- Brasier, A.R. The NF-kB regulatory network. Cardiovasc. Toxicol. 2006, 6, 111–130. [Google Scholar] [CrossRef]
- Pasparakis, M. Role of NF-κB in epithelial biology. Immunol. Rev. 2012, 246, 346–358. [Google Scholar] [CrossRef]
- Barkett, M.; Gilmore, T.D. Control of apoptosis by Rel/NF- κB transcription factors. Oncogene 1999, 18, 6910–6924. [Google Scholar] [CrossRef] [Green Version]
- Lassila, R.; Jouppila, A. Mast cell-derived heparin proteoglycans as a model for a local antithrombotic. Semin. Thromb. Hemost. 2014, 40, 837–844. [Google Scholar] [CrossRef] [Green Version]
- Sobue, M.; Nakashima, N.; Fukatsu, T.; Nagasaka, T.; Katoh, T.; Ogura, T.; Takeuchi, J. Production and characterization of monoclonal antibody to dermatan sulfate proteoglycan. J. Histochem. Cytochem. 1988, 36, 479–485. [Google Scholar] [CrossRef]
- Triggs-Raine, B.; Natowicz, M.R. Biology of hyaluronan: Insights from genetic disorders of metabolism. World J. Biol. Chem. 2015, 6, 110–120. [Google Scholar] [CrossRef]









| HARE VARIANT | HA UPTAKE (%) | HEP UPTAKE (%) |
|---|---|---|
| WT | 100 | 100 |
| EV | 0.2 | 0 |
| ΔM1 | 51 | 65 |
| ΔM2 | 61 | 135 |
| ΔM3 | 44 | 35 |
| ΔM4 | 119 | 68 |
| ΔM1M2 | 61 | - |
| ΔM1M2M4 | 58 | - |
| ΔM1M2M3M4 | 7 | - |
| WT(Y2519A) | 94 | 95 |
| ΔM1M2M3 + Y2519A | 5 | 0 |
| +M2 or +M4 | 0 | 0-5 |
| +M3 | 58 | 65 |
| +M3(Y2519A) | 5 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weigel, P.H. Systemic Glycosaminoglycan Clearance by HARE/Stabilin-2 Activates Intracellular Signaling. Cells 2020, 9, 2366. https://doi.org/10.3390/cells9112366
Weigel PH. Systemic Glycosaminoglycan Clearance by HARE/Stabilin-2 Activates Intracellular Signaling. Cells. 2020; 9(11):2366. https://doi.org/10.3390/cells9112366
Chicago/Turabian StyleWeigel, Paul H. 2020. "Systemic Glycosaminoglycan Clearance by HARE/Stabilin-2 Activates Intracellular Signaling" Cells 9, no. 11: 2366. https://doi.org/10.3390/cells9112366