Inhibition of Anti-Apoptotic Bcl-2 Proteins in Preclinical and Clinical Studies: Current Overview in Cancer
Abstract
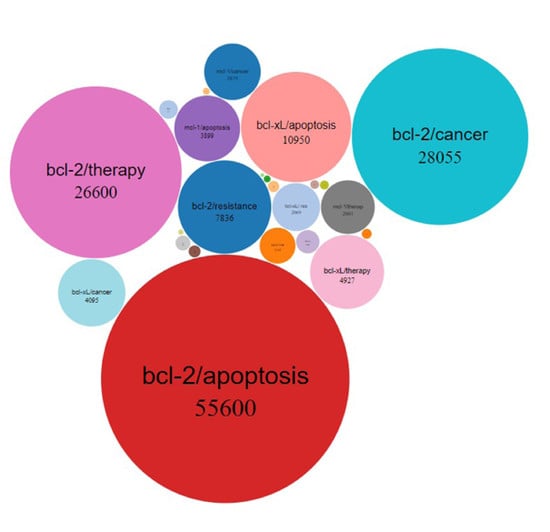
:1. Introduction
2. Relevance of Bcl-2 Anti-Apoptotic Family Proteins in Cancer
2.1. Bcl-2
2.2. Bcl-xL (BCL2-like 1 Gene, BCL2L1)
2.3. Mcl-1 (Myeloid Leukemia Sequence 1)
3. Anti-Apoptotic Bcl-2 Family Protein Inhibitors
3.1. Antisense Oligonucleotides
3.2. BH3 Mimetics
3.2.1. Rationale for the Use of BH3 Mimetics (Priming and Protein Addiction)
3.2.2. Multitarget BH3 Mimetics
3.2.3. Dual BH3 Inhibitors: Bcl-2/Bcl-xL, Bcl-2/Mcl-1, Bcl-xL/Mcl-1
3.2.4. Bcl-2 Specific Inhibition
3.2.5. Bcl-xL Specific Inhibition
3.2.6. Mcl-1 Specific Inhibition
3.3. BH3 Peptides
3.4. Molecules Promoting Protein Conformational Change
3.5. Bcl-2 Quadruplex Selective Approach
4. Vaccination Using Anti-Apoptotic Protein-Derived Peptides
5. Pro-Apoptotic Bcl-2 Family Protein Activations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hotchkiss, R.S.; Strasser, A.; McDunn, J.E.; Swanson, P.E. Cell death. N. Engl. J. Med. 2009, 361, 1570–1583. [Google Scholar] [CrossRef] [Green Version]
- Tait, S.W.; Green, D.R. Mitochondria and cell death: Outer membrane permeabilization and beyond. Nat. Rev. Mol. Cell Biol. 2010, 11, 621–632. [Google Scholar] [CrossRef]
- Yuan, S.; Akey, C.W. Apoptosome structure, assembly, and procaspase activation. Structure 2013, 21, 501–515. [Google Scholar] [CrossRef] [Green Version]
- Luna-Vargas, M.P.; Chipuk, J.E. The deadly landscape of pro-apoptotic BCL-2 proteins in the outer mitochondrial membrane. FEBS J. 2016, 283, 2676–2689. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Saez, A.J. The secrets of the Bcl-2 family. Cell Death Differ. 2012, 19, 1733–1740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabellini, C.; Trisciuoglio, D.; Del Bufalo, D. Non-canonical roles of Bcl-2 and Bcl-xL proteins: Relevance of BH4 domain. Carcinogenesis 2017, 38, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Kale, J.; Osterlund, E.J.; Andrews, D.W. BCL-2 family proteins: Changing partners in the dance towards death. Cell Death Differ. 2018, 25, 65–80. [Google Scholar] [CrossRef] [Green Version]
- Montero, J.; Letai, A. Why do BCL-2 inhibitors work and where should we use them in the clinic? Cell Death Differ. 2018, 25, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Hata, A.N.; Engelman, J.A.; Faber, A.C. The BCL2 Family: Key Mediators of the Apoptotic Response to Targeted Anticancer Therapeutics. Cancer Discov. 2015, 5, 475–487. [Google Scholar] [CrossRef] [Green Version]
- Adams, J.M.; Cory, S. The BCL-2 arbiters of apoptosis and their growing role as cancer targets. Cell Death Differ. 2018, 25, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Yogarajah, M.; Stone, R.M. A concise review of BCL-2 inhibition in acute myeloid leukemia. Expert Rev. Hematol. 2018, 11, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Jullien, M.; Gomez-Bougie, P.; Chiron, D.; Touzeau, C. Restoring Apoptosis with BH3 Mimetics in Mature B-Cell Malignancies. Cells 2020, 9, 717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fletcher, L.; Nabrinsky, E.; Liu, T.; Danilov, A. Cell Death Pathways in Lymphoid Malignancies. Curr. Oncol. Rep. 2020, 22, 10. [Google Scholar] [CrossRef] [PubMed]
- Cassier, P.A.; Castets, M.; Belhabri, A.; Vey, N. Targeting apoptosis in acute myeloid leukaemia. Br. J. Cancer 2017, 117, 1089–1098. [Google Scholar] [CrossRef] [Green Version]
- Vitagliano, O.; Addeo, R.; D’Angelo, V.; Indolfi, C.; Indolfi, P.; Casale, F. The Bcl-2/Bax and Ras/Raf/MEK/ERK signaling pathways: Implications in pediatric leukemia pathogenesis and new prospects for therapeutic approaches. Expert Rev. Hematol. 2013, 6, 587–597. [Google Scholar] [CrossRef]
- Sakamoto, K.M.; Grant, S.; Saleiro, D.; Crispino, J.D.; Hijiya, N.; Giles, F.; Platanias, L.; Eklund, E.A. Targeting novel signaling pathways for resistant acute myeloid leukemia. Mol. Genet. Metab. 2015, 114, 397–402. [Google Scholar] [CrossRef] [Green Version]
- Reed, J.C.; Meister, L.; Tanaka, S.; Cuddy, M.; Yum, S.; Geyer, C.; Pleasure, D. Differential expression of bcl2 protooncogene in neuroblastoma and other human tumor cell lines of neural origin. Cancer Res. 1991, 51, 6529–6538. [Google Scholar]
- Kuwashima, Y.; Uehara, T.; Kishi, K.; Shiromizu, K.; Matsuzawa, M.; Takayama, S. Immunohistochemical characterization of undifferentiated carcinomas of the ovary. J. Cancer Res. Clin. Oncol. 1994, 120, 672–677. [Google Scholar] [CrossRef]
- Monaghan, P.; Robertson, D.; Amos, T.A.; Dyer, M.J.; Mason, D.Y.; Greaves, M.F. Ultrastructural localization of bcl-2 protein. J. Histochem. Cytochem. 1992, 40, 1819–1825. [Google Scholar] [CrossRef] [Green Version]
- Pezzella, F.; Turley, H.; Kuzu, I.; Tungekar, M.F.; Dunnill, M.S.; Pierce, C.B.; Harris, A.; Gatter, K.C.; Mason, D.Y. Bcl-2 Protein in Non-Small-Cell Lung Carcinoma. N. Engl. J. Med. 1993, 329, 690–694. [Google Scholar] [CrossRef]
- Hartman, M.L.; Czyz, M. Anti-apoptotic proteins on guard of melanoma cell survival. Cancer Lett. 2013, 331, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, N.; Schwan, J.V.; Fujita, M.; Norris, D.A.; Shellman, Y.G. Alternative Treatments For Melanoma: Targeting BCL-2 Family Members to De-Bulk and Kill Cancer Stem Cells. J. Investig. Dermatol. 2015, 135, 2155–2161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, M.; Wang, L.; Li, P. Correlation between chemosensitivity to anticancer drugs and Bcl-2 expression in gastric cancer. Int. J. Clin. Exp. Pathol. 2013, 6, 2554–2559. [Google Scholar] [PubMed]
- Yang, X.K.; Zheng, F.; Chen, J.H.; Gao, Q.L.; Lu, Y.P.; Wang, S.X.; Wang, C.Y.; Ma, D. Relationship between expression of apoptosis-associated proteins and caspase-3 activity in cisplatin-resistant human ovarian cancer cell line. Ai Zheng 2002, 21, 1288–1291. [Google Scholar] [PubMed]
- Zhao, Y.; Zhang, C.L.; Zeng, B.F.; Wu, X.S.; Gao, T.T.; Oda, Y. Enhanced chemosensitivity of drug-resistant osteosarcoma cells by lentivirus-mediated Bcl-2 silencing. Biochem. Biophys. Res. Commun. 2009, 390, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Kim, K.O.; Shin, M.J.; Ha, J.H.; Seo, S.W.; Yang, J.; Lee, F.Y. siRNA-based targeting of antiapoptotic genes can reverse chemoresistance in P-glycoprotein expressing chondrosarcoma cells. Mol. Cancer 2009, 8, 28. [Google Scholar] [CrossRef] [Green Version]
- Maji, S.; Panda, S.; Samal, S.K.; Shriwas, O.; Rath, R.; Pellecchia, M.; Emdad, L.; Das, S.K.; Fisher, P.B.; Dash, R. Bcl-2 Antiapoptotic Family Proteins and Chemoresistance in Cancer. Adv. Cancer Res. 2018, 137, 37–75. [Google Scholar]
- Trisciuoglio, D.; Desideri, M.; Ciuffreda, L.; Mottolese, M.; Ribatti, D.; Vacca, A.; Del Rosso, M.; Marcocci, L.; Zupi, G.; Del Bufalo, D. Bcl-2 overexpression in melanoma cells increases tumor progression-associated properties and in vivo tumor growth. J. Cell. Physiol. 2005, 205, 414–421. [Google Scholar] [CrossRef]
- Trisciuoglio, D.; Iervolino, A.; Zupi, G.; Del Bufalo, D. Involvement of PI3K and MAPK signaling in bcl-2-induced vascular endothelial growth factor expression in melanoma cells. Mol. Biol. Cell 2005, 16, 4153–4162. [Google Scholar] [CrossRef] [Green Version]
- Trisciuoglio, D.; Gabellini, C.; Desideri, M.; Ziparo, E.; Zupi, G.; Del Bufalo, D. Bcl-2 regulates HIF-1alpha protein stabilization in hypoxic melanoma cells via the molecular chaperone HSP90. PLoS ONE 2010, 5, e11772. [Google Scholar] [CrossRef]
- Trisciuoglio, D.; Gabellini, C.; Desideri, M.; Ragazzoni, Y.; De Luca, T.; Ziparo, E.; Del Bufalo, D. Involvement of BH4 domain of bcl-2 in the regulation of HIF-1-mediated VEGF expression in hypoxic tumor cells. Cell Death Differ. 2011, 18, 1024–1035. [Google Scholar] [CrossRef] [Green Version]
- Trisciuoglio, D.; De Luca, T.; Desideri, M.; Passeri, D.; Gabellini, C.; Scarpino, S.; Liang, C.; Orlandi, A.; Del Bufalo, D. Removal of the BH4 domain from Bcl-2 protein triggers an autophagic process that impairs tumor growth. Neoplasia 2013, 15, 315–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabellini, C.; De Luca, T.; Trisciuoglio, D.; Desideri, M.; Di Martile, M.; Passeri, D.; Candiloro, A.; Biffoni, M.; Rizzo, M.G.; Orlandi, A.; et al. BH4 domain of bcl-2 protein is required for its proangiogenic function under hypoxic condition. Carcinogenesis 2013, 34, 2558–2567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trisciuoglio, D.; Tupone, M.G.; Desideri, M.; Di Martile, M.; Gabellini, C.; Buglioni, S.; Pallocca, M.; Alessandrini, G.; D’Aguanno, S.; Del Bufalo, D. BCL-XL overexpression promotes tumor progression-associated properties. Cell Death Dis. 2017, 8, 3216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Luca, T.; Pelosi, A.; Trisciuoglio, D.; D’Aguanno, S.; Desideri, M.; Farini, V.; Di Martile, M.; Bellei, B.; Tupone, M.G.; Candiloro, A.; et al. miR-211 and MITF modulation by Bcl-2 protein in melanoma cells. Mol. Carcinog. 2016, 55, 2304–2312. [Google Scholar] [CrossRef]
- D’Aguanno, S.; Valentini, E.; Tupone, M.G.; Desideri, M.; Di Martile, M.; Spagnuolo, M.; Buglioni, S.; Ercolani, C.; Falcone, I.; De Dominici, M.; et al. Semaphorin 5A drives melanoma progression: Role of Bcl-2, miR-204 and c-Myb. J. Exp. Clin. Cancer Res. 2018, 37, 278. [Google Scholar] [CrossRef]
- Tupone, M.G.; D’Aguanno, S.; Di Martile, M.; Valentini, E.; Desideri, M.; Trisciuoglio, D.; Donzelli, S.; Sacconi, A.; Buglioni, S.; Ercolani, C.; et al. microRNA-378a-5p iS a novel positive regulator of melanoma progression. Oncogenesis 2020, 9. [Google Scholar] [CrossRef]
- Di Martile, M.; Farini, V.; Consonni, F.M.; Trisciuoglio, D.; Desideri, M.; Valentini, E.; D’Aguanno, S.; Tupone, M.G.; Buglioni, S.; Ercolani, C.; et al. Melanoma-specific bcl-2 promotes a protumoral M2-like phenotype by tumor-associated macrophages. J. Immunother. Cancer 2020. [Google Scholar] [CrossRef]
- Trisciuoglio, D.; Desideri, M.; Farini, V.; De Luca, T.; Di Martile, M.; Tupone, M.G.; Urbani, A.; D’Aguanno, S.; Del Bufalo, D. Affinity purification-mass spectrometry analysis of bcl-2 interactome identified SLIRP as a novel interacting protein. Cell. Death Dis. 2016, 7, e2090. [Google Scholar] [CrossRef]
- Chong, S.J.F.; Marchi, S.; Petroni, G.; Kroemer, G.; Galluzzi, L.; Pervaiz, S. Noncanonical Cell Fate Regulation by Bcl-2 Proteins. Trends Cell Biol. 2020. [Google Scholar] [CrossRef]
- Oliver, L.; Cordel, S.; Barbieux, I.; LeCabellec, M.T.; Meflah, K.; Gregoire, M.; Vallette, F.M. Resistance to apoptosis is increased during metastatic dissemination of colon cancer. Clin. Exp. Metastasis 2002, 19, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Melucci, E.; Cosimelli, M.; Carpanese, L.; Pizzi, G.; Izzo, F.; Fiore, F.; Golfieri, R.; Giampalma, E.; Sperduti, I.; Ercolani, C.; et al. Italian Society of Locoregional Therapies in Oncology (S.I.T.I.L.O.) Decrease of survivin, p53 and Bcl-2 expression in chemorefractory colorectal liver metastases may be predictive of radiosensivity radiosensivity after radioembolization with yttrium-90 resin microspheres. J. Exp. Clin. Cancer Res. 2013, 32. [Google Scholar] [CrossRef] [Green Version]
- Neri, A.; Marrelli, D.; Roviello, F.; DeMarco, G.; Mariani, F.; DeStefano, A.; Megha, T.; Caruso, S.; Corso, G.; Cioppa, T.; et al. Bcl-2 expression correlates with lymphovascular invasion and long-term prognosis in breast cancer. Breast Cancer Res. Treat. 2006, 99, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, K.; Kimler, B.F.; Davis, M.K.; Fan, F.; Tawfik, O. Prognostic significance of Bcl-2 in invasive mammary carcinomas: A comparative clinicopathologic study between “triple-negative” and non-”triple-negative” tumors. Hum. Pathol. 2012, 43, 23–30. [Google Scholar] [CrossRef]
- Gryko, M.; Pryczynicz, A.; Zareba, K.; Kedra, B.; Kemona, A.; Guzinska-Ustymowicz, K. The expression of Bcl-2 and BID in gastric cancer cells. J. Immunol. Res. 2014, 2014, 953203. [Google Scholar] [CrossRef] [Green Version]
- Groeger, A.M.; Caputi, M.; Esposito, V.; De Luca, A.; Salat, A.; Murabito, M.; Giordano, G.G.; Baldi, F.; Giordano, A.; Wolner, E. Bcl-2 protein expression correlates with nodal status in non small cell lung cancer. Anticancer Res. 1999, 19, 821–824. [Google Scholar]
- Stevens, M.; Oltean, S. Modulation of the Apoptosis Gene Bcl-x Function Through Alternative Splicing. Front. Genet. 2019, 10, 804. [Google Scholar] [CrossRef] [Green Version]
- Warren, C.F.A.; Wong-Brown, M.W.; Bowden, N.A. BCL-2 family isoforms in apoptosis and cancer. Cell. Death Dis. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Giorgini, S.; Trisciuoglio, D.; Gabellini, C.; Desideri, M.; Castellini, L.; Colarossi, C.; Zangemeister-Wittke, U.; Zupi, G.; Del Bufalo, D. Modulation of bcl-xL in tumor cells regulates angiogenesis through CXCL8 expression. Mol. Cancer Res. 2007, 5, 761–771. [Google Scholar] [CrossRef] [Green Version]
- Gabellini, C.; Castellini, L.; Trisciuoglio, D.; Kracht, M.; Zupi, G.; Del Bufalo, D. Involvement of nuclear factor-kappa B in bcl-xL-induced interleukin 8 expression in glioblastoma. J. Neurochem. 2008, 107, 871–882. [Google Scholar] [CrossRef]
- Gabellini, C.; Gomez-Abenza, E.; Ibanez-Molero, S.; Tupone, M.G.; Perez-Oliva, A.B.; de Oliveira, S.; Del Bufalo, D.; Mulero, V. Interleukin 8 mediates bcl-xL-induced enhancement of human melanoma cell dissemination and angiogenesis in a zebrafish xenograft model. Int. J. Cancer 2018, 142, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Chen, Z.; Tang, L.H.; Fang, Y.; Shin, S.J.; Panarelli, N.C.; Chen, Y.T.; Li, Y.; Jiang, X.; Du, Y.N. Bcl-xL promotes metastasis independent of its anti-apoptotic activity. Nat. Commun. 2016, 7, 10384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bessou, M.; Lopez, J.; Gadet, R.; Deygas, M.; Popgeorgiev, N.; Poncet, D.; Nougarede, A.; Billard, P.; Mikaelian, I.; Gonzalo, P.; et al. The apoptosis inhibitor Bcl-xL controls breast cancer cell migration through mitochondria-dependent reactive oxygen species production. Oncogene 2020, 39, 3056–3074. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.; Pushker, N.; Saini, N.; Sen, S.; Sharma, A.; Bakhshi, S.; Chawla, B.; Kashyap, S. Expression of pro-apoptotic Bax and anti-apoptotic Bcl-2 proteins in human retinoblastoma. Clin. Exp. Ophthalmol. 2015, 43, 259–267. [Google Scholar] [CrossRef]
- Zhang, H.; Rosdahl, I. Bcl-xL and bcl-2 proteins in melanoma progression and UVB-induced apoptosis. Int. J. Oncol. 2006, 28, 661–666. [Google Scholar] [CrossRef]
- Keitel, U.; Scheel, A.; Thomale, J.; Halpape, R.; Kaulfuss, S.; Scheel, C.; Dobbelstein, M. Bcl-xL mediates therapeutic resistance of a mesenchymal breast cancer cell subpopulation. Oncotarget 2014, 5, 11778–11791. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.L.; Pang, L.Q.; Wu, Y.; Wang, X.Y.; Wang, C.Q.; Fan, Y. Significance of Bcl-xL in human colon carcinoma. World J. Gastroenterol. 2008, 14, 3069–3073. [Google Scholar] [CrossRef]
- Zhang, K.; Jiao, K.; Xing, Z.; Zhang, L.; Yang, J.; Xie, X.; Yang, L. Bcl-xL overexpression and its association with the progress of tongue carcinoma. Int. J. Clin. Exp. Pathol. 2014, 7, 7360–7377. [Google Scholar]
- Watanabe, J.; Kushihata, F.; Honda, K.; Sugita, A.; Tateishi, N.; Mominoki, K.; Matsuda, S.; Kobayashi, N. Prognostic significance of Bcl-xL in human hepatocellular carcinoma. Surgery 2004, 135, 604–612. [Google Scholar] [CrossRef]
- Kozopas, K.M.; Yang, T.; Buchan, H.L.; Zhou, P.; Craig, R.W. MCL1, a gene expressed in programmed myeloid cell differentiation, has sequence similarity to BCL2. Proc. Natl. Acad. Sci. USA 1993, 90, 3516–3520. [Google Scholar] [CrossRef] [Green Version]
- Beroukhim, R.; Mermel, C.H.; Porter, D.; Wei, G.; Raychaudhuri, S.; Donovan, J.; Barretina, J.; Boehm, J.S.; Dobson, J.; Urashima, M.; et al. The landscape of somatic copy-number alteration across human cancers. Nature 2010, 463, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.J.; Dhayade, S.; Ferrari, N.; Sims, A.H.; Johnson, E.; Mason, S.M.; Dickson, A.; Ryan, K.M.; Kalna, G.; Edwards, J.; et al. MCL-1 is a prognostic indicator and drug target in breast cancer. Cell. Death Dis. 2018, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Young, A.I.; Law, A.M.; Castillo, L.; Chong, S.; Cullen, H.D.; Koehler, M.; Herzog, S.; Brummer, T.; Lee, E.F.; Fairlie, W.D.; et al. MCL-1 inhibition provides a new way to suppress breast cancer metastasis and increase sensitivity to dasatinib. Breast Cancer Res. 2016, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zervantonakis, I.K.; Iavarone, C.; Chen, H.Y.; Selfors, L.M.; Palakurthi, S.; Liu, J.F.; Drapkin, R.; Matulonis, U.; Leverson, J.D.; Sampath, D.; et al. Systems analysis of apoptotic priming in ovarian cancer identifies vulnerabilities and predictors of drug response. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef]
- Reiner, T.; de Las Pozas, A.; Parrondo, R.; Palenzuela, D.; Cayuso, W.; Rai, P.; Perez-Stable, C. Mcl-1 protects prostate cancer cells from cell death mediated by chemotherapy-induced DNA damage. Oncoscience 2015, 2, 703–715. [Google Scholar] [CrossRef]
- Akgul, C. Mcl-1 is a potential therapeutic target in multiple types of cancer. Cell Mol. Life Sci. 2009, 66, 1326–1336. [Google Scholar] [CrossRef]
- He, K.; Chen, D.; Ruan, H.; Li, X.; Tong, J.; Xu, X.; Zhang, L.; Yu, J. BRAFV600E-dependent Mcl-1 stabilization leads to everolimus resistance in colon cancer cells. Oncotarget 2016, 7, 47699–47710. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Zhao, Z.; Wu, K.; Xu, Z.; Liu, K. MCL-1 is the key target of adjuvant chemotherapy to reverse the cisplatin-resistance in NSCLC. Gene 2016, 587, 147–154. [Google Scholar] [CrossRef]
- Awan, F.T.; Kay, N.E.; Davis, M.E.; Wu, W.; Geyer, S.M.; Leung, N.; Jelinek, D.F.; Tschumper, R.C.; Secreto, C.R.; Lin, T.S.; et al. Mcl-1 expression predicts progression-free survival in chronic lymphocytic leukemia patients treated with pentostatin, cyclophosphamide, and rituximab. Blood 2009, 113, 535–537. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.S.; Park, Y.L.; Kim, N.; Oh, H.H.; Son, D.J.; Kim, M.Y.; Oak, C.Y.; Chung, C.Y.; Park, H.C.; Kim, J.S.; et al. Myeloid cell leukemia-1 regulates the cell growth and predicts prognosis in gastric cancer. Int. J. Oncol. 2015, 46, 2154–2162. [Google Scholar] [CrossRef]
- Bashari, M.H.; Fan, F.; Vallet, S.; Sattler, M.; Arn, M.; Luckner-Minden, C.; Schulze-Bergkamen, H.; Zornig, I.; Marme, F.; Schneeweiss, A.; et al. Mcl-1 confers protection of Her2-positive breast cancer cells to hypoxia: Therapeutic implications. Breast Cancer Res. 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, Q.; Zhan, Y.; Zheng, H.; Zang, H.; Luo, J.; Zhang, Y.; Wang, W.; Feng, J.; Lu, J.; Chen, L.; et al. Elevated expression of mcl-1 inhibits apoptosis and predicts poor prognosis in patients with surgically resected non-small cell lung cancer. Diagn. Pathol. 2019, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Bufalo, D.; Rizzo, A.; Trisciuoglio, D.; Cardinali, G.; Torrisi, M.R.; Zangemeister-Wittke, U.; Zupi, G.; Biroccio, A. Involvement of hTERT in apoptosis induced by interference with Bcl-2 expression and function. Cell Death Differ. 2005, 12, 1429–1438. [Google Scholar] [CrossRef] [PubMed]
- Zangemeister-Wittke, U.; Leech, S.H.; Olie, R.A.; Simoes-Wust, A.P.; Gautschi, O.; Luedke, G.H.; Natt, F.; Haner, R.; Martin, P.; Hall, J.; et al. A novel bispecific antisense oligonucleotide inhibiting both bcl-2 and bcl-xL expression efficiently induces apoptosis in tumor cells. Clin. Cancer Res. 2000, 6, 2547–2555. [Google Scholar] [PubMed]
- Olie, R.A.; Hafner, C.; Kuttel, R.; Sigrist, B.; Willers, J.; Dummer, R.; Hall, J.; Stahel, R.A.; Zangemeister-Wittke, U. Bcl-2 and bcl-xL antisense oligonucleotides induce apoptosis in melanoma cells of different clinical stages. J. Investig. Dermatol. 2002, 118, 505–512. [Google Scholar] [CrossRef] [Green Version]
- Bedikian, A.Y.; Garbe, C.; Conry, R.; Lebbe, C.; Grob, J.J. Genasense Melanoma Study Group Dacarbazine with or without oblimersen (a Bcl-2 antisense oligonucleotide) in chemotherapy-naive patients with advanced melanoma and low-normal serum lactate dehydrogenase: ‘The AGENDA trial’. Melanoma Res. 2014, 24, 237–243. [Google Scholar] [CrossRef]
- Raab, R.; Sparano, J.A.; Ocean, A.J.; Christos, P.; Ramirez, M.; Vinciguerra, V.; Kaubisch, A. A phase I trial of oblimersen sodium in combination with cisplatin and 5-fluorouracil in patients with advanced esophageal, gastroesophageal junction, and gastric carcinoma. Am. J. Clin. Oncol. 2010, 33, 61–65. [Google Scholar] [CrossRef]
- Galatin, P.S.; Advani, R.H.; Fisher, G.A.; Francisco, B.; Julian, T.; Losa, R.; Sierra, M.I.; Sikic, B.I. Phase I trial of oblimersen (Genasense(R)) and gemcitabine in refractory and advanced malignancies. Investig. New Drugs 2011, 29, 971–977. [Google Scholar] [CrossRef]
- Ott, P.A.; Chang, J.; Madden, K.; Kannan, R.; Muren, C.; Escano, C.; Cheng, X.; Shao, Y.; Mendoza, S.; Gandhi, A.; et al. Oblimersen in combination with temozolomide and albumin-bound paclitaxel in patients with advanced melanoma: A phase I trial. Cancer Chemother. Pharmacol. 2013, 71, 183–191. [Google Scholar] [CrossRef]
- Leverson, J.D.; Sampath, D.; Souers, A.J.; Rosenberg, S.H.; Fairbrother, W.J.; Amiot, M.; Konopleva, M.; Letai, A. Found in Translation: How Preclinical Research Is Guiding the Clinical Development of the BCL2-Selective Inhibitor Venetoclax. Cancer Discov. 2017, 7, 1376–1393. [Google Scholar] [CrossRef] [Green Version]
- Delbridge, A.R.; Grabow, S.; Strasser, A.; Vaux, D.L. Thirty years of BCL-2: Translating cell death discoveries into novel cancer therapies. Nat. Rev. Cancer 2016, 16, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Merino, D.; Kelly, G.L.; Lessene, G.; Wei, A.H.; Roberts, A.W.; Strasser, A. BH3-Mimetic Drugs: Blazing the Trail for New Cancer Medicines. Cancer Cell 2018, 34, 879–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, A.W.; Seymour, J.F.; Brown, J.R.; Wierda, W.G.; Kipps, T.J.; Khaw, S.L.; Carney, D.A.; He, S.Z.; Huang, D.C.; Xiong, H.; et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: Results of a phase I study of navitoclax in patients with relapsed or refractory disease. J. Clin. Oncol. 2012, 30, 488–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue-Yamauchi, A.; Jeng, P.S.; Kim, K.; Chen, H.C.; Han, S.; Ganesan, Y.T.; Ishizawa, K.; Jebiwott, S.; Dong, Y.; Pietanza, M.C.; et al. Targeting the differential addiction to anti-apoptotic BCL-2 family for cancer therapy. Nat. Commun. 2017, 8, 16078. [Google Scholar] [CrossRef] [PubMed]
- Aubrey, B.J.; Kelly, G.L.; Kueh, A.J.; Brennan, M.S.; O’Connor, L.; Milla, L.; Wilcox, S.; Tai, L.; Strasser, A.; Herold, M.J. An inducible lentiviral guide RNA platform enables the identification of tumor-essential genes and tumor-promoting mutations in vivo. Cell. Rep. 2015, 10, 1422–1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotschy, A.; Szlavik, Z.; Murray, J.; Davidson, J.; Maragno, A.L.; Le Toumelin-Braizat, G.; Chanrion, M.; Kelly, G.L.; Gong, J.N.; Moujalled, D.M.; et al. The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature 2016, 538, 477–482. [Google Scholar] [CrossRef]
- Zhai, D.; Jin, C.; Satterthwait, A.C.; Reed, J.C. Comparison of chemical inhibitors of antiapoptotic Bcl-2-family proteins. Cell Death Differ. 2006, 13, 1419–1421. [Google Scholar] [CrossRef] [Green Version]
- Paulus, A.; Chitta, K.; Akhtar, S.; Personett, D.; Miller, K.C.; Thompson, K.J.; Carr, J.; Kumar, S.; Roy, V.; Ansell, S.M.; et al. AT-101 downregulates BCL2 and MCL1 and potentiates the cytotoxic effects of lenalidomide and dexamethasone in preclinical models of multiple myeloma and Waldenstrom macroglobulinaemia. Br. J. Haematol. 2014, 164, 352–365. [Google Scholar] [CrossRef]
- Oliver, C.L.; Miranda, M.B.; Shangary, S.; Land, S.; Wang, S.; Johnson, D.E. (-)-Gossypol acts directly on the mitochondria to overcome Bcl-2- and Bcl-X(L)-mediated apoptosis resistance. Mol. Cancer Ther. 2005, 4, 23–31. [Google Scholar]
- Nguyen, M.; Marcellus, R.C.; Roulston, A.; Watson, M.; Serfass, L.; Murthy Madiraju, S.R.; Goulet, D.; Viallet, J.; Belec, L.; Billot, X.; et al. Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc. Natl. Acad. Sci. USA 2007, 104, 19512–19517. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Yague, E.; Zhao, J.; Wang, L.; Bai, J.; Yang, Q.; Pan, T.; Zhao, H.; Liu, J.; Zhang, J. Sabutoclax, pan-active BCL-2 protein family antagonist, overcomes drug resistance and eliminates cancer stem cells in breast cancer. Cancer Lett. 2018, 423, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Quinn, B.A.; Dash, R.; Sarkar, S.; Azab, B.; Bhoopathi, P.; Das, S.K.; Emdad, L.; Wei, J.; Pellecchia, M.; Sarkar, D.; et al. Pancreatic Cancer Combination Therapy Using a BH3 Mimetic and a Synthetic Tetracycline. Cancer Res. 2015, 75, 2305–2315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, R.S., II; Placzek, W.; Fernandez, A.; Ziaee, S.; Chu, C.Y.; Wei, J.; Stebbins, J.; Kitada, S.; Fritz, G.; Reed, J.C.; et al. Sabutoclax, a Mcl-1 antagonist, inhibits tumorigenesis in transgenic mouse and human xenograft models of prostate cancer. Neoplasia 2012, 14, 656–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oltersdorf, T.; Elmore, S.W.; Shoemaker, A.R.; Armstrong, R.C.; Augeri, D.J.; Belli, B.A.; Bruncko, M.; Deckwerth, T.L.; Dinges, J.; Hajduk, P.J.; et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 2005, 435, 677–681. [Google Scholar] [CrossRef]
- Del Gaizo Moore, V.; Brown, J.R.; Certo, M.; Love, T.M.; Novina, C.D.; Letai, A. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J. Clin. Investig. 2007, 117, 112–121. [Google Scholar] [CrossRef] [Green Version]
- Sakakibara-Konishi, J.; Ikezawa, Y.; Oizumi, S.; Kikuchi, J.; Kikuchi, E.; Mizugaki, H.; Kinoshita, I.; Dosaka-Akita, H.; Nishimura, M. Combined antitumor effect of gamma-secretase inhibitor and ABT-737 in Notch-expressing non-small cell lung cancer. Int. J. Clin. Oncol. 2017, 22, 257–268. [Google Scholar] [CrossRef]
- Yu, X.; Dobrikov, M.; Keir, S.T.; Gromeier, M.; Pastan, I.H.; Reisfeld, R.; Bigner, D.D.; Chandramohan, V. Synergistic antitumor effects of 9.2.27-PE38KDEL and ABT-737 in primary and metastatic brain tumors. PLoS ONE 2019, 14, e0210608. [Google Scholar] [CrossRef]
- Serasinghe, M.N.; Missert, D.J.; Asciolla, J.J.; Podgrabinska, S.; Wieder, S.Y.; Izadmehr, S.; Belbin, G.; Skobe, M.; Chipuk, J.E. Anti-apoptotic BCL-2 proteins govern cellular outcome following B-RAF(V600E) inhibition and can be targeted to reduce resistance. Oncogene 2015, 34, 857–867. [Google Scholar] [CrossRef] [Green Version]
- Suryani, S.; Carol, H.; Chonghaile, T.N.; Frismantas, V.; Sarmah, C.; High, L.; Bornhauser, B.; Cowley, M.J.; Szymanska, B.; Evans, K.; et al. Cell and molecular determinants of in vivo efficacy of the BH3 mimetic ABT-263 against pediatric acute lymphoblastic leukemia xenografts. Clin. Cancer Res. 2014, 20, 4520–4531. [Google Scholar] [CrossRef] [Green Version]
- Faber, A.C.; Farago, A.F.; Costa, C.; Dastur, A.; Gomez-Caraballo, M.; Robbins, R.; Wagner, B.L.; Rideout, W.M., III; Jakubik, C.T.; Ham, J.; et al. Assessment of ABT-263 activity across a cancer cell line collection leads to a potent combination therapy for small-cell lung cancer. Proc. Natl. Acad. Sci. USA 2015, 112, 1288–1296. [Google Scholar] [CrossRef] [Green Version]
- Schulze, A.B.; Evers, G.; Kerkhoff, A.; Mohr, M.; Schliemann, C.; Berdel, W.E.; Schmidt, L.H. Future Options of Molecular-Targeted Therapy in Small Cell Lung Cancer. Cancers (Basel) 2019, 11, 690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.J.; Tsang, T.; Anderson, G.R.; Posimo, J.M.; Brady, D.C. Inhibition of BCL2 Family Members Increases the Efficacy of Copper Chelation in BRAF(V600E)-Driven Melanoma. Cancer Res. 2020, 80, 1387–1400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoenwaelder, S.M.; Jarman, K.E.; Gardiner, E.E.; Hua, M.; Qiao, J.; White, M.J.; Josefsson, E.C.; Alwis, I.; Ono, A.; Willcox, A.; et al. Bcl-xL-inhibitory BH3 mimetics can induce a transient thrombocytopathy that undermines the hemostatic function of platelets. Blood 2011, 118, 1663–1674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, S.; Zhang, X.; Lv, D.; Zhang, Q.; He, Y.; Zhang, P.; Liu, X.; Thummuri, D.; Yuan, Y.; Wiegand, J.S.; et al. A selective BCL-XL PROTAC degrader achieves safe and potent antitumor activity. Nat. Med. 2019, 25, 1938–1947. [Google Scholar] [CrossRef]
- Wilson, W.H.; O’Connor, O.A.; Czuczman, M.S.; LaCasce, A.S.; Gerecitano, J.F.; Leonard, J.P.; Tulpule, A.; Dunleavy, K.; Xiong, H.; Chiu, Y.L.; et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: A phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol. 2010, 11, 1149–1159. [Google Scholar] [CrossRef] [Green Version]
- Inao, T.; Iida, Y.; Moritani, T.; Okimoto, T.; Tanino, R.; Kotani, H.; Harada, M. Bcl-2 inhibition sensitizes triple-negative human breast cancer cells to doxorubicin. Oncotarget 2018, 9, 25545–25556. [Google Scholar] [CrossRef] [Green Version]
- Wali, V.B.; Langdon, C.G.; Held, M.A.; Platt, J.T.; Patwardhan, G.A.; Safonov, A.; Aktas, B.; Pusztai, L.; Stern, D.F.; Hatzis, C. Systematic Drug Screening Identifies Tractable Targeted Combination Therapies in Triple-Negative Breast Cancer. Cancer Res. 2017, 77, 566–578. [Google Scholar] [CrossRef] [Green Version]
- Fleury, H.; Malaquin, N.; Tu, V.; Gilbert, S.; Martinez, A.; Olivier, M.A.; Sauriol, A.; Communal, L.; Leclerc-Desaulniers, K.; Carmona, E.; et al. Exploiting interconnected synthetic lethal interactions between PARP inhibition and cancer cell reversible senescence. Nat. Commun. 2019, 10, 2556. [Google Scholar] [CrossRef] [Green Version]
- Faber, A.C.; Coffee, E.M.; Costa, C.; Dastur, A.; Ebi, H.; Hata, A.N.; Yeo, A.T.; Edelman, E.J.; Song, Y.; Tam, A.T.; et al. mTOR inhibition specifically sensitizes colorectal cancers with KRAS or BRAF mutations to BCL-2/BCL-XL inhibition by suppressing MCL-1. Cancer. Discov. 2014, 4, 42–52. [Google Scholar] [CrossRef] [Green Version]
- Bai, L.; Chen, J.; McEachern, D.; Liu, L.; Zhou, H.; Aguilar, A.; Wang, S. BM-1197: A novel and specific Bcl-2/Bcl-xL inhibitor inducing complete and long-lasting tumor regression in vivo. PLoS ONE 2014, 9, e99404. [Google Scholar] [CrossRef]
- Ye, L.; Yuan, G.; Xu, F.; Sun, Y.; Chen, Z.; Chen, M.; Li, T.; Sun, P.; Li, S.; Sun, J. The small-molecule compound BM-1197 inhibits the antiapoptotic regulators Bcl-2/Bcl-xL and triggers apoptotic cell death in human colorectal cancer cells. Tumour Biol. 2015, 36, 3447–3455. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.L.; Jiang, W.Q.; Luo, Q.Y.; Yang, D.J.; Cai, Y.C.; Huang, H.Q.; Sun, J. A novel Bcl-2 inhibitor, BM-1197, induces apoptosis in malignant lymphoma cells through the endogenous apoptotic pathway. BMC Cancer 2019, 20. [Google Scholar] [CrossRef] [Green Version]
- Loriot, Y.; Mordant, P.; Dugue, D.; Geneste, O.; Gombos, A.; Opolon, P.; Guegan, J.; Perfettini, J.L.; Pierre, A.; Berthier, L.K.; et al. Radiosensitization by a novel Bcl-2 and Bcl-XL inhibitor S44563 in small-cell lung cancer. Cell Death Dis. 2014, 5, e1423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemati, F.; de Montrion, C.; Lang, G.; Kraus-Berthier, L.; Carita, G.; Sastre-Garau, X.; Berniard, A.; Vallerand, D.; Geneste, O.; de Plater, L.; et al. Targeting Bcl-2/Bcl-XL induces antitumor activity in uveal melanoma patient-derived xenografts. PLoS ONE 2014, 9, e80836. [Google Scholar] [CrossRef]
- Wang, J.; Yang, D.; Luo, Q.; Qiu, M.; Zhang, L.; Li, B.; Chen, H.; Yi, H.; Yan, X.; Li, S.; et al. APG-1252-12A induces mitochondria-dependent apoptosis through inhibiting the antiapoptotic proteins Bcl-2/Bcl-xl in HL-60 cells. Int. J. Oncol. 2017, 51, 563–572. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Aguilar, A.; Chen, J.; Bai, L.; Liu, L.; Meagher, J.L.; Yang, C.Y.; McEachern, D.; Cong, X.; Stuckey, J.A.; et al. Structure-based design of potent Bcl-2/Bcl-xL inhibitors with strong in vivo antitumor activity. J. Med. Chem. 2012, 55, 6149–6161. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Wang, Z.; Guo, Z.; Zhang, X.; Song, T.; Guo, Y.; Ji, T.; Zhang, Z. Structure-based design, synthesis, and evaluation of Bcl-2/Mcl-1 dual inhibitors. Arch. Pharm. (Weinheim) 2020, 353, e2000005. [Google Scholar] [CrossRef]
- Abou Samra, A.; Robert, A.; Gov, C.; Favre, L.; Eloy, L.; Jacquet, E.; Bignon, J.; Wiels, J.; Desrat, S.; Roussi, F. Dual inhibitors of the pro-survival proteins Bcl-2 and Mcl-1 derived from natural compound meiogynin A. Eur. J. Med. Chem. 2018, 148, 26–38. [Google Scholar] [CrossRef]
- Desrat, S.; Pujals, A.; Colas, C.; Dardenne, J.; Geny, C.; Favre, L.; Dumontet, V.; Iorga, B.I.; Litaudon, M.; Raphael, M.; et al. Pro-apoptotic meiogynin A derivatives that target Bcl-xL and Mcl-1. Bioorg. Med. Chem. Lett. 2014, 24, 5086–5088. [Google Scholar] [CrossRef]
- Wang, Z.; He, N.; Guo, Z.; Niu, C.; Song, T.; Guo, Y.; Cao, K.; Wang, A.; Zhu, J.; Zhang, X.; et al. Proteolysis Targeting Chimeras for the Selective Degradation of Mcl-1/Bcl-2 Derived from Nonselective Target Binding Ligands. J. Med. Chem. 2019, 62, 8152–8163. [Google Scholar] [CrossRef]
- Lee, E.F.; Harris, T.J.; Tran, S.; Evangelista, M.; Arulananda, S.; John, T.; Ramnac, C.; Hobbs, C.; Zhu, H.; Gunasingh, G.; et al. BCL-XL and MCL-1 are the key BCL-2 family proteins in melanoma cell survival. Cell Death Dis. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Souers, A.J.; Leverson, J.D.; Boghaert, E.R.; Ackler, S.L.; Catron, N.D.; Chen, J.; Dayton, B.D.; Ding, H.; Enschede, S.H.; Fairbrother, W.J.; et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 2013, 19, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.W.; Davids, M.S.; Pagel, J.M.; Kahl, B.S.; Puvvada, S.D.; Gerecitano, J.F.; Kipps, T.J.; Anderson, M.A.; Brown, J.R.; Gressick, L.; et al. Targeting BCL2 with Venetoclax in Relapsed Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2016, 374, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Lachowiez, C.; DiNardo, C.D.; Konopleva, M. Venetoclax in acute myeloid leukemia-current and future directions. Leuk. Lymphoma 2020. [Google Scholar] [CrossRef] [PubMed]
- Seymour, J.F.; Kipps, T.J.; Eichhorst, B.; Hillmen, P.; D’Rozario, J.; Assouline, S.; Owen, C.; Gerecitano, J.; Robak, T.; De la Serna, J.; et al. Venetoclax-Rituximab in Relapsed or Refractory Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2018, 378, 1107–1120. [Google Scholar] [CrossRef] [PubMed]
- Flinn, I.W.; Gribben, J.G.; Dyer, M.J.S.; Wierda, W.; Maris, M.B.; Furman, R.R.; Hillmen, P.; Rogers, K.A.; Iyer, S.P.; Quillet-Mary, A.; et al. Phase 1b study of venetoclax-obinutuzumab in previously untreated and relapsed/refractory chronic lymphocytic leukemia. Blood 2019, 133, 2765–2775. [Google Scholar] [CrossRef] [Green Version]
- Jain, N.; Keating, M.; Thompson, P.; Ferrajoli, A.; Burger, J.; Borthakur, G.; Takahashi, K.; Estrov, Z.; Fowler, N.; Kadia, T.; et al. Ibrutinib and Venetoclax for First-Line Treatment of CLL. N. Engl. J. Med. 2019, 380, 2095–2103. [Google Scholar] [CrossRef]
- Kumar, S.; Kaufman, J.L.; Gasparetto, C.; Mikhael, J.; Vij, R.; Pegourie, B.; Benboubker, L.; Facon, T.; Amiot, M.; Moreau, P.; et al. Efficacy of venetoclax as targeted therapy for relapsed/refractory t(11;14) multiple myeloma. Blood 2017, 130, 2401–2409. [Google Scholar] [CrossRef] [Green Version]
- Moreau, P.; Chanan-Khan, A.; Roberts, A.W.; Agarwal, A.B.; Facon, T.; Kumar, S.; Touzeau, C.; Punnoose, E.A.; Cordero, J.; Munasinghe, W.; et al. Promising efficacy and acceptable safety of venetoclax plus bortezomib and dexamethasone in relapsed/refractory MM. Blood 2017, 130, 2392–2400. [Google Scholar] [CrossRef] [Green Version]
- Konopleva, M.; Pollyea, D.A.; Potluri, J.; Chyla, B.; Hogdal, L.; Busman, T.; McKeegan, E.; Salem, A.H.; Zhu, M.; Ricker, J.L.; et al. Efficacy and Biological Correlates of Response in a Phase II Study of Venetoclax Monotherapy in Patients with Acute Myelogenous Leukemia. Cancer. Discov. 2016, 6, 1106–1117. [Google Scholar] [CrossRef] [Green Version]
- Chan, S.M.; Thomas, D.; Corces-Zimmerman, M.R.; Xavy, S.; Rastogi, S.; Hong, W.J.; Zhao, F.; Medeiros, B.C.; Tyvoll, D.A.; Majeti, R. Isocitrate dehydrogenase 1 and 2 mutations induce BCL-2 dependence in acute myeloid leukemia. Nat. Med. 2015, 21, 178–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birkinshaw, R.W.; Gong, J.N.; Luo, C.S.; Lio, D.; White, C.A.; Anderson, M.A.; Blombery, P.; Lessene, G.; Majewski, I.J.; Thijssen, R.; et al. Structures of BCL-2 in complex with venetoclax reveal the molecular basis of resistance mutations. Nat. Commun. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Lok, S.W.; Whittle, J.R.; Vaillant, F.; Teh, C.E.; Lo, L.L.; Policheni, A.N.; Bergin, A.R.T.; Desai, J.; Ftouni, S.; Gandolfo, L.C.; et al. A Phase Ib Dose-Escalation and Expansion Study of the BCL2 Inhibitor Venetoclax Combined with Tamoxifen in ER and BCL2-Positive Metastatic Breast Cancer. Cancer Discov. 2019, 9, 354–369. [Google Scholar] [CrossRef] [PubMed]
- Casara, P.; Davidson, J.; Claperon, A.; Le Toumelin-Braizat, G.; Vogler, M.; Bruno, A.; Chanrion, M.; Lysiak-Auvity, G.; Le Diguarher, T.; Starck, J.B.; et al. S55746 is a novel orally active BCL-2 selective and potent inhibitor that impairs hematological tumor growth. Oncotarget 2018, 9, 20075–20088. [Google Scholar] [CrossRef] [Green Version]
- Karpel-Massler, G.; Ishida, C.T.; Bianchetti, E.; Shu, C.; Perez-Lorenzo, R.; Horst, B.; Banu, M.; Roth, K.A.; Bruce, J.N.; Canoll, P.; et al. Inhibition of Mitochondrial Matrix Chaperones and Antiapoptotic Bcl-2 Family Proteins Empower Antitumor Therapeutic Responses. Cancer Res. 2017, 77, 3513–3526. [Google Scholar] [CrossRef] [Green Version]
- Whittle, J.R.; Vaillant, F.; Surgenor, E.; Policheni, A.N.; Giner, G.; Capaldo, B.D.; Chen, H.R.; Liu, H.K.; Dekkers, J.F.; Sachs, N.; et al. Dual targeting of CDK4/6 and BCL2 pathways augments tumor response in estrogen receptor positive breast cancer. Clin. Cancer Res. 2020. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Krump, N.A.; Herlyn, M.; You, J. Combining DNA Damage Induction with BCL-2 Inhibition to Enhance Merkel Cell Carcinoma Cytotoxicity. Biology (Basel) 2020, 9, 35. [Google Scholar] [CrossRef] [Green Version]
- Tahir, S.K.; Smith, M.L.; Hessler, P.; Rapp, L.R.; Idler, K.B.; Park, C.H.; Leverson, J.D.; Lam, L.T. Potential mechanisms of resistance to venetoclax and strategies to circumvent it. BMC Cancer 2017, 17. [Google Scholar] [CrossRef]
- Leverson, J.D.; Phillips, D.C.; Mitten, M.J.; Boghaert, E.R.; Diaz, D.; Tahir, S.K.; Belmont, L.D.; Nimmer, P.; Xiao, Y.; Ma, X.M.; et al. Exploiting selective BCL-2 family inhibitors to dissect cell survival dependencies and define improved strategies for cancer therapy. Sci. Transl. Med. 2015, 7. [Google Scholar] [CrossRef]
- Bose, P.; Gandhi, V.; Konopleva, M. Pathways and mechanisms of venetoclax resistance. Leuk. Lymphoma 2017, 58, 1–17. [Google Scholar] [CrossRef]
- Lessene, G.; Czabotar, P.E.; Sleebs, B.E.; Zobel, K.; Lowes, K.N.; Adams, J.M.; Baell, J.B.; Colman, P.M.; Deshayes, K.; Fairbrother, W.J.; et al. Structure-guided design of a selective BCL-X(L) inhibitor. Nat. Chem. Biol. 2013, 9, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Baranski, Z.; de Jong, Y.; Ilkova, T.; Peterse, E.F.; Cleton-Jansen, A.M.; van de Water, B.; Hogendoorn, P.C.; Bovee, J.V.; Danen, E.H. Pharmacological inhibition of Bcl-xL sensitizes osteosarcoma to doxorubicin. Oncotarget 2015, 6, 36113–36125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, Z.F.; Hasvold, L.; Wang, L.; Wang, X.; Petros, A.M.; Park, C.H.; Boghaert, E.R.; Catron, N.D.; Chen, J.; Colman, P.M.; et al. Discovery of a Potent and Selective BCL-XL Inhibitor with in Vivo Activity. ACS Med. Chem. Lett. 2014, 5, 1088–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faqar-Uz-Zaman, S.F.; Heinicke, U.; Meister, M.T.; Vogler, M.; Fulda, S. BCL-xL-selective BH3 mimetic sensitizes rhabdomyosarcoma cells to chemotherapeutics by activation of the mitochondrial pathway of apoptosis. Cancer Lett. 2018, 412, 131–142. [Google Scholar] [CrossRef]
- Wei, A.H.; Roberts, A.W.; Spencer, A.; Rosenberg, A.S.; Siegel, D.; Walter, R.B.; Caenepeel, S.; Hughes, P.; McIver, Z.; Mezzi, K.; et al. Targeting MCL-1 in hematologic malignancies: Rationale and progress. Blood Rev. 2020. [Google Scholar] [CrossRef]
- Abulwerdi, F.; Liao, C.; Liu, M.; Azmi, A.S.; Aboukameel, A.; Mady, A.S.; Gulappa, T.; Cierpicki, T.; Owens, S.; Zhang, T.; et al. A novel small-molecule inhibitor of mcl-1 blocks pancreatic cancer growth in vitro and in vivo. Mol. Cancer Ther. 2014, 13, 565–575. [Google Scholar] [CrossRef] [Green Version]
- Leverson, J.D.; Zhang, H.; Chen, J.; Tahir, S.K.; Phillips, D.C.; Xue, J.; Nimmer, P.; Jin, S.; Smith, M.; Xiao, Y.; et al. Potent and selective small-molecule MCL-1 inhibitors demonstrate on-target cancer cell killing activity as single agents and in combination with ABT-263 (navitoclax). Cell Death Dis. 2015, 6, e1590. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Nimmer, P.; Sheppard, G.S.; Bruncko, M.; Hessler, P.; Lu, X.; Roberts-Rapp, L.; Pappano, W.N.; Elmore, S.W.; Souers, A.J.; et al. MCL-1 Is a Key Determinant of Breast Cancer Cell Survival: Validation of MCL-1 Dependency Utilizing a Highly Selective Small Molecule Inhibitor. Mol. Cancer Ther. 2015, 14, 1837–1847. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.; Bian, Z.; Zhao, B.; Hogdal, L.J.; Sensintaffar, J.L.; Goodwin, C.M.; Belmar, J.; Shaw, S.; Tarr, J.C.; Veerasamy, N.; et al. Discovery and biological characterization of potent myeloid cell leukemia-1 inhibitors. FEBS Lett. 2017, 591, 240–251. [Google Scholar] [CrossRef] [Green Version]
- Nangia, V.; Siddiqui, F.M.; Caenepeel, S.; Timonina, D.; Bilton, S.J.; Phan, N.; Gomez-Caraballo, M.; Archibald, H.L.; Li, C.; Fraser, C.; et al. Exploiting MCL1 Dependency with Combination MEK + MCL1 Inhibitors Leads to Induction of Apoptosis and Tumor Regression in KRAS-Mutant Non-Small Cell Lung Cancer. Cancer Discov. 2018, 8, 1598–1613. [Google Scholar] [CrossRef] [Green Version]
- Weeden, C.E.; Ah-Cann, C.; Holik, A.Z.; Pasquet, J.; Garnier, J.M.; Merino, D.; Lessene, G.; Asselin-Labat, M.L. Dual inhibition of BCL-XL and MCL-1 is required to induce tumour regression in lung squamous cell carcinomas sensitive to FGFR inhibition. Oncogene 2018, 37, 4475–4488. [Google Scholar] [CrossRef] [PubMed]
- Karpel-Massler, G.; Ishida, C.T.; Zhang, Y.; Halatsch, M.E.; Westhoff, M.A.; Siegelin, M.D. Targeting intrinsic apoptosis and other forms of cell death by BH3-mimetics in glioblastoma. Expert Opin. Drug Discov. 2017, 12, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Merino, D.; Whittle, J.R.; Vaillant, F.; Serrano, A.; Gong, J.N.; Giner, G.; Maragno, A.L.; Chanrion, M.; Schneider, E.; Pal, B.; et al. Synergistic action of the MCL-1 inhibitor S63845 with current therapies in preclinical models of triple-negative and HER2-amplified breast cancer. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdul Rahman, S.F.; Muniandy, K.; Soo, Y.K.; Tiew, E.Y.H.; Tan, K.X.; Bates, T.E.; Mohana-Kumaran, N. Co-inhibition of BCL-XL and MCL-1 with selective BCL-2 family inhibitors enhances cytotoxicity of cervical cancer cell lines. Biochem. Biophys. Rep. 2020, 22, 100756. [Google Scholar] [CrossRef]
- Kehr, S.; Haydn, T.; Bierbrauer, A.; Irmer, B.; Vogler, M.; Fulda, S. Targeting BCL-2 proteins in pediatric cancer: Dual inhibition of BCL-XL and MCL-1 leads to rapid induction of intrinsic apoptosis. Cancer Lett. 2020, 482, 19–32. [Google Scholar] [CrossRef]
- Tseng, H.Y.; Dreyer, J.; Emran, A.A.; Gunatilake, D.; Pirozyan, M.; Cullinane, C.; Dutton-Regester, K.; Rizos, H.; Hayward, N.K.; McArthur, G.; et al. Co-targeting bromodomain and extra-terminal proteins and MCL1 induces synergistic cell death in melanoma. Int. J. Cancer 2020. [Google Scholar] [CrossRef]
- Thomas, R.L.; Roberts, D.J.; Kubli, D.A.; Lee, Y.; Quinsay, M.N.; Owens, J.B.; Fischer, K.M.; Sussman, M.A.; Miyamoto, S.; Gustafsson, A.B. Loss of MCL-1 leads to impaired autophagy and rapid development of heart failure. Genes Dev. 2013, 27, 1365–1377. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Bathina, M.; Lynch, J.; Koss, B.; Calabrese, C.; Frase, S.; Schuetz, J.D.; Rehg, J.E.; Opferman, J.T. Deletion of MCL-1 causes lethal cardiac failure and mitochondrial dysfunction. Genes Dev. 2013, 27, 1351–1364. [Google Scholar] [CrossRef] [Green Version]
- Ramsey, H.E.; Fischer, M.A.; Lee, T.; Gorska, A.E.; Arrate, M.P.; Fuller, L.; Boyd, K.L.; Strickland, S.A.; Sensintaffar, J.; Hogdal, L.J.; et al. A Novel MCL1 Inhibitor Combined with Venetoclax Rescues Venetoclax-Resistant Acute Myelogenous Leukemia. Cancer Discov. 2018, 8, 1566–1581. [Google Scholar] [CrossRef] [Green Version]
- Williams, M.M.; Elion, D.L.; Rahman, B.; Hicks, D.J.; Sanchez, V.; Cook, R.S. Therapeutic inhibition of Mcl-1 blocks cell survival in estrogen receptor-positive breast cancers. Oncotarget 2019, 10, 5389–5402. [Google Scholar] [CrossRef] [Green Version]
- Cidado, J.; Boiko, S.; Proia, T.; Ferguson, D.; Criscione, S.W.; San Martin, M.; Pop-Damkov, P.; Su, N.; Roamio Franklin, V.N.; Sekhar Reddy Chilamakuri, C.; et al. AZD4573 Is a Highly Selective CDK9 Inhibitor That Suppresses MCL-1 and Induces Apoptosis in Hematologic Cancer Cells. Clin. Cancer Res. 2020, 26, 922–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berger, S.; Procko, E.; Margineantu, D.; Lee, E.F.; Shen, B.W.; Zelter, A.; Silva, D.A.; Chawla, K.; Herold, M.J.; Garnier, J.M.; et al. Computationally designed high specificity inhibitors delineate the roles of BCL2 family proteins in cancer. eLife 2016, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langan, R.A.; Boyken, S.E.; Ng, A.H.; Samson, J.A.; Dods, G.; Westbrook, A.M.; Nguyen, T.H.; Lajoie, M.J.; Chen, Z.; Berger, S.; et al. De novo design of bioactive protein switches. Nature 2019, 572, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Ryan, J.; Chen, T.S.; Kougentakis, C.; Letai, A.; Keating, A.E. Potent and specific peptide inhibitors of human pro-survival protein Bcl-xL. J. Mol. Biol. 2015, 427, 1241–1253. [Google Scholar] [CrossRef] [PubMed]
- Distelhorst, C.W. Targeting Bcl-2-IP3 receptor interaction to treat cancer: A novel approach inspired by nearly a century treating cancer with adrenal corticosteroid hormones. Biochim. Biophys. Acta Mol. Cell. Res. 2018, 1865, 1795–1804. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wild, C.; Ding, Y.; Ye, N.; Chen, H.; Wold, E.A.; Zhou, J. BH4 domain of Bcl-2 as a novel target for cancer therapy. Drug Discov. Today 2016, 21, 989–996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, B.; Park, D.; Li, R.; Xie, M.; Owonikoko, T.K.; Zhang, G.; Sica, G.L.; Ding, C.; Zhou, J.; Magis, A.T.; et al. Small-Molecule Bcl2 BH4 Antagonist for Lung Cancer Therapy. Cancer Cell 2015, 27, 852–863. [Google Scholar] [CrossRef] [Green Version]
- Deng, J.; Park, D.; Wang, M.; Nooka, A.; Deng, Q.; Matulis, S.; Kaufman, J.; Lonial, S.; Boise, L.H.; Galipeau, J.; et al. BCL2-BH4 antagonist BDA-366 suppresses human myeloma growth. Oncotarget 2016, 7, 27753–27763. [Google Scholar] [CrossRef]
- Song, T.; Wang, Z.; Ji, F.; Feng, Y.; Fan, Y.; Chai, G.; Li, X.; Li, Z.; Zhang, Z. Deactivation of Mcl-1 by Dual-Function Small-Molecule Inhibitors Targeting the Bcl-2 Homology 3 Domain and Facilitating Mcl-1 Ubiquitination. Angew. Chem. Int. Ed. Engl. 2016, 55, 14250–14256. [Google Scholar] [CrossRef]
- Lee, S.; Wales, T.E.; Escudero, S.; Cohen, D.T.; Luccarelli, J.; Gallagher, C.G.; Cohen, N.A.; Huhn, A.J.; Bird, G.H.; Engen, J.R.; et al. Allosteric inhibition of antiapoptotic MCL-1. Nat. Struct. Mol. Biol. 2016, 23, 600–607. [Google Scholar] [CrossRef] [Green Version]
- Akcay, G.; Belmonte, M.A.; Aquila, B.; Chuaqui, C.; Hird, A.W.; Lamb, M.L.; Rawlins, P.B.; Su, N.; Tentarelli, S.; Grimster, N.P.; et al. Inhibition of Mcl-1 through covalent modification of a noncatalytic lysine side chain. Nat. Chem. Biol. 2016, 12, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.; Chattopadhyay, S.; Chatterjee, S. G-Quadruplex surveillance in BCL-2 gene: A promising therapeutic intervention in cancer treatment. Drug Discov. Today 2017, 22, 1165–1186. [Google Scholar] [CrossRef] [PubMed]
- Onel, B.; Carver, M.; Wu, G.; Timonina, D.; Kalarn, S.; Larriva, M.; Yang, D. A New G-Quadruplex with Hairpin Loop Immediately Upstream of the Human BCL2 P1 Promoter Modulates Transcription. J. Am. Chem. Soc. 2016, 138, 2563–2570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahid, R.; Bugaut, A.; Balasubramanian, S. The BCL-2 5’ untranslated region contains an RNA G-quadruplex-forming motif that modulates protein expression. Biochemistry 2010, 49, 8300–8306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jana, J.; Mondal, S.; Bhattacharjee, P.; Sengupta, P.; Roychowdhury, T.; Saha, P.; Kundu, P.; Chatterjee, S. Chelerythrine down regulates expression of VEGFA, BCL2 and KRAS by arresting G-Quadruplex structures at their promoter regions. Sci. Rep. 2017, 7, 40706. [Google Scholar] [CrossRef]
- Casagrande, V.; Salvati, E.; Alvino, A.; Bianco, A.; Ciammaichella, A.; D’Angelo, C.; Ginnari-Satriani, L.; Serrilli, A.M.; Iachettini, S.; Leonetti, C.; et al. N-cyclic bay-substituted perylene G-quadruplex ligands have selective antiproliferative effects on cancer cells and induce telomere damage. J. Med. Chem. 2011, 54, 1140–1156. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, Q.; Li, Q.; Yang, L.; Chen, L.; Zhou, Y.; Liu, J. A ruthenium(II) complex capable of inducing and stabilizing bcl-2 G-quadruplex formation as a potential cancer inhibitor. J. Inorg. Biochem. 2014, 134, 1–11. [Google Scholar] [CrossRef]
- Pelliccia, S.; Amato, J.; Capasso, D.; Di Gaetano, S.; Massarotti, A.; Piccolo, M.; Irace, C.; Tron, G.C.; Pagano, B.; Randazzo, A.; et al. Bio-Inspired Dual-Selective BCL-2/c-MYC G-Quadruplex Binders: Design, Synthesis, and Anticancer Activity of Drug-like Imidazo [2,1-i]purine Derivatives. J. Med. Chem. 2020, 63, 2035–2050. [Google Scholar] [CrossRef]
- Amato, J.; Pagano, A.; Capasso, D.; Di Gaetano, S.; Giustiniano, M.; Novellino, E.; Randazzo, A.; Pagano, B. Targeting the BCL2 Gene Promoter G-Quadruplex with a New Class of Furopyridazinone-Based Molecules. Chem. Med. Chem. 2018, 13, 406–410. [Google Scholar] [CrossRef]
- Gunaratnam, M.; Collie, G.W.; Reszka, A.P.; Todd, A.K.; Parkinson, G.N.; Neidle, S. A naphthalene diimide G-quadruplex ligand inhibits cell growth and down-regulates BCL-2 expression in an imatinib-resistant gastrointestinal cancer cell line. Bioorg. Med. Chem. 2018, 26, 2958–2964. [Google Scholar] [CrossRef]
- Andersen, M.H.; Reker, S.; Kvistborg, P.; Becker, J.C.; thor Straten, P. Spontaneous immunity against Bcl-xL in cancer patients. J. Immunol. 2005, 175, 2709–2714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsen, H.L.; Andersen, M.H.; Wandall, H.H.; Madsen, C.B.; Christensen, R.E.; Petersen, T.R.; Pedersen, A.E. Induction of Bcl-xL-specific cytotoxic T lymphocytes in mice. Scand. J. Immunol. 2014, 80, 111–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorgensen, N.G.; Ahmad, S.M.; Abildgaard, N.; Straten, P.T.; Svane, I.M.; Andersen, M.H.; Knudsen, L.M. Peptide vaccination against multiple myeloma using peptides derived from anti-apoptotic proteins: A phase I trial. Stem Cell Investig. 2016, 3, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robin, A.Y.; Krishna Kumar, K.; Westphal, D.; Wardak, A.Z.; Thompson, G.V.; Dewson, G.; Colman, P.M.; Czabotar, P.E. Crystal structure of Bax bound to the BH3 peptide of Bim identifies important contacts for interaction. Cell Death Dis. 2015, 6, e1809. [Google Scholar] [CrossRef] [Green Version]
- Garner, T.P.; Reyna, D.E.; Priyadarshi, A.; Chen, H.C.; Li, S.; Wu, Y.; Ganesan, Y.T.; Malashkevich, V.N.; Cheng, E.H.; Gavathiotis, E. An Autoinhibited Dimeric Form of BAX Regulates the BAX Activation Pathway. Mol. Cell 2016, 63, 485–497. [Google Scholar] [CrossRef] [Green Version]
- Garner, T.P.; Lopez, A.; Reyna, D.E.; Spitz, A.Z.; Gavathiotis, E. Progress in targeting the BCL-2 family of proteins. Curr. Opin. Chem. Biol. 2017, 39, 133–142. [Google Scholar] [CrossRef]
- Hadji, A.; Schmitt, G.K.; Schnorenberg, M.R.; Roach, L.; Hickey, C.M.; Leak, L.B.; Tirrell, M.V.; LaBelle, J.L. Preferential targeting of MCL-1 by a hydrocarbon-stapled BIM BH3 peptide. Oncotarget 2019, 10, 6219–6233. [Google Scholar] [CrossRef]
- Schnorenberg, M.R.; Bellairs, J.A.; Samaeekia, R.; Acar, H.; Tirrell, M.V.; LaBelle, J.L. Activating the Intrinsic Pathway of Apoptosis Using BIM BH3 Peptides Delivered by Peptide Amphiphiles with Endosomal Release. Materials (Basel) 2019, 12, 2567. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Wang, J.; Zhang, J.; Wang, W. Binding modes of Bcl-2 homology 3 (BH3) peptides with anti-apoptotic protein A1 and redesign of peptide inhibitors: A computational study. J. Biomol. Struct. Dyn. 2018, 36, 3967–3977. [Google Scholar] [CrossRef]
- Vila-Julia, G.; Granadino-Roldan, J.M.; Perez, J.J.; Rubio-Martinez, J. Molecular Determinants for the Activation/Inhibition of Bak Protein by BH3 Peptides. J. Chem. Inf. Model. 2020, 60, 1632–1643. [Google Scholar] [CrossRef]
- Banta, K.L.; Wang, X.; Das, P.; Winoto, A. B cell lymphoma 2 (Bcl-2) residues essential for Bcl-2’s apoptosis-inducing interaction with Nur77/Nor-1 orphan steroid receptors. J. Biol. Chem. 2018, 293, 4724–4734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolluri, S.K.; Zhu, X.; Zhou, X.; Lin, B.; Chen, Y.; Sun, K.; Tian, X.; Town, J.; Cao, X.; Lin, F.; et al. A short Nur77-derived peptide converts Bcl-2 from a protector to a killer. Cancer Cell 2008, 14, 285–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Inhibitor | Specificity | Clinical Trial Identifier | Tumor Histotype | Phase | Combination with |
|---|---|---|---|---|---|
| Navitoclax | Bcl-2/Bcl-xL/ Bcl-w | NCT04041050 | myelo- proliferative neoplasm | I | Ruxolitinib or single agent |
| NCT03222609 | myelofibrosis | II | Ruxolitinib or single agent | ||
| NCT00788684 | lymphoid cancers | I | Ruxolitinib | ||
| NCT02520778 | advanced or metastatic non-small lung cancer | I | Osimertinib | ||
| NCT02143401 | relapsed or refractory solid tumors | I | Sorafenib | ||
| NCT02079740 | advanced or metastatic solid tumors | I/II | Trametinib | ||
| NCT01989585 | BRAF mutant melanoma | I/II | Dabrafenib/ Trametinib | ||
| APG-1252 | Bcl-2/Bcl-xL | NCT03080311 | small cell lung cancer or other solid tumors | I | single agent |
| NCT04210037 | relapsed/ refractory small cell lung cancer | I/II | Paclitaxel | ||
| AZD0466 | Bcl-2/Bcl-xL | NCT04214093 | hematologic or solid tumors | I | single agent |
| Venetoclax | Bcl-2 | NCT03000257 | advanced solid tumors | I | ABBV-181 |
| NCT03082209 | previously treated solid tumors and hematologic malignancies | I | ABBV-621 | ||
| NCT03181126 | relapsed/ refractory acute lymphoblastic leukemia and relapsed/ refractory lymphoblastic lymphoma | I | Navitoclax | ||
| NCT04029688 | relapsed/refractory acute leukemias or solid tumors | I/II | Idasanutlin | ||
| S65487 | Bcl-2 | NCT03755154 | acute myeloid leukemia, non-Hodgkin lymphoma or multiple myeloma | I | single agent |
| AMG-176 | Mcl-1 | NCT02675452 | relapsed or refractory multiple myeloma and subjects with relapsed or refractory acute myeloid leukemia | I | single agent |
| AZD5991 | Mcl-1 | NCT03218683 | relapsed or refractory hematologic malignancies | II | Venetoclax |
| S64315/ MIK665 | Mcl-1 | NCT02979366 | acute myeloid leukemia or myelodysplastic syndrome | I | single agent |
| NCT02992483 | refractory or relapsed lymphoma or multiple myeloma | I | single agent | ||
| NCT03672695 | acute myeloid leukemia | I | Venetoclax |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Aguanno, S.; Del Bufalo, D. Inhibition of Anti-Apoptotic Bcl-2 Proteins in Preclinical and Clinical Studies: Current Overview in Cancer. Cells 2020, 9, 1287. https://doi.org/10.3390/cells9051287
D’Aguanno S, Del Bufalo D. Inhibition of Anti-Apoptotic Bcl-2 Proteins in Preclinical and Clinical Studies: Current Overview in Cancer. Cells. 2020; 9(5):1287. https://doi.org/10.3390/cells9051287
Chicago/Turabian StyleD’Aguanno, Simona, and Donatella Del Bufalo. 2020. "Inhibition of Anti-Apoptotic Bcl-2 Proteins in Preclinical and Clinical Studies: Current Overview in Cancer" Cells 9, no. 5: 1287. https://doi.org/10.3390/cells9051287