Metabolic Signature of Hepatic Fibrosis: From Individual Pathways to Systems Biology
Abstract
:1. Introduction
2. Hepatic Stellate Cells: Crucial Cells for Hepatic Fibrosis
3. Metabolic Pathways Associated with Hepatic Fibrosis
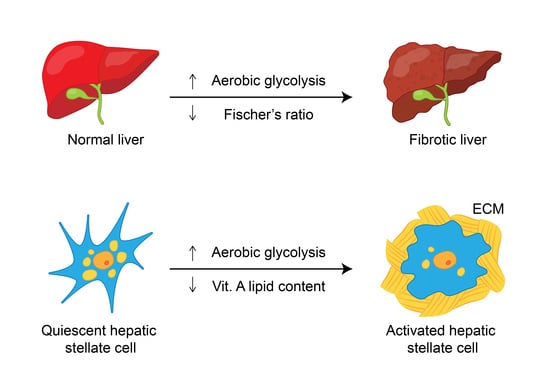
3.1. Carbohydrate-Associated Pathways
3.2. Amino Acid-Associated Pathways
3.3. Lipid-Associated Pathways
4. Metabolic Alterations Caused by Hepatic Fibrosis Based on Systems Biology Data
4.1. In Vitro Studies
4.2. Animal Studies
4.2.1. CCl4-Treated Rats
4.2.2. DMN-Treated Rats
4.2.3. TAA-Treated Rats and Mice
4.2.4. Rats Treated with Three Fibrogenic Compounds
4.2.5. Mouse Model of Primary Biliary Cholangitis
4.3. Human Studies
4.3.1. HBV-Related Hepatic Fibrosis
4.3.2. HCV-Related Hepatic Fibrosis
4.3.3. NAFLD-Related Hepatic Fibrosis
4.3.4. Nonspecific Cirrhosis
5. Concluding Remarks and Future Prospective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bataller, R.; Brenner, D.A. Liver fibrosis. J. Clin. Investig. 2005, 115, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Kisseleva, T.; Brenner, D.A. Mechanisms of fibrogenesis. Exp. Biol. Med. 2008, 233, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Kisseleva, T. Reversibility of liver fibrosis. Clin. Res. Hepatol. Gastroenterol. 2015, 39 (Suppl. S1), S60–S63. [Google Scholar] [CrossRef]
- GBD 2013 Risk Factors Collaborators; Forouzanfar, M.H.; Alexander, L.; Anderson, H.R.; Bachman, V.F.; Biryukov, S.; Brauer, M.; Burnett, R.; Casey, D.; Casey, M.M.; et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 2287–2323. [Google Scholar] [CrossRef]
- Tsochatzis, E.A.; Bosch, J.; Burroughs, A.K. Liver cirrhosis. Lancet 2014, 383, 1749–1761. [Google Scholar] [CrossRef]
- Cheung, K.J.; Tilleman, K.; Deforce, D.; Colle, I.; Van Vlierberghe, H. Proteomics in liver fibrosis is more than meets the eye. Eur. J. Gastroenterol. Hepatol. 2008, 20, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.A. Biomarkers of liver fibrosis. J. Gastroenterol. Hepatol. 2011, 26, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.L. Metabolic alterations and hepatitis C: From bench to bedside. World J. Gastroenterol. 2016, 22, 1461–1476. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.T.; Cheng, J.S.; Lin, C.H.; Chen, T.H.; Lee, K.C.; Chang, M.L. Rheumatoid factor and immunoglobulin M mark hepatitis C-associated mixed cryoglobulinaemia: An 8-year prospective study. Clin. Microbiol. Infect. 2019. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Zwighaft, Z.; Reinke, H.; Asher, G. The Liver in the Eyes of a Chronobiologist. J. Biol. Rhythm. 2016, 31, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.W.; Cheng, M.L.; Shiao, M.S.; Yeh, C.T.; Wang, C.H.; Fan, C.M.; Chiu, C.T.; Chang, M.L. Recovery of lipid metabolic alterations in hepatitis C patients after viral clearance: Incomplete restoration with accelerated ω-oxidation. J. Clin. Lipidol. 2018, 12, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.L.; Cheng, M.L.; Chang, S.W.; Tang, H.Y.; Chiu, C.T.; Yeh, C.T.; Shiao, M.S. Recovery of pan-genotypic and genotype-specific amino acid alterations in chronic hepatitis C after viral clearance: Transition at the crossroad of metabolism and immunity. Amino Acids 2017, 49, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Tavassoly, I.; Goldfarb, J.; Iyengar, R. Systems biology primer: The basic methods and approaches. Essays Biochem. 2018, 62, 487–500. [Google Scholar] [PubMed]
- Khan, V.; Putluri, N.; Sreekumar, A.; Mindikoglu, A.L. Current Applications of Metabolomics in Cirrhosis. Metabolites 2018, 8, 67. [Google Scholar] [CrossRef] [PubMed]
- Procopet, B.; Fischer, P.; Farcau, O.; Stefanescu, H. Metabolomics: From liver chiromancy to personalized precision medicine in advanced chronic liver disease. World J. Hepatol. 2018, 10, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Saxena, N.K.; Anania, F.A. Adipocytokines and hepatic fibrosis. Trends Endocrinol. Metab. 2015, 26, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Panebianco, C.; Oben, J.A.; Vinciguerra, M.; Pazienza, V. Senescence in hepatic stellate cells as a mechanism of liver fibrosis reversal: A putative synergy between retinoic acid and PPAR-gamma signalings. Clin. Exp. Med. 2017, 17, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L. Hepatic stellate cells: Protean, multifunctional, and enigmatic cells of the liver. Physiol. Rev. 2008, 88, 125–172. [Google Scholar] [CrossRef] [PubMed]
- Duffield, J.S.; Forbes, S.J.; Constandinou, C.M.; Clay, S.; Partolina, M.; Vuthoori, S.; Wu, S.; Lang, R.; Iredale, J.P. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J. Clin. Investig. 2005, 115, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Kawelke, N.; Vasel, M.; Sens, C.; Au, A.; Dooley, S.; Nakchbandi, I.A. Fibronectin protects from excessive liver fibrosis by modulating the availability of and responsiveness of stellate cells to active TGF-β. PLoS ONE 2011, 6, e28181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lian, N.; Jin, H.; Zhang, F.; Wu, L.; Shao, J.; Lu, Y.; Zheng, S. Curcumin inhibits aerobic glycolysis in hepatic stellate cells associated with activation of adenosine monophosphate-activated protein kinase. IUBMB Life 2016, 68, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Jia, Y.; Li, M.; Wang, L.; Shao, J.; Guo, Q.; Tan, S.; Ding, H.; Chen, A.; Zhang, F.; et al. Blockade of glycolysis-dependent contraction by oroxylin a via inhibition of lactate dehydrogenase-a in hepatic stellate cells. Cell Commun. Signal. 2019, 17, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Choi, S.S.; Michelotti, G.A.; Chan, I.S.; Swiderska-Syn, M.; Karaca, G.F.; Xie, G.; Moylan, C.A.; Garibaldi, F.; Premont, R.; et al. Hedgehog controls hepatic stellate cell fate by regulating metabolism. Gastroenterology 2012, 143, 1319–1329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olson, A.L.; Pessin, J.E. Structure, function, and regulation of the mammalian facilitative glucose transporter gene family. Annu. Rev. Nutr. 1996, 16, 235–256. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Xia, T.; Du, Y.; Liu, J.; Xie, Y.; Zhang, Y.; Guan, F.; Wu, J.; Wang, X.; Shi, C. Exosomes from activated hepatic stellate cells contain GLUT1 and PKM2: A role for exosomes in metabolic switch of liver nonparenchymal cells. FASEB J. 2019, 33, 8530–8542. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Ye, J.; Wang, Z.J. Embryonic liver fordin is involved in glucose glycolysis of hepatic stellate cell by regulating PI3K/Akt signaling. World J. Gastroenterol. 2016, 22, 8519–8527. [Google Scholar] [CrossRef] [PubMed]
- Bugianesi, E.; Kalhan, S.; Burkett, E.; Marchesini, G.; McCullough, A. Quantification of gluconeogenesis in cirrhosis: Response to glucagon. Gastroenterology 1998, 115, 1530–1540. [Google Scholar] [CrossRef]
- Anand, A.C. Nutrition and Muscle in Cirrhosis. J. Clin. Exp. Hepatol. 2017, 7, 340–357. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, J.B.; Mortensen, C.; Bendtsen, F.; Møller, S. Lactate metabolism in chronic liver disease. Scand. J. Clin. Lab. Investig. 2013, 73, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, H.; Mei, Y.; Ding, X.; Yang, X.; Li, C.; Deng, M.; Gong, J. Clinicopathological and prognostic significance of PKM2 protein expression in cirrhotic hepatocellular carcinoma and non-cirrhotic hepatocellular carcinoma. Sci. Rep. 2017, 7, 15294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pusec, C.M.; De Jesus, A.; Khan, M.W.; Terry, A.R.; Ludvik, A.E.; Xu, K.; Giancola, N.; Pervaiz, H.; Daviau Smith, E.; Ding, X.; et al. Hepatic HKDC1 Expression Contributes to Liver Metabolism. Endocrinology 2019, 160, 313–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krähenbühl, L.; Lang, C.; Lüdes, S.; Seiler, C.; Schäfer, M.; Zimmermann, A.; Krähenbühl, S. Reduced hepatic glycogen stores in patients with liver cirrhosis. Liver Int. 2003, 23, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Hudert, C.A.; Selinski, S.; Rudolph, B.; Bläker, H.; Loddenkemper, C.; Thielhorn, R.; Berndt, N.; Golka, K.; Cadenas, C.; Reinders, J.; et al. Genetic determinants of steatosis and fibrosis progression in paediatric non-alcoholic fatty liver disease. Liver Int. 2019, 39, 540–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheen, J.M.; Chen, Y.C.; Tain, Y.L.; Huang, L.T. Increased circulatory asymmetric dimethylarginine and multiple organ failure: Bile duct ligation in rat as a model. Int. J. Mol. Sci. 2014, 15, 3989–4006. [Google Scholar] [CrossRef] [PubMed]
- Ferrigno, A.; Di Pasqua, L.G.; Berardo, C.; Richelmi, P.; Vairetti, M. Liver plays a central role in asymmetric dimethylarginine-mediated organ injury. World J. Gastroenterol. 2015, 21, 5131–5137. [Google Scholar] [CrossRef] [PubMed]
- Holeček, M.; Vodeničarovová, M. Muscle wasting and branched-chain amino acid, alpha-ketoglutarate, and ATP depletion in a rat model of liver cirrhosis. Int. J. Exp. Pathol. 2018, 99, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Holeček, M.; Mráz, J.; Tilšer, I. Plasma amino acids in four models of experimental liver injury in rats. Amino Acids 1996, 10, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, L.; Yin, L.; Qi, Y.; Xu, Y.; Han, X.; Peng, J. Quantitative chemical proteomics for investigating the biomarkers of dioscin against liver fibrosis caused by CCl4 in rats. Chem. Commun. 2015, 51, 11064–11067. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Fang, S.; Liu, Q.; Gao, J.; Wang, X.; Zhu, H.; Zhu, Z.; Ji, F.; Wu, J.; Ma, Y.; et al. TGF-β1/p65/MAT2A pathway regulates liver fibrogenesis via intracellular SAM. EBioMedicine 2019, 42, 458–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fontana, L.; Moreira, E.; Torres, M.I.; Fernández, M.I.; Ríos, A.; Sánchez de Medina, F.; Gil, A. Serum amino acid changes in rats with thioacetamide-induced liver cirrhosis. Toxicology 1996, 106, 197–206. [Google Scholar] [CrossRef]
- Low, T.Y.; Leow, C.K.; Salto-Tellez, M.; Chung, M.C. A proteomic analysis of thioacetamide-induced hepatotoxicity and cirrhosis in rat livers. Proteomics 2004, 4, 3960–3974. [Google Scholar] [CrossRef] [PubMed]
- Soeters, P.B.; Fischer, J.E. Insulin, glucagon, aminoacid imbalance, and hepatic encephalopathy. Lancet 1976, 2, 880–882. [Google Scholar] [CrossRef]
- Holeček, M. Branched-chain amino acids in health and disease: Metabolism, alterations in blood plasma, and as supplements. Nutr. Metab. 2018, 15, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanco Vela, C.I.; Bosques Padilla, F.J. Determination of ammonia concentrations in cirrhosis patients-still confusing after all these years? Ann. Hepatol. 2011, 10 (Suppl. S2), S60–S65. [Google Scholar] [CrossRef]
- Long, J.; Wang, H.; Lang, Z.; Wang, T.; Long, M.; Wang, B. Expression level of glutamine synthetase is increased in hepatocellular carcinoma and liver tissue with cirrhosis and chronic hepatitis B. Hepatol. Int. 2011, 5, 698–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleming, K.E.; Wanless, I.R. Glutamine synthetase expression in activated hepatocyte progenitor cells and loss of hepatocellular expression in congestion and cirrhosis. Liver Int. 2013, 33, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, T.; Taniguchi, E.; Sata, M. Effects of oral branched-chain amino acids on hepatic encephalopathy and outcome in patients with liver cirrhosis. Nutr. Clin. Pract. 2013, 28, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Holecek, M. Ammonia and amino acid profiles in liver cirrhosis: Effects of variables leading to hepatic encephalopathy. Nutrition 2015, 31, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Kakazu, E.; Kondo, Y.; Kogure, T.; Ninomiya, M.; Kimura, O.; Ueno, Y.; Shimosegawa, T. Plasma amino acids imbalance in cirrhotic patients disturbs the tricarboxylic acid cycle of dendritic cell. Sci. Rep. 2013, 3, 3459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Chiara, F.; Thomsen, K.L.; Habtesion, A.; Jones, H.; Davies, N.; Gracia-Sancho, J.; Manicardi, N.; Hall, A.; Andreola, F.; Paish, H.L.; et al. Ammonia scavenging prevents progression of fibrosis in experimental non-alcoholic fatty liver disease. Hepatology 2019. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Ko, J.M.; Kim, Y.M.; Seo, G.H.; Kim, G.H.; Lee, B.H.; Yoo, H.W. Low prevalence of argininosuccinate lyase deficiency among inherited urea cycle disorders in Korea. J. Hum. Genet. 2018, 63, 911–917. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Hong, Y.; Wang, X.; Zhang, X.; Long, J.; Li, H.; Zhang, B.; Chen, S.; Liu, Q.; Li, H.; et al. Identification and clinical significance of an elevated level of serum aminoacylase-1 autoantibody in patients with hepatitis B virus-related liver cirrhosis. Mol. Med. Rep. 2016, 14, 4255–4262. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Gao, L.; Zhou, Z.; Lin, H.; Chen, C.; Huang, P.; Huang, W.; Zhou, C.; Huang, S.; Nie, L.; et al. Indoleamine 2,3-dioxygenase 1 deficiency attenuates CCl4-induced fibrosis through Th17 cells down-regulation and tryptophan 2,3-dioxygenase compensation. Oncotarget 2017, 8, 40486–40500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clària, J.; Moreau, R.; Fenaille, F.; Amorós, A.; Junot, C.; Gronbaek, H.; Coenraad, M.J.; Pruvost, A.; Ghettas, A.; Chu-Van, E.; et al. CANONIC Study Investigators of the EASL Clif Consortium, Grifols Chair and the European Foundation for the Study of Chronic Liver Failure (EF Clif). Orchestration of Tryptophan-Kynurenine Pathway, Acute Decompensation, and Acute-on-Chronic Liver Failure in Cirrhosis. Hepatology 2019, 69, 1686–1701. [Google Scholar] [PubMed]
- Jing, X.Y.; Yang, X.F.; Qing, K.; Ou-yang, Y. Roles of the lipid metabolism in hepatic stellate cells activation. Chin. Med. Sci. J. 2013, 28, 233–236. [Google Scholar] [CrossRef]
- Shirakami, Y.; Lee, S.A.; Clugston, R.D.; Blaner, W.S. Hepatic metabolism of retinoids and disease associations. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 2012, 1821, 124–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saeed, A.; Hoekstra, M.; Hoeke, M.O.; Heegsma, J.; Faber, K.N. The interrelationship between bile acid and vitamin A homeostasis. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 2017, 1862, 496–512. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, M.; Lefebvre, P.; Staels, B. Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J. Hepatol. 2015, 62, 720–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zardi, E.M.; Navarini, L.; Sambataro, G.; Piccinni, P.; Sambataro, F.M.; Spina, C.; Dobrina, A. Hepatic PPARs: Their role in liver physiology, fibrosis and treatment. Curr. Med. Chem. 2013, 20, 3370–3396. [Google Scholar] [CrossRef] [PubMed]
- Arain, S.Q.; Talpur, F.N.; Channa, N.A.; Ali, M.S.; Afridi, H.I. Serum lipid profile as a marker of liver impairment in hepatitis B Cirrhosis patients. Lipids Health Dis. 2017, 16, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Liu, J.; Lin, C.; Wang, H.; Jiang, Y.; Wang, J.; Yang, P.; He, F. Peroxiredoxin 2: A potential biomarker for early diagnosis of hepatitis B virus related liver fibrosis identified by proteomic analysis of the plasma. BMC Gastroenterol. 2010, 10, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katrinli, S.; Ozdil, K.; Sahin, A.; Ozturk, O.; Kir, G.; Baykal, A.T.; Akgun, E.; Sarac, O.S.; Sokmen, M.; Doğanay, H.L.; et al. Proteomic profiling of HBV infected liver biopsies with different fibrotic stages. Proteome Sci. 2017, 15, 7. [Google Scholar] [CrossRef] [PubMed]
- Trieb, M.; Horvath, A.; Birner-Gruenberger, R.; Spindelboeck, W.; Stadlbauer, V.; Taschler, U.; Curcic, S.; Stauber, R.E.; Holzer, M.; Pasterk, L.; et al. Liver disease alters high-density lipoprotein composition, metabolism and function. Biochim. Biophys. Acta 2016, 1861, 630–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gangadharan, B.; Bapat, M.; Rossa, J.; Antrobus, R.; Chittenden, D.; Kampa, B.; Barnes, E.; Klenerman, P.; Dwek, R.A.; Zitzmann, N. Discovery of novel biomarker candidates for liver fibrosis in hepatitis C patients: A preliminary study. PLoS ONE 2012, 7, e39603. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.W.; Hung, Y.C.; Wu, T.H.; Chen, M.H.; Yeh, C.T.; Pan, T.L. Proteome-based identification of apolipoprotein A-IV as an early diagnostic biomarker in liver fibrosis. Oncotarget 2017, 8, 88951–88964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Chen, F.; Fan, X.; Lin, C.; Hao, Y.; Wei, H.; Lin, W.; Jiang, Y.; He, F. Quantitative Proteomic analysis on Activated Hepatic Stellate Cells reversion Reveal STAT1 as a key regulator between Liver Fibrosis and recovery. Sci. Rep. 2017, 7, 44910. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, P.; Chen, F.; Hao, Y.; Lao, Y.; Ren, L.; Sun, L.; Sun, W.; Wei, H.; Chan, D.W.; et al. SILAC-based quantitative proteomic analysis of secretome between activated and reverted hepatic stellate cells. Proteomics 2014, 14, 1977–1986. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Zhong, Y.; Dang, L.; Zhu, M.; Yu, H.; Chen, W.; Cui, J.; Bian, H.; Li, Z. Alteration of protein glycosylation in human hepatic stellate cells activated with transforming growth factor-β1. J. Proteom. 2012, 75, 4114–4123. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Yu, F.; Ji, Q.; Li, Z.; Wang, K.; Zhang, J.; Lu, J.; Chen, L.; Zeng, Y.; Ji, Y. Comparative proteomic analysis of rat hepatic stellate cell activation: A comprehensive view and suppressed immune response. Hepatology 2012, 56, 332–349. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Chen, Q.L.; Song, Y.N.; Sun, Y.; Wei, B.; Li, X.Y.; Hu, Y.Y.; Liu, P.; Su, S.B. Mechanisms of CCl4-induced liver fibrosis with combined transcriptomic and proteomic analysis. J. Toxicol. Sci. 2016, 41, 561–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teufel, A.; Becker, D.; Weber, S.N.; Dooley, S.; Breitkopf-Heinlein, K.; Maass, T.; Hochrath, K.; Krupp, M.; Marquardt, J.U.; Kolb, M.; et al. Identification of RARRES1 as a core regulator in liver fibrosis. J. Mol. Med. 2012, 90, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; He, J.Q.; Chen, D.Y.; Pan, Q.L.; Yang, J.F.; Cao, H.C.; Li, L.J. Dynamic changes of key metabolites during liver fibrosis in rats. World J. Gastroenterol. 2019, 25, 941–954. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Meng, H.Y.; Liu, S.M.; Wang, Y.; Yang, X.X.; Lu, F.; Wang, H.Y. Identification of key metabolic changes during liver fibrosis progression in rats using a urine and serum metabolomics approach. Sci. Rep. 2017, 7, 11433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.N.; Dong, S.; Wei, B.; Liu, P.; Zhang, Y.Y.; Su, S.B. Metabolomic mechanisms of gypenoside against liver fibrosis in rats: An integrative analysis of proteomics and metabolomics data. PLoS ONE 2017, 12, e0173598. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Song, J.M.; Gao, P.F.; Qin, X.J.; Xu, S.Z.; Zhang, J.F. Metabolic characterization of the early stage of hepatic fibrosis in rat using GC-TOF/MS and multivariate data analyses. Biomed. Chromatogr. 2017, 31. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zheng, H.; Yang, Z.T.; Cheng, B.; Wu, J.X.; Liu, X.W.; Tang, C.L.; Lu, S.Y.; Chen, Z.N.; Song, F.M.; et al. Urinary metabonomics study of the hepatoprotective effects of total alkaloids from Corydalis saxicola Bunting on carbon tetrachloride-induced chronic hepatotoxicity in rats using 1H NMR analysis. J. Pharm. Biomed. Anal. 2017, 140, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Qin, X.J.; Jiang, H.; Chen, J.F.; Wang, T.; Zhang, T.; Xu, S.Z.; Song, J.M. Detecting serum and urine metabolic profile changes of CCl4-liver fibrosis in rats at 12 weeks based on gas chromatography-mass spectrometry. Exp. Ther. Med. 2017, 14, 1496–1504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Dai, R.; Ke, M.; Suheryani, I.; Meng, W.; Deng, Y. Differential Proteomic Analysis of Dimethylnitrosamine (DMN)-Induced Liver Fibrosis. Proteomics 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.D.; Wang, J.S.; Wang, P.R.; Li, M.H.; Yang, M.H.; Kong, L.Y. Toxic effects of chronic low-dose exposure of thioacetamide on rats based on NMR metabolic profiling. J. Pharm. Biomed. Anal. 2014, 98, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, K.; Takami, T.; Matsumoto, T.; Yamamoto, N.; Sakaida, I. Profiling of the circadian metabolome in thioacetamide-induced liver cirrhosis in mice. Hepatol. Commun. 2017, 1, 704–718. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, D.L.; AbdulHameed, M.D.; Tawa, G.J.; Baer, C.E.; Permenter, M.G.; McDyre, B.C.; Dennis, W.E.; Boyle, M.H.; Hobbs, C.A.; Streicker, M.A.; et al. Gene Expression Patterns Associated with Histopathology in Toxic Liver Fibrosis. Toxicol. Sci. 2016, 149, 67–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, G.; Hu, C.; Zhu, H.; Li, X.; Zhao, L.; Zhou, R.; Zhang, X.; Zhang, F.; Wu, L.; Li, Y. Comparative proteomics study on liver mitochondria of primary biliary cirrhosis mouse model. BMC Gastroenterol. 2013, 13, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, R.; Zhao, Q.; Hu, D.D.; Xiao, X.R.; Huang, J.F.; Li, F. Metabolomic analysis of cholestatic liver damage in mice. Food Chem. Toxicol. 2018, 120, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, Z.; Deng, B.; Wu, X.; Liu, J.; Feng, X. Identification of Enolase 1 and Thrombospondin-1 as serum biomarkers in HBV hepatic fibrosis by proteomics. Proteome Sci. 2013, 11, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, Z.G.; Zhao, W.; Zhang, J.; Wu, X.; Hu, J.; Yin, G.C.; Xu, Y.J. Metabolomics and eicosanoid analysis identified serum biomarkers for distinguishing hepatocellular carcinoma from hepatitis B virus-related cirrhosis. Oncotarget 2017, 8, 63890–63900. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Bellance, N.; Damm, A.; Bing, H.; Zhu, Z.; Handa, K.; Yovchev, M.I.; Sehgal, V.; Moss, T.J.; Oertel, M.; et al. A switch in the source of ATP production and a loss in capacity to perform glycolysis are hallmarks of hepatocyte failure in advance liver disease. J. Hepatol. 2014, 60, 1203–1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diamond, D.L.; Jacobs, J.M.; Paeper, B.; Proll, S.C.; Gritsenko, M.A.; Carithers, R.L., Jr.; Larson, A.M.; Yeh, M.M.; Camp, D.G., 2nd. Proteomic profiling of human liver biopsies: Hepatitis C virus-induced fibrosis and mitochondrial dysfunction. Hepatology 2007, 46, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Mariño, Z.; Millet, O.; Martínez-Arranz, I.; Navasa, M.; Falcón-Pérez, J.M.; Pérez-Cormenzana, M.; Caballería, J.; Embade, N. Metabolomics Signature Linked to Liver Fibrosis in The Serum of Transplanted Hepatitis C Patients. Sci. Rep. 2017, 7, 10497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitian, A.I.; Nelson, D.R.; Liu, C.; Xu, Y.; Ararat, M.; Cabrera, R. Integrated metabolomic profiling of hepatocellular carcinoma in hepatitis C cirrhosis through GC/MS and UPLC/MS-MS. Liver Int. 2014, 34, 1428–1444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubio-Gozalbo, M.E.; Bakker, J.A.; Waterham, H.R.; Wanders, R.J. Carnitine-acylcarnitine translocase deficiency, clinical, biochemical and genetic aspects. Mol. Aspects Med. 2004, 25, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Embade, N.; Mariño, Z.; Diercks, T.; Cano, A.; Lens, S.; Cabrera, D.; Navasa, M.; Falcón-Pérez, J.M.; Caballería, J.; Castro, A.; et al. Metabolic Characterization of Advanced Liver Fibrosis in HCV Patients as Studied by Serum 1H-NMR Spectroscopy. PLoS ONE 2016, 11, e0155094. [Google Scholar] [CrossRef] [PubMed]
- Sands, C.J.; Guha, I.N.; Kyriakides, M.; Wright, M.; Beckonert, O.; Holmes, E.; Rosenberg, W.M.; Coen, M. Metabolic phenotyping for enhanced mechanistic stratification of chronic hepatitis C-induced liver fibrosis. Am. J. Gastroenterol. 2015, 110, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Baranova, A.; Stepanova, M.; Page, S.; Calvert, V.S.; Afendy, A.; Goodman, Z.; Chandhoke, V.; Liotta, L.; Petricoin, E. Phosphoproteomic biomarkers predicting histologic nonalcoholic steatohepatitis and fibrosis. J. Proteome Res. 2010, 9, 3218–3224. [Google Scholar] [CrossRef] [PubMed]
- Traussnigg, S.; Kienbacher, C.; Gajdošík, M.; Valkovič, L.; Halilbasic, E.; Stift, J.; Rechling, C.; Hofer, H.; Steindl-Munda, P.; Ferenci, P.; et al. Ultra-high-field magnetic resonance spectroscopy in non-alcoholic fatty liver disease: Novel mechanistic and diagnostic insights of energy metabolism in non-alcoholic steatohepatitis and advanced fibrosis. Liver Int. 2017, 37, 1544–1553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Antolín, G.; Almohalla-Alvarez, C.; Bueno, P.; Almansa, R.; Iglesias, V.; Rico, L.; Ortega, A.; Muñoz-Conejero, E.; García-Pajares, F.; Bermejo-Martin, J.F. Evidence of Active Pro-Fibrotic Response in Blood of Patients with Cirrhosis. PLoS ONE 2015, 10, e0137128. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Jiang, S.; Chen, Y.; Zhao, X.; Li, H.; Lin, W.; Li, B.; Wang, X.; Yuan, J.; Sun, Y. Cirrhosis related functionality characteristic of the fecal microbiota as revealed by a metaproteomic approach. BMC Gastroenterol. 2016, 16, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, W.; Sanda, M.; Wu, J.; Koomen, J.; Goldman, R. Quantitative analysis of immunoglobulin subclasses and subclass specific glycosylation by LC-MS-MRM in liver disease. J. Proteom. 2015, 116, 24–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, K.F.; Krssak, M.; Navarro, V.; Chandramouli, V.; Hundal, R.; Schumann, W.C.; Landau, B.R.; Shulman, G.I. Contributions of net hepatic glycogenolysis and gluconeogenesis to glucose production in cirrhosis. Am. J. Physiol. 1999, 276, E529–E535. [Google Scholar] [CrossRef] [PubMed]
- Rachakonda, V.; Argemi, J.; Borhani, A.A.; Bataller, R.; Tevar, A.; Behari, J. Reduced Serum Sphingolipids Constitute a Molecular Signature of Malnutrition in Hospitalized Patients with Decompensated Cirrhosis. Clin. Transl. Gastroenterol. 2019, 10, e00013. [Google Scholar] [CrossRef] [PubMed]
- Osman, D.; Ali, O.; Obada, M.; El-Mezayen, H.; El-Said, H. Chromatographic determination of some biomarkers of liver cirrhosis and hepatocellular carcinoma in Egyptian patients. Biomed. Chromatogr. 2017, 31. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, Z.; Huang, M.; Sun, X.; Liu, B.; Guo, Q.; Chang, Q.; Duan, Z. Taurocholic acid is an active promoting factor, not just a biomarker of progression of liver cirrhosis: Evidence from a human metabolomic study and in vitro experiments. BMC Gastroenterol. 2018, 18, 112. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Ling, Z.; Chen, D.; Liu, Y.; Yang, F.; Li, L. Disorganized Gut Microbiome Contributed to Liver Cirrhosis Progression: A Meta-Omics-Based Study. Front. Microbiol. 2018, 9, 3166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soga, T.; Sugimoto, M.; Honma, M.; Mori, M.; Igarashi, K.; Kashikura, K.; Ikeda, S.; Hirayama, A.; Yamamoto, T.; Yoshida, H.; et al. Serum metabolomics reveals γ-glutamyl dipeptides as biomarkers for discrimination among different forms of liver disease. J. Hepatol. 2011, 55, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.; Wan, D.; Zhao, C.; Chen, J.; Zhao, X.; Wang, W.; Lu, X.; Yang, S.; Gu, J.; Xu, G. A metabonomic study of hepatitis B-induced liver cirrhosis and hepatocellular carcinoma by using RP-LC and HILIC coupled with mass spectrometry. Mol. Biosyst. 2009, 5, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Lu, Q.; Liu, X.; Cong, H.; Zhao, L.; Wang, H.; Lin, D. Application of 1H NMR-based metabonomics in the study of metabolic profiling of human hepatocellular carcinoma and liver cirrhosis. Cancer Sci. 2009, 100, 782–785. [Google Scholar] [CrossRef] [PubMed]
- Dabos, K.J.; Parkinson, J.A.; Sadler, I.H.; Plevris, J.N.; Hayes, P.C. 1H nuclear magnetic resonance spectroscopy-based metabonomic study in patients with cirrhosis and hepatic encephalopathy. World J. Hepatol. 2015, 7, 1701–1707. [Google Scholar] [CrossRef] [PubMed]
- Schofield, Z.; Reed, M.A.; Newsome, P.N.; Adams, D.H.; Günther, U.L.; Lalor, P.F. Changes in human hepatic metabolism in steatosis and cirrhosis. World J. Gastroenterol. 2017, 23, 2685–2695. [Google Scholar] [CrossRef] [PubMed]
- Beyoğlu, D.; Idle, J.R. The metabolomic window into hepatobiliary disease. J. Hepatol. 2013, 59, 842–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Fibrogenic Chemicals/Animals | Methods | Altered Pathways or Metabolites | Refs |
|---|---|---|---|
| CCl4/rats | Transcriptomics and proteomics | Pathways: retinol metabolism, PPAR signaling, glycolysis, gluconeogenesis, arachidonic acid metabolism, xenobiotic metabolism via cytochrome P450 and drug metabolism Enzymes: cytochrome P450, family 4, subfamily a, polypeptide 3; aldehyde dehydrogenase 2; and aldehyde dehydrogenase 7 family, member A1 | [71] |
| Microarray and Western blot | RARRES1 mRNA and protein | [72] | |
| LC-QTOF-MS | β-Muricholic acid and cervonoyl ethanolamide | [73] | |
| LC-QTOF-MS | Serum tryptophan, valine, leucine, isoleucine, TCA cycle metabolites, sphingolipid and glycerophospholipid metabolites, valine and bile acid metabolites Urinary kynurenic acid, 5-hydroxyindoleacetic acid, glycine, and 4-(2-amino-3-hydroxyphenyl)-2,4-dioxobutanoic acid and serum sphinganine, SM, l-leucine, l-tryptophan, and lysoPC(17:0) | [74] | |
| GC/MS | Pathways: glycolysis, gluconeogenesis, fructose and mannose metabolism, glycine, serine and threonine metabolism, lysine degradation, arginine and proline metabolism, glutathione metabolism, and sulfur metabolism | [75] | |
| GC-TOF-MS | Succinic acid, threonine and lactose | [76] | |
| 1H NMR | Urinary 2-oxoglutarate, citrate, dimethylamine, taurine, phenylacetylglycine, creatinine and hippurate | [77] | |
| GC/MS | Serum metabolites: isoleucine, L-malic acid, α-copper, carnitine, hippuric acid, glutaric acid and glucose Urine metabolites: 2-hydroxy butyric acid, isoleucine, N-acetyl-β-alanine, cytidine and corticoid Pathways: glucose, amino acid, P450, fatty acid, nucleic acid, electrolyte and glutathione biosynthesis | [78] | |
| DMN/rats | iTRAQ-based proteomic analysis | Key enzymes in fatty acid metabolism (acyl-CoA synthetase long chain family member 1), the TCA cycle (succinate dehydrogenase), glycogenolysis, and gluconeogenesis (pyruvate carboxylase and phosphoenolpyruvate carboxykinase in the cytosol) and an increase in glycolysis enzymes (HK-1) | [79] |
| TAA/rats | NMR-based metabolomics | Pathways: TCA cycle, pyruvate metabolism, starch and sucrose metabolism, glycolysis or gluconeogenesis, ketone body degradation, butanoate metabolism, and BCAAs and AAAs biosynthesis Serum metabolites: 2-hydroxybutyrate, 3-hydroxybutyrate and adipate in urine and phenylalanine, N,N-dimethyl glycine, O-acetyl glycoprotein, N-acetyl glycoprotein and choline in serum | [80] |
| TAA/mice | LC and GC separation coupled with MS-MS | Pathways: glycolysis and β-oxidation Metabolites: Serotonin and other vitamin A-related metabolites; metabolic products related to energetics, redox homeostasis, bile acid production, inflammation, and other processes | [81] |
| allyl alcohol, CCl4, and 4,4’-methylenedianiline/rats | MS | Insulin-like growth factor-binding protein | [82] |
| poly(I:C)/mice | iTRAQ | Mitochondrial proteins associated with reduced oxidation, lipid metabolism, and steroid hormone and primary bile acid biosynthesis. Four proteins (hydroxyacyl-coenzyme A dehydrogenase, carnitine O-palmitoyltransferase 1, 2,4-dienoyl-CoA reductase, and isoform 1 of Enoyl-CoA hydratase domain-containing protein 2) involved in fatty acid β-oxidation | [83] |
| alpha-naphthylisothiocyanate/mice | UPLC-ESI-QTOF-MS | Plasma bile acids, phospholipids, arginine and glutathione | [84] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, M.-L.; Yang, S.-S. Metabolic Signature of Hepatic Fibrosis: From Individual Pathways to Systems Biology. Cells 2019, 8, 1423. https://doi.org/10.3390/cells8111423
Chang M-L, Yang S-S. Metabolic Signature of Hepatic Fibrosis: From Individual Pathways to Systems Biology. Cells. 2019; 8(11):1423. https://doi.org/10.3390/cells8111423
Chicago/Turabian StyleChang, Ming-Ling, and Sien-Sing Yang. 2019. "Metabolic Signature of Hepatic Fibrosis: From Individual Pathways to Systems Biology" Cells 8, no. 11: 1423. https://doi.org/10.3390/cells8111423