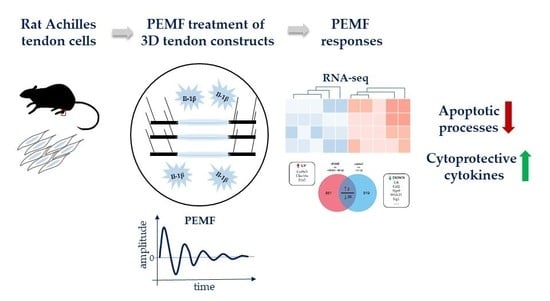
Global Responses of Il-1β-Primed 3D Tendon Constructs to Treatment with Pulsed Electromagnetic Fields
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Cell Culture
2.2. In Vitro Tendon-Like Construct Formation
2.3. Pro-Inflammatory Stimulation/Conditioning of Tendon-Like Constructs
2.4. PEMF Treatment
2.5. Cell Viability, Metabolic Activity, and Cytotoxicity Assays
2.6. RNA Isolation, Quantification and Validation
2.7. RNASeq and Data Analyses
2.8. Reverse Transcription and Gene Expression Analysis
2.9. Protein Lysates, SDS-PAGE and Western Blot
2.10. Cryo-Embedding and Sectioning
2.11. TUNEL Staining and Caspase3/7 Activity Assay
2.12. Quantification of Nuclear Aspect Ratio, Cell Orientation and Collagen Density
2.13. Interleukin-6 ELISA and Quantification of NO Production
2.14. MMP2 and MMP9 in Gel Zymography
2.15. Statisical Analysis
3. Results
3.1. Experimental Design and RNASeq Data Analysis
3.2. Global Responses in Il-1β-Primed TDSPCs after PEMF Exposure
3.3. PEMF Exposure Does not Alter the Structure or Turn-Over of the Extracellular Matrix in 3D Cultures
3.4. Treatment with PEMF Drives Expression of Cytoprotective Cytokines
3.5. PEMF limits Il-1β-Induced Apoptosis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schneider, M.; Angele, P.; Jarvinen, T.A.H.; Docheva, D. Rescue plan for Achilles: Therapeutics steering the fate and functions of stem cells in tendon wound healing. Adv. Drug Deliv. Rev. 2018, 129, 352–375. [Google Scholar] [CrossRef]
- Riley, G. Tendinopathy--from basic science to treatment. Nat. Clin. Pract. Rheumatol. 2008, 4, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Tempfer, H.; Lehner, C.; Grütz, M.; Gehwolf, R.; Traweger, A. Biological Augmentation for Tendon Repair: Lessons to be Learned from Development, Disease, and Tendon Stem Cell Research. Cell Eng. Regener. 2017. [Google Scholar] [CrossRef]
- Andres, B.M.; Murrell, G.A. Treatment of tendinopathy: What works, what does not, and what is on the horizon. Clin. Orthop. Relat. Res. 2008, 466, 1539–1554. [Google Scholar] [CrossRef]
- Millar, N.L.; Murrell, G.A.; McInnes, I.B. Inflammatory mechanisms in tendinopathy—Towards translation. Nat. Rev. Rheumatol. 2017, 13, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Dean, B.J.; Gettings, P.; Dakin, S.G.; Carr, A.J. Are inflammatory cells increased in painful human tendinopathy? A systematic review. Br. J. Sports Med. 2016, 50, 216–220. [Google Scholar] [CrossRef]
- Schulze-Tanzil, G.; Al-Sadi, O.; Wiegand, E.; Ertel, W.; Busch, C.; Kohl, B.; Pufe, T. The role of pro-inflammatory and immunoregulatory cytokines in tendon healing and rupture: New insights. Scand. J. Med. Sci. Sports 2011, 21, 337–351. [Google Scholar] [CrossRef]
- Tsuzaki, M.; Guyton, G.; Garrett, W.; Archambault, J.M.; Herzog, W.; Almekinders, L.; Bynum, D.; Yang, X.; Banes, A.J. IL-1 beta induces COX2, MMP-1, -3 and -13, ADAMTS-4, IL-1 beta and IL-6 in human tendon cells. J. Orthop. Res. 2003, 21, 256–264. [Google Scholar] [CrossRef]
- Archambault, J.; Tsuzaki, M.; Herzog, W.; Banes, A.J. Stretch and interleukin-1beta induce matrix metalloproteinases in rabbit tendon cells In vitro. J. Orthop. Res. 2002, 20, 36–39. [Google Scholar] [CrossRef]
- Funk, R.H. Coupling of pulsed electromagnetic fields (PEMF) therapy to molecular grounds of the cell. Am. J. Transl. Res. 2018, 10, 1260–1272. [Google Scholar] [PubMed]
- Rohde, C.; Chiang, A.; Adipoju, O.; Casper, D.; Pilla, A.A. Effects of pulsed electromagnetic fields on interleukin-1 beta and postoperative pain: A double-blind, placebo-controlled, pilot study in breast reduction patients. Plast. Reconstructive Surg. 2010, 125, 1620–1629. [Google Scholar] [CrossRef]
- Bassett, C.A. Fundamental and practical aspects of therapeutic uses of pulsed electromagnetic fields (PEMFs). Crit. Rev. Biomed. Eng. 1989, 17, 451–529. [Google Scholar]
- Goudarzi, I.; Hajizadeh, S.; Salmani, M.E.; Abrari, K. Pulsed electromagnetic fields accelerate wound healing in the skin of diabetic rats. Bioelectromagnetics 2010, 31, 318–323. [Google Scholar] [CrossRef]
- Sorrell, R.G.; Muhlenfeld, J.; Moffett, J.; Stevens, G.; Kesten, S. Evaluation of pulsed electromagnetic field therapy for the treatment of chronic postoperative pain following lumbar surgery: A pilot, double-blind, randomized, sham-controlled clinical trial. J. Pain. Res. 2018, 11, 1209–1222. [Google Scholar] [CrossRef] [PubMed]
- Ryang We, S.; Koog, Y.H.; Jeong, K.I.; Wi, H. Effects of pulsed electromagnetic field on knee osteoarthritis: A systematic review. Rheumatology 2013, 52, 815–824. [Google Scholar] [CrossRef]
- Veronesi, F.; Torricelli, P.; Giavaresi, G.; Sartori, M.; Cavani, F.; Setti, S.; Cadossi, M.; Ongaro, A.; Fini, M. In vivo effect of two different pulsed electromagnetic field frequencies on osteoarthritis. J. Orthop. Res. 2014, 32, 677–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Mattei, M.; Caruso, A.; Pezzetti, F.; Pellati, A.; Stabellini, G.; Sollazzo, V.; Traina, G.C. Effects of pulsed electromagnetic fields on human articular chondrocyte proliferation. Connective Tissue Res. 2001, 42, 269–279. [Google Scholar] [CrossRef]
- Pezzetti, F.; De Mattei, M.; Caruso, A.; Cadossi, R.; Zucchini, P.; Carinci, F.; Traina, G.C.; Sollazzo, V. Effects of pulsed electromagnetic fields on human chondrocytes: An In vitro study. Calcified Tissue Int. 1999, 65, 396–401. [Google Scholar] [CrossRef]
- De Mattei, M.; Varani, K.; Masieri, F.F.; Pellati, A.; Ongaro, A.; Fini, M.; Cadossi, R.; Vincenzi, F.; Borea, P.A.; Caruso, A. Adenosine analogs and electromagnetic fields inhibit prostaglandin E2 release in bovine synovial fibroblasts. Osteoarthritis Cartilage 2009, 17, 252–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Girolamo, L.; Vigano, M.; Galliera, E.; Stanco, D.; Setti, S.; Marazzi, M.G.; Thiebat, G.; Corsi Romanelli, M.M.; Sansone, V. In vitro functional response of human tendon cells to different dosages of low-frequency pulsed electromagnetic field. Knee Surg. Sports Traumatol. Arthroscopy 2015, 23, 3443–3453. [Google Scholar] [CrossRef] [PubMed]
- Strauch, B.; Patel, M.K.; Rosen, D.J.; Mahadevia, S.; Brindzei, N.; Pilla, A.A. Pulsed magnetic field therapy increases tensile strength in a rat Achilles’ tendon repair model. J. Hand Surg. 2006, 31, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.W.; Maffulli, N.; Li, C.K.; Chan, K.M. Pulsed magnetic and electromagnetic fields in experimental achilles tendonitis in the rat: A prospective randomized study. Arch. Phys. Med. Rehabil. 1997, 78, 399–404. [Google Scholar] [CrossRef]
- Osti, L.; Buono, A.D.; Maffulli, N. Pulsed electromagnetic fields after rotator cuff repair: A randomized, controlled study. Orthopedics 2015, 38, e223–228. [Google Scholar] [CrossRef]
- Kunkel, N.; Wagner, A.; Gehwolf, R.; Heimel, P.; Tempfer, H.; Korntner, S.; Augat, P.; Resch, H.; Redl, H.; Betz, O.; et al. Comparing the osteogenic potential of bone marrow and tendon-derived stromal cells to repair a critical-sized defect in the rat femur. J. Tissue Eng. Regenerative Med. 2015. [Google Scholar] [CrossRef] [PubMed]
- Gehwolf, R.; Spitzer, G.; Wagner, A.; Lehner, C.; Weissenbacher, N.; Tempfer, H.; Traweger, A. 3D-Embedded Cell Cultures to Study Tendon Biology. Methods Mol. Biol 2019. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, RESEARCH0034. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 15, 680–685. [Google Scholar] [CrossRef]
- Tempfer, H.; Kaser-Eichberger, A.; Lehner, C.; Gehwolf, R.; Korntner, S.; Kunkel, N.; Wagner, A.; Gruetz, M.; Heindl, L.M.; Schroedl, F.; et al. Bevacizumab improves Achilles tendon repair in a rat model. Cell. Physiol. Biochem. 2018, 46, 1148–1158. [Google Scholar] [CrossRef]
- Hsieh, C.F.; Alberton, P.; Loffredo-Verde, E.; Volkmer, E.; Pietschmann, M.; Muller, P.; Schieker, M.; Docheva, D. Scaffold-free Scleraxis-programmed tendon progenitors aid in significantly enhanced repair of full-size Achilles tendon rupture. Nanomedicine (Lond) 2016, 11, 1153–1167. [Google Scholar] [CrossRef]
- Gaynor, J.S.; Hagberg, S.; Gurfein, B.T. Veterinary applications of pulsed electromagnetic field therapy. Res. Vet. Sci. 2018, 119, 1–8. [Google Scholar] [CrossRef]
- de Girolamo, L.; Stanco, D.; Galliera, E.; Vigano, M.; Colombini, A.; Setti, S.; Vianello, E.; Corsi Romanelli, M.M.; Sansone, V. Low frequency pulsed electromagnetic field affects proliferation, tissue-specific gene expression, and cytokines release of human tendon cells. Cell Biochem. Biophys. 2013, 66, 697–708. [Google Scholar] [CrossRef]
- Huegel, J.; Choi, D.S.; Nuss, C.A.; Minnig, M.C.C.; Tucker, J.J.; Kuntz, A.F.; Waldorff, E.I.; Zhang, N.; Ryaby, J.T.; Soslowsky, L.J. Effects of pulsed electromagnetic field therapy at different frequencies and durations on rotator cuff tendon-to-bone healing in a rat model. J. Shoulder Elbow Surg. 2018, 27, 553–560. [Google Scholar] [CrossRef]
- Strauch, B.; Herman, C.; Dabb, R.; Ignarro, L.J.; Pilla, A.A. Evidence-based use of pulsed electromagnetic field therapy in clinical plastic surgery. Aesthet. Surg. J. 2009, 29, 135–143. [Google Scholar] [CrossRef]
- Galace de Freitas, D.; Marcondes, F.B.; Monteiro, R.L.; Rosa, S.G.; Maria de Moraes Barros Fucs, P.; Fukuda, T.Y. Pulsed electromagnetic field and exercises in patients with shoulder impingement syndrome: A randomized, double-blind, placebo-controlled clinical trial. Arch. Phys. Med. Rehabil. 2014, 95, 345–352. [Google Scholar] [CrossRef]
- D’Addona, A.; Maffulli, N.; Formisano, S.; Rosa, D. Inflammation in tendinopathy. Surgeon 2017, 15, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Asai, S.; Yu, B.; Enomoto-Iwamoto, M. IL-1beta irreversibly inhibits tenogenic differentiation and alters metabolism in injured tendon-derived progenitor cells In vitro. Biochem. Biophys. Res. Commun. 2015, 463, 667–672. [Google Scholar] [CrossRef]
- McClellan, A.; Evans, R.; Sze, C.; Kan, S.; Paterson, Y.; Guest, D. A novel mechanism for the protection of embryonic stem cell derived tenocytes from inflammatory cytokine interleukin 1 beta. Sci. Rep. 2019, 9, 2755. [Google Scholar] [CrossRef] [PubMed]
- Busch, F.; Mobasheri, A.; Shayan, P.; Lueders, C.; Stahlmann, R.; Shakibaei, M. Resveratrol modulates interleukin-1beta-induced phosphatidylinositol 3-kinase and nuclear factor kappaB signaling pathways in human tenocytes. J. Biol. Chem. 2012, 287, 38050–38063. [Google Scholar] [CrossRef]
- Ongaro, A.; Varani, K.; Masieri, F.F.; Pellati, A.; Massari, L.; Cadossi, R.; Vincenzi, F.; Borea, P.A.; Fini, M.; Caruso, A.; et al. Electromagnetic fields (EMFs) and adenosine receptors modulate prostaglandin E(2) and cytokine release in human osteoarthritic synovial fibroblasts. J. Cell Physiol. 2012, 227, 2461–2469. [Google Scholar] [CrossRef] [PubMed]
- de Girolamo, L.; Stanco, D.; Galliera, E.; Vigano, M.; Lovati, A.B.; Marazzi, M.G.; Romeo, P.; Sansone, V. Soft-focused extracorporeal shock waves increase the expression of tendon-specific markers and the release of anti-inflammatory cytokines in an adherent culture model of primary human tendon cells. Ultrasound. Med. Biol. 2014, 40, 1204–1215. [Google Scholar] [CrossRef]
- Andersen, M.B.; Pingel, J.; Kjaer, M.; Langberg, H. Interleukin-6: A growth factor stimulating collagen synthesis in human tendon. J. Appl. Physiol. 2011, 110, 1549–1554. [Google Scholar] [CrossRef] [PubMed]
- Szomor, Z.L.; Wang, M.X.; Kruller, A.; Murrell, G.A.; Farmer, K.M.; Kirkham, B.W.; Bonar, F. Differential expression of cytokines and nitric oxide synthase isoforms in human rotator cuff bursae. Ann. Rheumatic Dis. 2001, 60, 431–432. [Google Scholar] [CrossRef] [Green Version]
- Lu, Z.; Xu, S. ERK1/2 MAP kinases in cell survival and apoptosis. IUBMB Life 2006, 58, 621–631. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Liu, W.Z.; Liu, T.; Feng, X.; Yang, N.; Zhou, H.F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept Signal Transduct. Res. 2015, 35, 600–604. [Google Scholar] [CrossRef] [PubMed]









| Biological Process | % Genes | Fold Enrichment | Corrected p Value |
|---|---|---|---|
| response to hypoxia | 3.044 | 2.032 | 6.18 × 10−12 |
| negative regulation of apoptotic process | 4.959 | 1.698 | 3.06 × 10−11 |
| positive regulation of gene expression | 4.330 | 1.765 | 3.93 × 10−11 |
| response to drug | 4.776 | 1.708 | 5.34 × 10−11 |
| cellular response to tumor necrosis factor | 1.942 | 2.293 | 7.95 × 10−10 |
| extracellular matrix organization | 1.837 | 2.350 | 8.19 × 10−10 |
| response to estradiol stimulus | 2.362 | 2.092 | 1.55 × 10−9 |
| wound healing | 1.679 | 2.398 | 2.92 × 10−9 |
| response to lipopolysaccharide | 2.729 | 1.949 | 5.02 × 10−10 |
| positive regulation of cell proliferation | 4.907 | 1.606 | 1.64 × 10−8 |
| negative regulation of gene expression | 2.650 | 1.907 | 5.10 × 10−8 |
| in utero embryonic development | 2.755 | 1.853 | 1.57 × 10−7 |
| Aging | 3.201 | 1.755 | 2.4 × 10−7 |
| cytoplasmic translation | 0.787 | 3.223 | 4.20 × 10−7 |
| positive regulation of cell migration | 2.467 | 1.893 | 4.53 × 10−7 |
| protein transport | 2.309 | 1.925 | 6.84 × 10−7 |
| regulation of cell proliferation | 2.020 | 2.012 | 8.89 × 10−7 |
| apoptotic process | 3.385 | 1.690 | 1.25 × 10−6 |
| collagen fibril organization | 0.813 | 3.059 | 1.48 × 10−6 |
| negative regulation of cell proliferation | 3.437 | 1.667 | 2.58 × 10−7 |
| Gene Symbol | Gene Name | Fold Change | Gene Symbol | Gene Name | Fold Change |
|---|---|---|---|---|---|
| Ltk | Tyrosine-protein kinase receptor | −10.36 | Irg1 | Immune-Responsive Gene 1 Protein | −2.45 |
| Csf2 | Granulocyte-macrophage colony-stimulating factor | −10.16 | Ereg | Proepiregulin | −2.44 |
| Rgs9 | Regulator of G-protein signaling 9 | −4.61 | Egr3 | Early growth response protein 3 | −2.43 |
| Wfdc21 | WAP four-disulfide core domain 21 | −4.41 | Fam84a | Family with sequence similarity 84, member A | −2.41 |
| Itga7 | Integrin alpha-7 | −3.86 | Cited1 | Melanocyte-Specific Protein 1 | −2.29 |
| Scgb1c1 | Secretoglobin family 1C member 1 | −3.80 | Csf3 | Colony-stimulating factor 3 | −2.28 |
| Fam71e2 | Family with sequence similarity 71, member E2 | −3.60 | Cnn1 | Calponin-1 | −2.27 |
| Adgb | Androglobin | −3.59 | Gdf15 | Growth/differentiation factor 15 | −2.16 |
| Cd40 | CD40 molecule | −3.56 | Ptgs2 | Prostaglandin G/H synthase 2 | −2.15 |
| Car12 | Carbonic anhydrase 12 | −3.51 | Actg2 | Actin, gamma-enteric smooth muscle | −2.14 |
| Cxcl2 | C-X-C motif chemokine 2 | −3.40 | Scube1 | Signal Peptide, CUB Domain, EGF-Like 1 | −2.14 |
| Ip6k3 | Kinase | −3.21 | Pvalb | Parvalbumin alpha | −2.14 |
| Rdh5 | Retinol dehydrogenase 5 | −3.05 | Ggt1 | Glutathione hydrolase 1 proenzyme | −2.13 |
| Nsg2 | Neuronal vesicle trafficking-associated protein 2 | −2.84 | Igsf9b | Protein turtle homolog B | −2.09 |
| Fam64a | Family with sequence similarity 64, member A | −2.84 | Myb | MYB proto-oncogene, transcription factor | −2.07 |
| Ass1 | Argininosuccinate synthase | −2.78 | Dlgap2 | Disks large-associated protein 2 | −2.04 |
| Prom1 | Prominin 1 | −2.61 | Neurl3 | E3 ubiquitin-protein ligase NEURL3 | −2.03 |
| RGD1561143 | Similar to cell surface receptor FDFACT | −2.54 | Col9a3 | Collagen type IX alpha 3 chain | 2.05 |
| Ces1a | Carboxylic ester hydrolase | −2.49 | Clec10a | C-type lectin domain family 10 member A | 2.05 |
| Fbn2 | −2.47 | Il1r2 | Interleukin-1 receptor type 2 | 2.78 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gehwolf, R.; Schwemberger, B.; Jessen, M.; Korntner, S.; Wagner, A.; Lehner, C.; Weissenbacher, N.; Tempfer, H.; Traweger, A. Global Responses of Il-1β-Primed 3D Tendon Constructs to Treatment with Pulsed Electromagnetic Fields. Cells 2019, 8, 399. https://doi.org/10.3390/cells8050399
Gehwolf R, Schwemberger B, Jessen M, Korntner S, Wagner A, Lehner C, Weissenbacher N, Tempfer H, Traweger A. Global Responses of Il-1β-Primed 3D Tendon Constructs to Treatment with Pulsed Electromagnetic Fields. Cells. 2019; 8(5):399. https://doi.org/10.3390/cells8050399
Chicago/Turabian StyleGehwolf, Renate, Bettina Schwemberger, Malik Jessen, Stefanie Korntner, Andrea Wagner, Christine Lehner, Nadja Weissenbacher, Herbert Tempfer, and Andreas Traweger. 2019. "Global Responses of Il-1β-Primed 3D Tendon Constructs to Treatment with Pulsed Electromagnetic Fields" Cells 8, no. 5: 399. https://doi.org/10.3390/cells8050399