Voltage-Gated T-Type Calcium Channel Modulation by Kinases and Phosphatases: The Old Ones, the New Ones, and the Missing Ones
Abstract
:1. Introduction
1.1. Voltage-Gated Calcium Channels
1.2. T-Type Calcium Channels
1.3. Protein Kinases
1.4. Protein Phosphatases
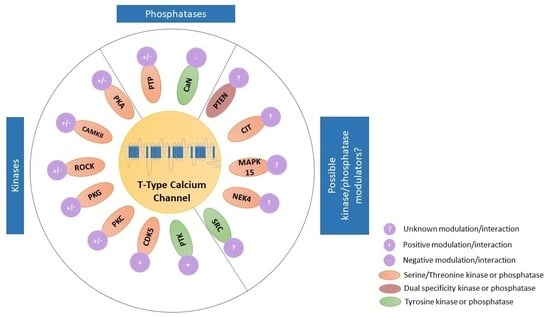
2. Kinase Modulators of T-Type Ca2+ Channels
2.1. Modulation of T-Type Ca2+ Channels by Serine/Threonine Kinase Family
2.1.1. Modulation of T-Type Ca2+ Channels by Protein Kinase A
2.1.2. Modulation of T-Type Ca2+ Channels by Ca2+/Calmodulin-Dependent Protein Kinase II
2.1.3. Modulation of T-Type Ca2+ Channels by Rho/Rho-Kinase
2.1.4. Modulation of T-Type Ca2+ Channels by Protein Kinase G
2.1.5. Modulation of T-Type Ca2+ Channels by Protein Kinase C
2.1.6. Modulation of T-Type Ca2+ Channels by Cyclin-Dependent Kinase 5
2.2. Modulation of T-Type Ca2+ Channels by Protein Tyrosine Kinase Family
3. Modulation of T-Type Ca2+ Channels by Phosphatases
3.1. Modulation of T-Type Ca2+ Channels by Tyrosine Phosphatases
3.2. Modulation of T-Type Ca2+ Channels by Calcineurin
4. Kinase and Phosphatase Regulation of TTCCs That Is Plausible but Yet to Be Investigated
4.1. Possible Direct Modulation of Cav3.2 by Mitogen-Activated Protein Kinases
4.2. Possible Modulation of Cav3.2 by Never in Mitosis A—Related Kinase
4.3. Possible Citron Kinase Modulation of Cav3.3
4.4. Possible Modulation of Cav3.2 by SRC Kinase
4.5. Possible Modulation of Cav3.1 by Phosphatase and Tensin Homolog Deleted on Chromosome 10
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Catterall, W.A. Voltage-Gated Calcium Channels. Cold Spring Harb. Perspect. Biol. 2011, 3, a003947. [Google Scholar] [CrossRef] [PubMed]
- Satheesh, N.; Büsselberg, D. The Role of Intracellular Calcium for the Development and Treatment of Neuroblastoma. Cancers 2015, 7, 823–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuttler, L.; Glaum, S.R.; Collins, B.A.; Miller, R.J. Calcium Signalling in Single Growth Hormone-Releasing Factor-Responsive Pituitary Cells. Endocrinology 1992, 130, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Sudhof, T.C. Calcium Control of Neurotransmitter Release. Cold Spring Harb. Perspect. Biol. 2012, 4, a011353. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D.G.; Groth, R.D.; Ma, H.; Barrett, C.F.; Owen, S.F.; Safa, P.; Tsien, R.W. CaV1 and CaV2 Channels Engage Distinct Modes of Ca2+ Signaling to Control CREB-Dependent Gene Expression. Cell 2012, 149, 1112–1124. [Google Scholar] [CrossRef] [Green Version]
- Tanabe, T.; Beam, K.G.; Adams, B.A.; Niidome, T.; Numa, S. Regions of the Skeletal Muscle Dihydropyridine Receptor Critical for Excitation–Contraction Coupling. Nature 1990, 346, 567–569. [Google Scholar] [CrossRef]
- Phan, N.N.; Wang, C.-Y.; Chen, C.-F.; Sun, Z.; Lai, M.-D.; Lin, Y.-C. Voltage-Gated Calcium Channels: Novel Targets for Cancer Therapy. Oncol. Lett. 2017, 14, 2059–2074. [Google Scholar] [CrossRef] [Green Version]
- Zamponi, G.W. Targeting Voltage-Gated Calcium Channels in Neurological and Psychiatric Diseases. Nat. Rev. Drug Discov. 2016, 15, 19–34. [Google Scholar] [CrossRef]
- Yang, S.-N.; Berggren, P.-O. The Role of Voltage-Gated Calcium Channels in Pancreatic β-Cell Physiology and Pathophysiology. Endocr. Rev. 2006, 27, 621–676. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.-Y.; Lai, M.-D.; Phan, N.N.; Sun, Z.; Lin, Y.-C. Meta-Analysis of Public Microarray Datasets Reveals Voltage-Gated Calcium Gene Signatures in Clinical Cancer Patients. PLoS ONE 2015, 10, e0125766. [Google Scholar] [CrossRef]
- Dolphin, A.C. Voltage-Gated Calcium Channels: Their Discovery, Function and Importance as Drug Targets. Brain Neurosci. Adv. 2018, 2, 239821281879480. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A.; Perez-Reyes, E.; Snutch, T.P.; Striessnig, J. International Union of Pharmacology. XLVIII. Nomenclature and Structure-Function Relationships of Voltage-Gated Calcium Channels. Pharmacol. Rev. 2005, 57, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, M.J.; Clapham, D.E. Ion Channels—Basic Science and Clinical Disease. N. Engl. J. Med. 1997, 336, 1575–1586. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.M.; Nimigean, C.M. Voltage-Gated Potassium Channels: A Structural Examination of Selectivity and Gating. Cold Spring Harb. Perspect. Biol. 2016, 8, a029231. [Google Scholar] [CrossRef] [Green Version]
- Chang, X.; Wang, J.; Jiang, H.; Shi, L.; Xie, J. Hyperpolarization-Activated Cyclic Nucleotide-Gated Channels: An Emerging Role in Neurodegenerative Diseases. Front. Mol. Neurosci. 2019, 12, 141. [Google Scholar] [CrossRef] [Green Version]
- Lambert, R.C.; Maulet, Y.; Mouton, J.; Beattie, R.; Volsen, S.; De Waard, M.; Feltz, A. T-Type Ca2+ Current Properties Are Not Modified by Ca2+ Channel β Subunit Depletion in Nodosus Ganglion Neurons. J. Neurosci. 1997, 17, 6621–6628. [Google Scholar] [CrossRef] [Green Version]
- Catterall, W.A.; Striessnig, J.; Snutch, T.P.; Perez-Reyes, E. International Union of Pharmacology. XL. Compendium of Voltage-Gated Ion Channels: Calcium Channels. Pharmacol. Rev. 2003, 55, 579–581. [Google Scholar] [CrossRef] [Green Version]
- Blesneac, I.; Chemin, J.; Bidaud, I.; Huc-Brandt, S.; Vandermoere, F.; Lory, P. Phosphorylation of the Cav3.2 T-Type Calcium Channel Directly Regulates Its Gating Properties. Proc. Natl. Acad. Sci. USA 2015, 112, 13705–13710. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Jiang, X.; Snutch, T.P.; Tao, J. Modulation of Low-Voltage-Activated T-Type Ca2+ Channels. Biochim. Biophys. Acta BBA-Biomembr. 2013, 1828, 1550–1559. [Google Scholar] [CrossRef] [Green Version]
- Weiss, N.; Zamponi, G.W. T-Type Calcium Channels: From Molecule to Therapeutic Opportunities. Int. J. Biochem. Cell Biol. 2019, 108, 34–39. [Google Scholar] [CrossRef]
- Billups, S.J.; Carter, B.L. Mibefradil: A New Class of Calcium-Channel Antagonists. Ann. Pharmacother. 1998, 32, 659–671. [Google Scholar] [CrossRef]
- Hayashi, K.; Wakino, S.; Homma, K.; Sugano, N.; Saruta, T. Pathophysiological Significance of T-Type Ca2+ Channels: Role of T-Type Ca2+ Channels in Renal Microcirculation. J. Pharmacol. Sci. 2005, 99, 221–227. [Google Scholar] [CrossRef] [Green Version]
- Timic Stamenic, T.; Todorovic, S.M. Thalamic T-Type Calcium Channels as Targets for Hypnotics and General Anesthetics. Int. J. Mol. Sci. 2022, 23, 2349. [Google Scholar] [CrossRef]
- Iftinca, M. Neuronal T–Type Calcium Channels: What’s New? Iftinca: T–Type Channel Regulation. J. Med. Life 2011, 4, 126–138. [Google Scholar]
- Lenglet, S.; Louiset, E.; Delarue, C.; Vaudry, H.; Contesse, V. Activation of 5-HT7 Receptor in Rat Glomerulosa Cells Is Associated with an Increase in Adenylyl Cyclase Activity and Calcium Influx through T-Type Calcium Channels. Endocrinology 2002, 143, 1748–1760. [Google Scholar] [CrossRef] [PubMed]
- Talley, E.M.; Cribbs, L.L.; Lee, J.-H.; Daud, A.; Perez-Reyes, E.; Bayliss, D.A. Differential Distribution of Three Members of a Gene Family Encoding Low Voltage-Activated (T-Type) Calcium Channels. J. Neurosci. 1999, 19, 1895–1911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monteil, A.; Chemin, J.; Bourinet, E.; Mennessier, G.; Lory, P.; Nargeot, J. Molecular and Functional Properties of the Human A1G Subunit That Forms T-Type Calcium Channels. J. Biol. Chem. 2000, 275, 6090–6100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohn, G.; Moosmang, S.; Conrad, H.; Ludwig, A.; Hofmann, F.; Klugbauer, N. Expression of T- and L-Type Calcium Channel MRNA in Murine Sinoatrial Node. FEBS Lett. 2000, 481, 73–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuang, H.; Bhattacharjee, A.; Hu, F.; Zhang, M.; Goswami, T.; Wang, L.; Wu, S.; Berggren, P.O.; Li, M. Cloning of a T-Type Ca2+ Channel Isoform in Insulin-Secreting Cells. Diabetes 2000, 49, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Vignali, S.; Leiss, V.; Karl, R.; Hofmann, F.; Welling, A. Characterization of Voltage-Dependent Sodium and Calcium Channels in Mouse Pancreatic A- and B-Cells. J. Physiol. 2006, 572, 691–706. [Google Scholar] [CrossRef]
- Levitsky, K.L.; López-Barneo, J. Developmental Change of T-Type Ca2+ Channel Expression and Its Role in Rat Chromaffin Cell Responsiveness to Acute Hypoxia: T-Type Channels and Chromaffin Cell O2 Sensing. J. Physiol. 2009, 587, 1917–1929. [Google Scholar] [CrossRef] [PubMed]
- Rose, K.E.; Lunardi, N.; Boscolo, A.; Dong, X.; Erisir, A.; Jevtovic-Todorovic, V.; Todorovic, S.M. Immunohistological Demonstration of CaV3.2 T-Type Voltage-Gated Calcium Channel Expression in Soma of Dorsal Root Ganglion Neurons and Peripheral Axons of Rat and Mouse. Neuroscience 2013, 250, 263–274. [Google Scholar] [CrossRef] [Green Version]
- Cribbs, L.L.; Lee, J.-H.; Yang, J.; Satin, J.; Zhang, Y.; Daud, A.; Barclay, J.; Williamson, M.P.; Fox, M.; Rees, M.; et al. Cloning and Characterization of A1H From Human Heart, a Member of the T-Type Ca2+ Channel Gene Family. Circ. Res. 1998, 83, 103–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barghouth, M.; Ye, Y.; Karagiannopoulos, A.; Ma, Y.; Cowan, E.; Wu, R.; Eliasson, L.; Renström, E.; Luan, C.; Zhang, E. The T-Type Calcium Channel CaV3.2 Regulates Insulin Secretion in the Pancreatic β-Cell. Cell Calcium 2022, 108, 102669. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.E.; Washburn, M.S.; Hans, M.; Urrutia, A.; Brust, P.F.; Prodanovich, P.; Harpold, M.M.; Stauderman, K.A. Structure and Functional Characterization of a Novel Human Low-Voltage Activated Calcium Channel. J. Neurochem. 1999, 72, 791–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrier, A.D.; Wang, H.; Talley, E.M.; Perez-Reyes, E.; Barrett, P.Q. α 1H T-Type Ca2+ Channel Is the Predominant Subtype Expressed in Bovine and Rat Zona Glomerulosa. Am. J. Physiol.-Cell Physiol. 2001, 280, C265–C272. [Google Scholar] [CrossRef]
- Lee, J.-H.; Daud, A.N.; Cribbs, L.L.; Lacerda, A.E.; Pereverzev, A.; Klöckner, U.; Schneider, T.; Perez-Reyes, E. Cloning and Expression of a Novel Member of the Low Voltage-Activated T-Type Calcium Channel Family. J. Neurosci. 1999, 19, 1912–1921. [Google Scholar] [CrossRef] [Green Version]
- Han, W.; Bao, W.; Wang, Z.; Nattel, S. Comparison of Ion-Channel Subunit Expression in Canine Cardiac Purkinje Fibers and Ventricular Muscle. Circ. Res. 2002, 91, 790–797. [Google Scholar] [CrossRef] [Green Version]
- Toyota, M.; Ho, C.; Ohe-Toyota, M.; Baylin, S.B.; Issa, J.-P.J. Inactivation of CACNA1G, a T-Type Calcium Channel Gene, by Aberrant Methylation of Its 5′ CpG Island in Human Tumors1. Cancer Res. 1999, 59, 4535–4541. [Google Scholar]
- Ohkubo, T. T-Type Voltage-Activated Calcium Channel Cav3.1, but Not Cav3.2, Is Involved in the Inhibition of Proliferation and Apoptosis in MCF-7 Human Breast Cancer Cells. Int. J. Oncol. 2012, 41, 267–275. [Google Scholar] [CrossRef]
- Chemin, J.; Siquier-Pernet, K.; Nicouleau, M.; Barcia, G.; Ahmad, A.; Medina-Cano, D.; Hanein, S.; Altin, N.; Hubert, L.; Bole-Feysot, C.; et al. De Novo Mutation Screening in Childhood-Onset Cerebellar Atrophy Identifies Gain-of-Function Mutations in the CACNA1G Calcium Channel Gene. Brain 2018, 141, 1998–2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, A.; Pushparaj, C.; Bahí, N.; Sorolla, A.; Herreros, J.; Pamplona, R.; Vilella, R.; Matias-Guiu, X.; Martí, R.M.; Cantí, C. Functional Expression of Voltage-Gated Calcium Channels in Human Melanoma: Functional Expression of VGCCs in Human Melanoma. Pigment Cell Melanoma Res. 2012, 25, 200–212. [Google Scholar] [CrossRef]
- Li, R.-F.; Man, Q.-W.; Liu, J.-Y.; Zheng, Y.-Y.; Gao, X.; Liu, H.-M. Overexpression of T-Type Calcium Channel Cav3.1 in Oral Squamous Cell Carcinoma: Association with Proliferation and Anti-Apoptotic Activity. J. Mol. Histol. 2021, 52, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Dziegielewska, B.; Casarez, E.V.; Yang, W.Z.; Gray, L.S.; Dziegielewski, J.; Slack-Davis, J.K. T-Type Ca2+ Channel Inhibition Sensitizes Ovarian Cancer to Carboplatin. Mol. Cancer Ther. 2016, 15, 460–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, F.; Chen, H.; Zhou, C.; Liu, S.; Guo, M.; Chen, P.; Zhuang, H.; Xie, D.; Wu, S. T-Type Ca2+ Channel Expression in Human Esophageal Carcinomas: A Functional Role in Proliferation. Cell Calcium 2008, 43, 49–58. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Cruickshanks, N.; Yuan, F.; Wang, B.; Pahuski, M.; Wulfkuhle, J.; Gallagher, I.; Koeppel, A.F.; Hatef, S.; Papanicolas, C.; et al. Targetable T-Type Calcium Channels Drive Glioblastoma. Cancer Res. 2017, 77, 3479–3490. [Google Scholar] [CrossRef] [Green Version]
- Fukami, K.; Sekiguchi, F.; Yasukawa, M.; Asano, E.; Kasamatsu, R.; Ueda, M.; Yoshida, S.; Kawabata, A. Functional Upregulation of the H2S/Cav3.2 Channel Pathway Accelerates Secretory Function in Neuroendocrine-Differentiated Human Prostate Cancer Cells. Biochem. Pharmacol. 2015, 97, 300–309. [Google Scholar] [CrossRef]
- Basson, M.D.; Zeng, B.; Downey, C.; Sirivelu, M.P.; Tepe, J.J. Increased Extracellular Pressure Stimulates Tumor Proliferation by a Mechanosensitive Calcium Channel and PKC-β. Mol. Oncol. 2015, 9, 513–526. [Google Scholar] [CrossRef]
- Rzhepetskyy, Y.; Lazniewska, J.; Blesneac, I.; Pamphlett, R.; Weiss, N. CACNA1H Missense Mutations Associated with Amyotrophic Lateral Sclerosis Alter Ca v 3.2 T-Type Calcium Channel Activity and Reticular Thalamic Neuron Firing. Channels 2016, 10, 466–477. [Google Scholar] [CrossRef] [Green Version]
- Daniil, G.; Fernandes-Rosa, F.L.; Chemin, J.; Blesneac, I.; Beltrand, J.; Polak, M.; Jeunemaitre, X.; Boulkroun, S.; Amar, L.; Strom, T.M.; et al. CACNA1H Mutations Are Associated With Different Forms of Primary Aldosteronism. EBioMedicine 2016, 13, 225–236. [Google Scholar] [CrossRef] [Green Version]
- Pera, E.; Kaemmerer, E.; Milevskiy, M.J.G.; Yapa, K.T.D.S.; O’Donnell, J.S.; Brown, M.A.; Simpson, F.; Peters, A.A.; Roberts-Thomson, S.J.; Monteith, G.R. The Voltage Gated Ca2+-Channel Cav3.2 and Therapeutic Responses in Breast Cancer. Cancer Cell Int. 2016, 16, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huse, M.; Kuriyan, J. The Conformational Plasticity of Protein Kinases. Cell 2002, 109, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Kostich, M.; English, J.; Madison, V.; Gheyas, F.; Wang, L.; Qiu, P.; Greene, J.; Laz, T.M. Human Members of the Eukaryotic Protein Kinase Family. Genome Biol. 2002, 3, research0043.1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manning, G.; Whyte, D.B.; Martinez, R.; Hunter, T.; Sudarsanam, S. The Protein Kinase Complement of the Human Genome. Science 2002, 298, 1912–1934. [Google Scholar] [CrossRef] [Green Version]
- Hanks, S.K. Genomic Analysis of the Eukaryotic Protein Kinase Superfamily: A Perspective. Genome Biol. 2003, 4, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, P. The Role of Protein Phosphorylation in Human Health and Disease.: Delivered on June 30th 2001 at the FEBS Meeting in Lisbon. Eur. J. Biochem. 2001, 268, 5001–5010. [Google Scholar] [CrossRef]
- Levinson, A.D.; Oppermann, H.; Levintow, L.; Varmus, H.E.; Bishop, J.M. Evidence That the Transforming Gene of Avian Sarcoma Virus Encodes a Protein Kinase Associated with a Phosphoprotein. Cell 1978, 15, 561–572. [Google Scholar] [CrossRef]
- Li, X.; Wilmanns, M.; Thornton, J.; Köhn, M. Elucidating Human Phosphatase-Substrate Networks. Sci. Signal. 2013, 6, rs10. [Google Scholar] [CrossRef]
- Tonks, N.K. Protein Tyrosine Phosphatases—From Housekeeping Enzymes to Master Regulators of Signal Transduction. FEBS J. 2013, 280, 346–378. [Google Scholar] [CrossRef] [Green Version]
- Stoker, A.W. Protein Tyrosine Phosphatases and Signalling. J. Endocrinol. 2005, 185, 19–33. [Google Scholar] [CrossRef] [Green Version]
- Brautigan, D.L. Protein Ser/ Thr Phosphatases—The Ugly Ducklings of Cell Signalling: Protein Ser/Thr Phosphatases. FEBS J. 2013, 280, 324–325. [Google Scholar] [CrossRef] [PubMed]
- Tonks, N.K. Protein Tyrosine Phosphatases: From Genes, to Function, to Disease. Nat. Rev. Mol. Cell Biol. 2006, 7, 833–846. [Google Scholar] [CrossRef]
- Walsh, D.A.; Perkins, J.P.; Krebs, E.G. An Adenosine 3′,5′-Monophosphate-Dependant Protein Kinase from Rabbit Skeletal Muscle. J. Biol. Chem. 1968, 243, 3763–3765. [Google Scholar] [CrossRef] [PubMed]
- Shoji, S.; Ericsson, L.H.; Walsh, K.A.; Fischer, E.H.; Titani, K. Amino Acid Sequence of the Catalytic Subunit of Bovine Type II Adenosine Cyclic 3′,5′-Phosphate-Dependent Protein Kinase. Biochemistry 1983, 22, 3702–3709. [Google Scholar] [CrossRef] [PubMed]
- Uhler, M.D.; Carmichael, D.F.; Lee, D.C.; Chrivia, J.C.; Krebs, E.G.; McKnight, G.S. Isolation of CDNA Clones Coding for the Catalytic Subunit of Mouse CAMP-Dependent Protein Kinase. Proc. Natl. Acad. Sci. USA 1986, 83, 1300–1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knighton, D.R.; Zheng, J.; Ten Eyck, L.F.; Ashford, V.A.; Xuong, N.-H.; Taylor, S.S.; Sowadski, J.M. Crystal Structure of the Catalytic Subunit of Cyclic Adenosine Monophosphate-Dependent Protein Kinase. Science 1991, 253, 407–414. [Google Scholar] [CrossRef]
- Chemin, J.; Mezghrani, A.; Bidaud, I.; Dupasquier, S.; Marger, F.; Barrère, C.; Nargeot, J.; Lory, P. Temperature-Dependent Modulation of CaV3 T-Type Calcium Channels by Protein Kinases C and A in Mammalian Cells. J. Biol. Chem. 2007, 282, 32710–32718. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Wei, Y.; Pu, Y.; Jiang, D.; Jiang, X.; Zhang, Y.; Tao, J. Brain-Derived Neurotrophic Factor Stimulation of T-Type Ca2+ Channels in Sensory Neurons Contributes to Increased Peripheral Pain Sensitivity. Sci. Signal. 2019, 12, eaaw2300. [Google Scholar] [CrossRef]
- Zhang, Y.; Qian, Z.; Jiang, D.; Sun, Y.; Gao, S.; Jiang, X.; Wang, H.; Tao, J. Neuromedin B Receptor Stimulation of Cav3.2 T-Type Ca2+ Channels in Primary Sensory Neurons Mediates Peripheral Pain Hypersensitivity. Theranostics 2021, 11, 9342–9357. [Google Scholar] [CrossRef]
- Yue, J.; Zhang, Y.; Li, X.; Gong, S.; Tao, J.; Jiang, X. Activation of G-Protein-Coupled Receptor 30 Increases T-Type Calcium Currents in Trigeminal Ganglion Neurons via the Cholera Toxin-Sensitive Protein Kinase A Pathway. Pharm. 2014, 69, 804–808. [Google Scholar]
- Lenglet, S.; Louiset, E.; Delarue, C.; Vaudry, H.; Contesse, V. Involvement of T-type calcium channels in the mechanism of action of 5-HT in rat glomerulosa cells: A novel signaling pathway for the 5-HT7 receptor. Endocr. Res. 2002, 28, 651–655. [Google Scholar] [CrossRef]
- Louiset, E.; Duparc, C.; Lenglet, S.; Gomez-Sanchez, C.E.; Lefebvre, H. Role of CAMP/PKA Pathway and T-Type Calcium Channels in the Mechanism of Action of Serotonin in Human Adrenocortical Cells. Mol. Cell. Endocrinol. 2017, 441, 99–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Xu, R.; Clarke, I.J.; Ruan, M.; Loneragan, K.; Roh, S. Diverse Intracellular Signalling Systems Used by Growth Hormone-releasing Hormone in Regulating Voltage-gated Ca2+ or K + Channels in Pituitary Somatotropes. Immunol. Cell Biol. 2000, 78, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.R.; Varanda, W.A. Intracellular Calcium Changes in Mice Leydig Cells Are Dependent on Calcium Entry through T-Type Calcium Channels: T-Type Calcium Channels in Leydig Cells. J. Physiol. 2007, 585, 339–349. [Google Scholar] [CrossRef]
- Costa, R.R.; dos Reis, R.I.; Aguiar, J.F.; Varanda, W.A. Luteinizing Hormone (LH) Acts through PKA and PKC to Modulate T-Type Calcium Currents and Intracellular Calcium Transients in Mice Leydig Cells. Cell Calcium 2011, 49, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-A.; Park, J.-Y.; Kang, H.-W.; Huh, S.-U.; Jeong, S.-W.; Lee, J.-H. Augmentation of Ca v 3.2 T-Type Calcium Channel Activity by CAMP-Dependent Protein Kinase A. J. Pharmacol. Exp. Ther. 2006, 318, 230–237. [Google Scholar] [CrossRef]
- Si, W.; Zhang, Y.; Chen, K.; Hu, D.; Qian, Z.; Gong, S.; Li, H.; Hao, Y.; Tao, J. Fibroblast Growth Factor Type 1 Receptor Stimulation of T-Type Ca2+ Channels in Sensory Neurons Requires the Phosphatidylinositol 3-Kinase and Protein Kinase A Pathways, Independently of Akt. Cell. Signal. 2018, 45, 93–101. [Google Scholar] [CrossRef]
- Pemberton, K.E.; Hill-Eubanks, L.J.; Jones, S.V.P. Modulation of Low-Threshold T-Type Calcium Channels by the Five Muscarinic Receptor Subtypes in NIH 3T3 Cells. Pflüg. Arch.-Eur. J. Physiol. 2000, 440, 452–461. [Google Scholar] [CrossRef]
- Wu, H.; Xie, X.; Sun, M.; Chen, M.; Tao, X.; Fang, X.; Meng, X.; Wei, W.; Yu, M. Modification of Mesenchymal Stem Cells by HMGB1 Promotes the Activity of Cav3.2 T-Type Calcium Channel via PKA/β-Catenin/γ-Cystathionase Pathway. Stem Cell Res. Ther. 2022, 13, 4. [Google Scholar] [CrossRef]
- Pfeiffer-Linn, C.; Lasater, E.M. Dopamine Modulates in a Differential Fashion T- and L-Type Calcium Currents in Bass Retinal Horizontal Cells. J. Gen. Physiol. 1993, 102, 277–294. [Google Scholar] [CrossRef]
- Pfeiffer-Linn, C.L.; Lasater, E.M. Multiple Second-Messenger System Modulation of Voltage-Activated Calcium Currents in Teleost Retinal Horizontal Cells. J. Neurophysiol. 1998, 80, 377–388. [Google Scholar] [CrossRef] [Green Version]
- Kawai, F.; Kurahashi, T.; Kaneko, A. Adrenaline Enhances Odorant Contrast by Modulating Signal Encoding in Olfactory Receptor Cells. Nat. Neurosci. 1999, 2, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; DePuy, S.D.; Yao, J.; McIntire, W.E.; Barrett, P.Q. Protein Kinase A Activity Controls the Regulation of T-Type CaV3.2 Channels by Gβγ Dimers. J. Biol. Chem. 2009, 284, 7465–7473. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, Y.; Jiang, X.; Zhang, Y.; Zhang, L.; Gong, S.; Liu, C.; Zhou, L.; Tao, J. Neuromedin U Inhibits T-Type Ca2+ Channel Currents and Decreases Membrane Excitability in Small Dorsal Root Ganglia Neurons in Mice. Cell Calcium 2011, 49, 12–22. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Jiang, D.; Reid, P.F.; Jiang, X.; Qin, Z.; Tao, J. Alpha-Cobratoxin Inhibits T-Type Calcium Currents through Muscarinic M4 Receptor and Go-Protein Βγ Subunits-Dependent Protein Kinase A Pathway in Dorsal Root Ganglion Neurons. Neuropharmacology 2012, 62, 1062–1072. [Google Scholar] [CrossRef]
- Qian, W.-J.; Yin, N.; Gao, F.; Miao, Y.; Li, Q.; Li, F.; Sun, X.-H.; Yang, X.-L.; Wang, Z. Cannabinoid CB1 and CB2 Receptors Differentially Modulate L- and T-Type Ca2+ Channels in Rat Retinal Ganglion Cells. Neuropharmacology 2017, 124, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D.E. Calcium Signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef] [Green Version]
- Chao, A.C.; Kouyama, K.; Heist, E.K.; Dong, Y.J.; Gardner, P. Calcium- and CaMKII-Dependent Chloride Secretion Induced by the Microsomal Ca(2+)-ATPase Inhibitor 2,5-Di-(Tert-Butyl)-1,4-Hydroquinone in Cystic Fibrosis Pancreatic Epithelial Cells. J. Clin. Investig. 1995, 96, 1794–1801. [Google Scholar] [CrossRef] [Green Version]
- Lin, M.Y.; Zal, T.; Ch’en, I.L.; Gascoigne, N.R.J.; Hedrick, S.M. A Pivotal Role for the Multifunctional Calcium/Calmodulin-Dependent Protein Kinase II in T Cells: From Activation to Unresponsiveness. J. Immunol. 2005, 174, 5583–5592. [Google Scholar] [CrossRef] [Green Version]
- Ji, Y.; Zhao, W.; Li, B.; Desantiago, J.; Picht, E.; Kaetzel, M.A.; Schultz, J.E.J.; Kranias, E.G.; Bers, D.M.; Dedman, J.R. Targeted Inhibition of Sarcoplasmic Reticulum CaMKII Activity Results in Alterations of Ca2+ Homeostasis and Cardiac Contractility. Am. J. Physiol.-Heart Circ. Physiol. 2006, 290, H599–H606. [Google Scholar] [CrossRef] [Green Version]
- Lisman, J.; Yasuda, R.; Raghavachari, S. Mechanisms of CaMKII Action in Long-Term Potentiation. Nat. Rev. Neurosci. 2012, 13, 169–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, W.; Lee, J.; Chen, X.; Díaz-Alonso, J.; Zhou, J.; Pleasure, S.; Nicoll, R.A. Synaptic Memory Requires CaMKII. eLife 2021, 10, e60360. [Google Scholar] [CrossRef] [PubMed]
- Howard, T.; Greer-Short, A.; Satroplus, T.; Patel, N.; Nassal, D.; Mohler, P.J.; Hund, T.J. CaMKII-Dependent Late Na + Current Increases Electrical Dispersion and Arrhythmia in Ischemia-Reperfusion. Am. J. Physiol.-Heart Circ. Physiol. 2018, 315, H794–H801. [Google Scholar] [CrossRef] [PubMed]
- Luczak, E.D.; Wu, Y.; Granger, J.M.; Joiner, M.A.; Wilson, N.R.; Gupta, A.; Umapathi, P.; Murphy, K.R.; Reyes Gaido, O.E.; Sabet, A.; et al. Mitochondrial CaMKII Causes Adverse Metabolic Reprogramming and Dilated Cardiomyopathy. Nat. Commun. 2020, 11, 4416. [Google Scholar] [CrossRef] [PubMed]
- Pasek, J.G.; Wang, X.; Colbran, R.J. Differential CaMKII Regulation by Voltage-Gated Calcium Channels in the Striatum. Mol. Cell. Neurosci. 2015, 68, 234–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudmon, A.; Schulman, H.; Kim, J.; Maltez, J.M.; Tsien, R.W.; Pitt, G.S. CaMKII Tethers to L-Type Ca2+ Channels, Establishing a Local and Dedicated Integrator of Ca2+ Signals for Facilitation. J. Cell Biol. 2005, 171, 537–547. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.K.; Fern, R.J.; Nee, J.J.; Barrett, P.Q. Ca(2+)-Dependent Activation of T-Type Ca2+ Channels by Calmodulin-Dependent Protein Kinase II. Am. J. Physiol.-Ren. Physiol. 1994, 267, F183–F189. [Google Scholar] [CrossRef] [PubMed]
- Barrett, P.Q.; Lu, H.-K.; Colbran, R.; Czernik, A.; Pancrazio, J.J. Stimulation of Unitary T-Type Ca2+ Channel Currents by Calmodulin-Dependent Protein Kinase II. Am. J. Physiol.-Cell Physiol. 2000, 279, C1694–C1703. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, J.T.; Wang, H.; Perez-Reyes, E.; Barrett, P.Q. Stimulation of Recombinant Ca v 3.2, T-type, Ca2+ Channel Currents by CaMKIIγ C. J. Physiol. 2002, 538, 343–355. [Google Scholar] [CrossRef]
- Welsby, P.J.; Wang, H.; Wolfe, J.T.; Colbran, R.J.; Johnson, M.L.; Barrett, P.Q. A Mechanism for the Direct Regulation of T-Type Calcium Channels by Ca2+ /Calmodulin-Dependent Kinase II. J. Neurosci. 2003, 23, 10116–10121. [Google Scholar] [CrossRef] [Green Version]
- Yao, J. Molecular Basis for the Modulation of Native T-Type Ca2+ Channels in Vivo by Ca2+/Calmodulin-Dependent Protein Kinase II. J. Clin. Investig. 2006, 116, 2403–2412. [Google Scholar] [CrossRef] [PubMed]
- Moriguchi, S.; Shioda, N.; Yamamoto, Y.; Tagashira, H.; Fukunaga, K. The T-Type Voltage-Gated Calcium Channel as a Molecular Target of the Novel Cognitive Enhancer ST101: Enhancement of Long-Term Potentiation and CaMKII Autophosphorylation in Rat Cortical Slices: ST101 Enhances Cortical LTP and CaMKII Activity via T-Type VGCCs. J. Neurochem. 2012, 121, 44–53. [Google Scholar] [CrossRef]
- Xu, J.; Yabuki, Y.; Yu, M.; Fukunaga, K. T-Type Calcium Channel Enhancer SAK3 Produces Anti-Depressant-like Effects by Promoting Adult Hippocampal Neurogenesis in Olfactory Bulbectomized Mice. J. Pharmacol. Sci. 2018, 137, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Hu, Y.; Lu, Y.; Yang, Q.; Fu, D.; Chen, F.; Li, Y. CaMKII and CaV3.2 T-Type Calcium Channel Mediate Connexin-43-Dependent Inflammation by Activating Astrocytes in Vincristine-Induced Neuropathic Pain. Cell Biol. Toxicol. 2021, 1–24. [Google Scholar] [CrossRef]
- Leung, T.; Manser, E.; Tan, L.; Lim, L. A Novel Serine/Threonine Kinase Binding the Ras-Related RhoA GTPase Which Translocates the Kinase to Peripheral Membranes. J. Biol. Chem. 1995, 270, 29051–29054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endo, M.; Ohashi, K.; Sasaki, Y.; Goshima, Y.; Niwa, R.; Uemura, T.; Mizuno, K. Control of Growth Cone Motility and Morphology by LIM Kinase and Slingshot via Phosphorylation and Dephosphorylation of Cofilin. J. Neurosci. 2003, 23, 2527–2537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukata, Y.; Oshiro, N.; Kinoshita, N.; Kawano, Y.; Matsuoka, Y.; Bennett, V.; Matsuura, Y.; Kaibuchi, K. Phosphorylation of Adducin by Rho-Kinase Plays a Crucial Role in Cell Motility. J. Cell Biol. 1999, 145, 347–361. [Google Scholar] [CrossRef] [Green Version]
- Katoh, K.; Kano, Y.; Amano, M.; Onishi, H.; Kaibuchi, K.; Fujiwara, K. Rho-Kinase--Mediated Contraction of Isolated Stress Fibers. J. Cell Biol. 2001, 153, 569–584. [Google Scholar] [CrossRef]
- Leung, T.; Chen, X.Q.; Manser, E.; Lim, L. The P160 RhoA-Binding Kinase ROK Alpha Is a Member of a Kinase Family and Is Involved in the Reorganization of the Cytoskeleton. Mol. Cell. Biol. 1996, 16, 5313–5327. [Google Scholar] [CrossRef] [Green Version]
- Marlow, F.; Topczewski, J.; Sepich, D.; Solnica-Krezel, L. Zebrafish Rho Kinase 2 Acts Downstream of Wnt11 to Mediate Cell Polarity and Effective Convergence and Extension Movements. Curr. Biol. 2002, 12, 876–884. [Google Scholar] [CrossRef] [Green Version]
- Inoue, M.; Rashid, M.H.; Fujita, R.; Contos, J.J.A.; Chun, J.; Ueda, H. Initiation of Neuropathic Pain Requires Lysophosphatidic Acid Receptor Signaling. Nat. Med. 2004, 10, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Iftinca, M.; Hamid, J.; Chen, L.; Varela, D.; Tadayonnejad, R.; Altier, C.; Turner, R.W.; Zamponi, G.W. Regulation of T-Type Calcium Channels by Rho-Associated Kinase. Nat. Neurosci. 2007, 10, 854–860. [Google Scholar] [CrossRef] [PubMed]
- González-Rodríguez, P.; Falcón, D.; Castro, M.J.; Ureña, J.; López-Barneo, J.; Castellano, A. Hypoxic Induction of T-Type Ca2+ Channels in Rat Cardiac Myocytes: Role of HIF-1α and RhoA/ROCK Signalling: HIF-1α and RhoA/ROCK Signalling in Cardiac Myocytes. J. Physiol. 2015, 593, 4729–4745. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, D.; Wang, F.; Shi, S.; Chen, Y.; Yang, B.; Tang, Y.; Huang, C. Exendin-4 Inhibits Structural Remodeling and Improves Ca2+ Homeostasis in Rats with Heart Failure via the GLP-1 Receptor through the ENOS/CGMP/PKG Pathway. Peptides 2017, 90, 69–77. [Google Scholar] [CrossRef]
- Krill, J.L.; Dawson-Scully, K. Characterization of a Novel Stimulus-Induced Glial Calcium Wave in Drosophila Larval Peripheral Segmental Nerves and Its Role in PKG-Modulated Thermoprotection. J. Neurogenet. 2021, 35, 221–235. [Google Scholar] [CrossRef]
- Weinmeister, P.; Lukowski, R.; Linder, S.; Traidl-Hoffmann, C.; Hengst, L.; Hofmann, F.; Feil, R. Cyclic Guanosine Monophosphate-Dependent Protein Kinase I Promotes Adhesion of Primary Vascular Smooth Muscle Cells. Mol. Biol. Cell 2008, 19, 4434–4441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, L.S.; Elbatarny, H.S.; Crawley, S.W.; Bennett, B.M.; Maurice, D.H. Compartmentation and Compartment-Specific Regulation of PDE5 by Protein Kinase G Allows Selective CGMP-Mediated Regulation of Platelet Functions. Proc. Natl. Acad. Sci. USA 2008, 105, 13650–13655. [Google Scholar] [CrossRef] [Green Version]
- Schramm, A.; Schweda, F.; Sequeira-Lopez, M.L.S.; Hofmann, F.; Sandner, P.; Schlossmann, J. Protein Kinase G Is Involved in Acute but Not in Long-Term Regulation of Renin Secretion. Front. Pharmacol. 2019, 10, 800. [Google Scholar] [CrossRef] [Green Version]
- Harraz, O.F.; Brett, S.E.; Welsh, D.G. Nitric Oxide Suppresses Vascular Voltage-Gated T-Type Ca2+ Channels through CGMP/PKG Signaling. Am. J. Physiol.-Heart Circ. Physiol. 2014, 306, H279–H285. [Google Scholar] [CrossRef] [Green Version]
- Samuel, S.; Zhang, K.; Tang, Y.-D.; Gerdes, A.M.; Carrillo-Sepulveda, M.A. Triiodothyronine Potentiates Vasorelaxation via PKG/VASP Signaling in Vascular Smooth Muscle Cells. Cell. Physiol. Biochem. 2017, 41, 1894–1904. [Google Scholar] [CrossRef] [Green Version]
- Buisson, B.; Laflamme, L.; Bottari, S.P.; de Gasparo, M.; Gallo-Payet, N.; Payet, M.D. A G Protein Is Involved in the Angiotensin AT2 Receptor Inhibition of the T-Type Calcium Current in Non-Differentiated NG108-15 Cells. J. Biol. Chem. 1995, 270, 1670–1674. [Google Scholar] [CrossRef] [Green Version]
- Kawai, F.; Miyachi, E. Modulation by CGMP of the Voltage-Gated Currents in Newt Olfactory Receptor Cells. Neurosci. Res. 2001, 39, 327–337. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Y.; Wu, N.; Yin, N.; Sun, X.-H.; Wang, Z. Activation of Somatostatin Receptor 5 Suppresses T-Type Ca2+ Channels through NO/CGMP/PKG Signaling Pathway in Rat Retinal Ganglion Cells. Neurosci. Lett. 2019, 708, 134337. [Google Scholar] [CrossRef]
- Bkaily, G.; Sculptoreanu, A.; Wang, S.; Nader, M.; Hazzouri, K.M.; Jacques, D.; Regoli, D.; D’Orleans-Juste, P.; Avedanian, L. Angiotensin II-Induced Increase of T-Type Ca2+ Current and Decrease of L-Type Ca2+ Current in Heart Cells. Peptides 2005, 26, 1410–1417. [Google Scholar] [CrossRef]
- Doerner, D.; Pitler, T.; Alger, B. Protein Kinase C Activators Block Specific Calcium and Potassium Current Components in Isolated Hippocampal Neurons. J. Neurosci. 1988, 8, 4069–4078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, M.; Wang, Y.; Kang, L.; Shimaoka, T.; Marni, F.; Ono, K. Intracellular Ca2+- and PKC-Dependent Upregulation of T-Type Ca2+ Channels in LPC-Stimulated Cardiomyocytes. J. Mol. Cell. Cardiol. 2010, 48, 131–139. [Google Scholar] [CrossRef]
- Lu, X.; Bean, J.S.; Kassab, G.S.; Rekhter, M.D. Protein Kinase C Inhibition Ameliorates Functional Endothelial Insulin Resistance and Vascular Smooth Muscle Cell Hypersensitivity to Insulin in Diabetic Hypertensive Rats. Cardiovasc. Diabetol. 2011, 10, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furukawa, T.; Ito, H.; Nitta, J.; Tsujino, M.; Adachi, S.; Hiroe, M.; Marumo, F.; Sawanobori, T.; Hiraoka, M. Endothelin-1 Enhances Calcium Entry through T-Type Calcium Channels in Cultured Neonatal Rat Ventricular Myocytes. Circ. Res. 1992, 71, 1242–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.-Y.; Kang, H.-W.; Moon, H.-J.; Huh, S.-U.; Jeong, S.-W.; Soldatov, N.M.; Lee, J.-H. Activation of Protein Kinase C Augments T-Type Ca2+ Channel Activity without Changing Channel Surface Density. J. Physiol. 2006, 577, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-Y.; Jeong, S.-W.; Perez-Reyes, E.; Lee, J.-H. Modulation of Ca v 3.2 T-Type Ca2+ Channels by Protein Kinase C. FEBS Lett. 2003, 547, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Herrington, J.; Lingle, C.J. Kinetic and Pharmacological Properties of Low Voltage-Activated Ca2+ Current in Rat Clonal (GH3) Pituitary Cells. J. Neurophysiol. 1992, 68, 213–232. [Google Scholar] [CrossRef]
- Hockberger, P.; Toselli, M.; Swandulla, D.; Lux, H.D. A Diacylglycerol Analogue Reduces Neuronal Calcium Currents Independently of Protein Kinase C Activation. Nature 1989, 338, 340–342. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, C.; Brown, A.M. Protein Kinase Activator 1-Oleoyl-2-Acetyl-Sn-Glycerol Inhibits Two Types of Calcium Currents in GH3 Cells. Am. J. Physiol.-Cell Physiol. 1988, 254, C206–C210. [Google Scholar] [CrossRef]
- Schroeder, J.; Fischbach, P.; McCleskey, E. T-Type Calcium Channels: Heterogeneous Expression in Rat Sensory Neurons and Selective Modulation by Phorbol Esters. J. Neurosci. 1990, 10, 947–951. [Google Scholar] [CrossRef] [PubMed]
- Toselli, M.; Lux, H.D. Opposing Effects of Acetylcholine on the Two Classes of Voltage-Dependent Calcium Channels in Hippocampal Neurons. In Central Cholinergic Synaptic Transmission; Frotscher, M., Misgeld, U., Eds.; Experientia Supplementum; Birkhäuser Basel: Basel, Switzerland, 1989; Volume 57, pp. 97–103. ISBN 978-3-0348-9922-2. [Google Scholar]
- Tseng, G.N.; Boyden, P.A. Different Effects of Intracellular Ca and Protein Kinase C on Cardiac T and L Ca Currents. Am. J. Physiol.-Heart Circ. Physiol. 1991, 261, H364–H379. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Wang, F.; Zhang, Y.; Wang, J.; Qin, Z.; Jiang, X.; Tao, J. Activation of M3 Muscarinic Receptors Inhibits T-Type Ca2+ Channel Currents via Pertussis Toxin-Sensitive Novel Protein Kinase C Pathway in Small Dorsal Root Ganglion Neurons. Cell. Signal. 2011, 23, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- Rossier, M.F.; Aptel, H.B.C.; Python, C.P.; Burnay, M.M.; Vallotton, M.B.; Capponi, A.M. Inhibition of Low Threshold Calcium Channels by Angiotensin II in Adrenal Glomerulosa Cells through Activation of Protein Kinase C. J. Biol. Chem. 1995, 270, 15137–15142. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Park, M.K.; Uhm, D.-Y.; Chung, S. Modulation of T-Type Ca2+ Channels by Corticotropin-Releasing Factor through Protein Kinase C Pathway in MN9D Dopaminergic Cells. Biochem. Biophys. Res. Commun. 2007, 358, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Tai, C.-H.; Yang, Y.-C.; Pan, M.-K.; Huang, C.-S.; Kuo, C.-C. Modulation of Subthalamic T-Type Ca2+ Channels Remedies Locomotor Deficits in a Rat Model of Parkinson Disease. J. Clin. Investig. 2011, 121, 3289–3305. [Google Scholar] [CrossRef]
- Rangel, A.; Sánchez-Armass, S.; Meza, U. Protein Kinase C-Mediated Inhibition of Recombinant T-Type Ca V 3.2 Channels by Neurokinin 1 Receptors. Mol. Pharmacol. 2010, 77, 202–210. [Google Scholar] [CrossRef]
- Shan, H.Q.; Hammarback, J.A.; Godwin, D.W. Ethanol Inhibition of a T-Type Ca2+ Channel Through Activity of Protein Kinase C. Alcohol. Clin. Exp. Res. 2013, 37, 1333–1342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Morishima, M.; Li, D.; Takahashi, N.; Saikawa, T.; Nattel, S.; Ono, K. Binge Alcohol Exposure Triggers Atrial Fibrillation Through T-Type Ca2+ Channel Upregulation via Protein Kinase C (PKC)/Glycogen Synthesis Kinase 3β (GSK3β)/Nuclear Factor of Activated T-Cells (NFAT) Signaling—An Experimental Account of Holiday Heart Syndrome. Circ. J. 2020, 84, 1931–1940. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ji, H.; Wang, J.; Sun, Y.; Qian, Z.; Jiang, X.; Snutch, T.P.; Sun, Y.; Tao, J. Melatonin-Mediated Inhibition of Cav3.2 T-Type Ca2+ Channels Induces Sensory Neuronal Hypoexcitability through the Novel Protein Kinase C-Eta Isoform. J. Pineal Res. 2018, 64, e12476. [Google Scholar] [CrossRef]
- Hellmich, M.R.; Pant, H.C.; Wada, E.; Battey, J.F. Neuronal Cdc2-like Kinase: A Cdc2-Related Protein Kinase with Predominantly Neuronal Expression. Proc. Natl. Acad. Sci. USA 1992, 89, 10867–10871. [Google Scholar] [CrossRef] [Green Version]
- Lew, J.; Beaudette, K.; Litwin, C.M.; Wang, J.H. Purification and Characterization of a Novel Proline-Directed Protein Kinase from Bovine Brain. J. Biol. Chem. 1992, 267, 13383–13390. [Google Scholar] [CrossRef]
- Meyerson, M.; Enders, G.H.; Wu, C.L.; Su, L.K.; Gorka, C.; Nelson, C.; Harlow, E.; Tsai, L.H. A Family of Human Cdc2-Related Protein Kinases. EMBO J. 1992, 11, 2909–2917. [Google Scholar] [CrossRef] [PubMed]
- Kesavapany, S.; Li, B.-S.; Pant, H.C. Cyclin-Dependent Kinase 5 in Neurofilament Function and Regulation. Neurosignals 2003, 12, 252–264. [Google Scholar] [CrossRef]
- Lai, K.-O.; Ip, N. Cdk5: A Key Player at Neuronal Synapse with Diverse Functions. Mini-Rev. Med. Chem. 2015, 15, 390–395. [Google Scholar] [CrossRef]
- Pao, P.-C.; Tsai, L.-H. Three Decades of Cdk5. J. Biomed. Sci. 2021, 28, 79. [Google Scholar] [CrossRef]
- Su, S.C.; Tsai, L.-H. Cyclin-Dependent Kinases in Brain Development and Disease. Annu. Rev. Cell Dev. Biol. 2011, 27, 465–491. [Google Scholar] [CrossRef]
- Calderón-Rivera, A.; Sandoval, A.; González-Ramírez, R.; González-Billault, C.; Felix, R. Regulation of Neuronal Cav3.1 Channels by Cyclin-Dependent Kinase 5 (Cdk5). PLoS ONE 2015, 10, e0119134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez, K.; Calderón-Rivera, A.; Sandoval, A.; González-Ramírez, R.; Vargas-Parada, A.; Ojeda-Alonso, J.; Granados-Soto, V.; Delgado-Lezama, R.; Felix, R. Cdk5-Dependent Phosphorylation of Ca V 3.2 T-Type Channels: Possible Role in Nerve Ligation-Induced Neuropathic Allodynia and the Compound Action Potential in Primary Afferent C Fibers. J. Neurosci. 2020, 40, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, S.R.; Till, J.H. Protein Tyrosine Kinase Structure and Function. Annu. Rev. Biochem. 2000, 69, 373–398. [Google Scholar] [CrossRef] [PubMed]
- Arnoult, C.; Lemos, J.R.; Florman, H.M. Voltage-Dependent Modulation of T-Type Calcium Channels by Protein Tyrosine Phosphorylation. EMBO J. 1997, 16, 1593–1599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morikawa, H.; Fukuda, K.; Mima, H.; Shoda, T.; Kato, S.; Mori, K. Tyrosine Kinase Inhibitors Suppress N-Type and T-Type Ca2+ Channel Currents in NG108-15 Cells. Pflugers Arch. Eur. J. Physiol. 1998, 436, 127–132. [Google Scholar] [CrossRef]
- Tao, J.; Zhang, Y.; Li, S.; Sun, W.; Soong, T.W. Tyrosine Kinase-Independent Inhibition by Genistein on Spermatogenic T-Type Calcium Channels Attenuates Mouse Sperm Motility and Acrosome Reaction. Cell Calcium 2009, 45, 133–143. [Google Scholar] [CrossRef]
- Kurejová, M.; Lacinová, L. Effect of Protein Tyrosine Kinase Inhibitors on the Current through the CaV3.1 Channel. Arch. Biochem. Biophys. 2006, 446, 20–27. [Google Scholar] [CrossRef]
- Lau, K.H.W.; Farley, J.R.; Baylink, D.J. Phosphotyrosyl Protein Phosphatases. Biochem. J. 1989, 257, 23–36. [Google Scholar] [CrossRef]
- Klee, C.B.; Ren, H.; Wang, X. Regulation of the Calmodulin-Stimulated Protein Phosphatase, Calcineurin. J. Biol. Chem. 1998, 273, 13367–13370. [Google Scholar] [CrossRef] [Green Version]
- Rusnak, F.; Mertz, P. Calcineurin: Form and Function. Physiol. Rev. 2000, 80, 1483–1521. [Google Scholar] [CrossRef]
- Park, Y.-J.; Yoo, S.-A.; Kim, M.; Kim, W.-U. The Role of Calcium–Calcineurin–NFAT Signaling Pathway in Health and Autoimmune Diseases. Front. Immunol. 2020, 11, 195. [Google Scholar] [CrossRef] [PubMed]
- Creamer, T.P. Calcineurin. Cell Commun. Signal. 2020, 18, 137. [Google Scholar] [CrossRef] [PubMed]
- Hemenway, C.S.; Heitman, J. Calcineurin: Structure, Function, and Inhibition. Cell Biochem. Biophys. 1999, 30, 115–151. [Google Scholar] [CrossRef]
- Jorgensen, K.A.; Koefoed-Nielsen, P.B.; Karamperis, N. Calcineurin Phosphatase Activity and Immunosuppression. A Review on the Role of Calcineurin Phosphatase Activity and the Immunosuppressive Effect of Cyclosporin A and Tacrolimus. Scand. J. Immunol. 2003, 57, 93–98. [Google Scholar] [CrossRef]
- Perrino, B.A.; Wilson, A.J.; Ellison, P.; Clapp, L.H. Substrate Selectivity and Sensitivity to Inhibition by FK506 and Cyclosporin A of Calcineurin Heterodimers Composed of the α or β Catalytic Subunit: Enzymatic Characteristics of Calcineurin Isoforms. Eur. J. Biochem. 2002, 269, 3540–3548. [Google Scholar] [CrossRef]
- Huang, C.-H.; Chen, Y.-C.; Chen, C.-C. Physical Interaction between Calcineurin and Cav3.2 T-type Ca2+ Channel Modulates Their Functions. FEBS Lett. 2013, 587, 1723–1730. [Google Scholar] [CrossRef] [Green Version]
- Tomono, M.; Toyoshima, K.; Ito, M.; Amano, H.; Kiss, Z. Inhibitors of Calcineurin Block Expression of Cyclins A and E Induced by Fibroblast Growth Factor in Swiss 3T3 Fibroblasts. Arch. Biochem. Biophys. 1998, 353, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Hogan, P.G.; Chen, L.; Nardone, J.; Rao, A. Transcriptional Regulation by Calcium, Calcineurin, and NFAT. Genes Dev. 2003, 17, 2205–2232. [Google Scholar] [CrossRef] [Green Version]
- Najafi, M.; Ahmadi, A.; Mortezaee, K. Extracellular-signal-regulated Kinase/Mitogen-activated Protein Kinase Signaling as a Target for Cancer Therapy: An Updated Review. Cell Biol. Int. 2019, 43, 1206–1222. [Google Scholar] [CrossRef]
- Papa, S.; Choy, P.M.; Bubici, C. The ERK and JNK Pathways in the Regulation of Metabolic Reprogramming. Oncogene 2019, 38, 2223–2240. [Google Scholar] [CrossRef] [Green Version]
- Abe, M.K.; Saelzler, M.P.; Espinosa, R.; Kahle, K.T.; Hershenson, M.B.; Le Beau, M.M.; Rosner, M.R. ERK8, a New Member of the Mitogen-Activated Protein Kinase Family. J. Biol. Chem. 2002, 277, 16733–16743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colecchia, D.; Dapporto, F.; Tronnolone, S.; Salvini, L.; Chiariello, M. MAPK15 Is Part of the ULK Complex and Controls Its Activity to Regulate Early Phases of the Autophagic Process. J. Biol. Chem. 2018, 293, 15962–15976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, M.; Colecchia, D.; Ilardi, G.; Acunzo, M.; Nigita, G.; Sasdelli, F.; Celetti, A.; Strambi, A.; Staibano, S.; Croce, C.M.; et al. MAPK15 Upregulation Promotes Cell Proliferation and Prevents DNA Damage in Male Germ Cell Tumors. Oncotarget 2016, 7, 20981–20998. [Google Scholar] [CrossRef] [PubMed]
- Strobeck, M.W.; Okuda, M.; Yamaguchi, H.; Schwartz, A.; Fukasawa, K. Morphological Transformation Induced by Activation of the Mitogen-Activated Protein Kinase Pathway Requires Suppression of the T-Type Ca2+ Channel. J. Biol. Chem. 1999, 274, 15694–15700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mor, M.; Beharier, O.; Levy, S.; Kahn, J.; Dror, S.; Blumenthal, D.; Gheber, L.A.; Peretz, A.; Katz, A.; Moran, A.; et al. ZnT-1 Enhances the Activity and Surface Expression of T-Type Calcium Channels through Activation of Ras-ERK Signaling. Am. J. Physiol.-Cell Physiol. 2012, 303, C192–C203. [Google Scholar] [CrossRef] [Green Version]
- Varjosalo, M.; Keskitalo, S.; Van Drogen, A.; Nurkkala, H.; Vichalkovski, A.; Aebersold, R.; Gstaiger, M. The Protein Interaction Landscape of the Human CMGC Kinase Group. Cell Rep. 2013, 3, 1306–1320. [Google Scholar] [CrossRef] [Green Version]
- Peres de Oliveira, A.; Kazuo Issayama, L.; Betim Pavan, I.C.; Riback Silva, F.; Diniz Melo-Hanchuk, T.; Moreira Simabuco, F.; Kobarg, J. Checking NEKs: Overcoming a Bottleneck in Human Diseases. Molecules 2020, 25, 1778. [Google Scholar] [CrossRef]
- Fry, A.M.; Nigg, E.A. Cell Cycle: The NIMA Kinase Joins Forces with Cdc2. Curr. Biol. 1995, 5, 1122–1125. [Google Scholar] [CrossRef] [Green Version]
- O’Connell, M.J.; Krien, M.J.E.; Hunter, T. Never Say Never. The NIMA-Related Protein Kinases in Mitotic Control. Trends Cell Biol. 2003, 13, 221–228. [Google Scholar] [CrossRef]
- Basei, F.L.; Meirelles, G.V.; Righetto, G.L.; dos Santos Migueleti, D.L.; Smetana, J.H.C.; Kobarg, J. New Interaction Partners for Nek4.1 and Nek4.2 Isoforms: From the DNA Damage Response to RNA Splicing. Proteome Sci. 2015, 13, 11. [Google Scholar] [CrossRef] [Green Version]
- Antal, L.; Martin-Caraballo, M. T-Type Calcium Channels in Cancer. Cancers 2019, 11, 134. [Google Scholar] [CrossRef] [Green Version]
- Capalbo, L.; Bassi, Z.I.; Geymonat, M.; Todesca, S.; Copoiu, L.; Enright, A.J.; Callaini, G.; Riparbelli, M.G.; Yu, L.; Choudhary, J.S.; et al. The Midbody Interactome Reveals Unexpected Roles for PP1 Phosphatases in Cytokinesis. Nat. Commun. 2019, 10, 4513. [Google Scholar] [CrossRef] [Green Version]
- Madaule, P.; Eda, M.; Watanabe, N.; Fujisawa, K.; Matsuoka, T.; Bito, H.; Ishizaki, T.; Narumiya, S. Role of Citron Kinase as a Target of the Small GTPase Rho in Cytokinesis. Nature 1998, 394, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Basit, S.; Al-Harbi, K.M.; Alhijji, S.A.M.; Albalawi, A.M.; Alharby, E.; Eldardear, A.; Samman, M.I. CIT, a Gene Involved in Neurogenic Cytokinesis, Is Mutated in Human Primary Microcephaly. Hum. Genet. 2016, 135, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Zhang, B.; Lu, W.; Peng, L.; Yang, Q.; Cao, W.; Lin, S.; Yu, W.; Li, X.; Ke, Y.; et al. Increased Src Family Kinase Activity Disrupts Excitatory Synaptic Transmission and Impairs Remote Fear Memory in Forebrain Shp2-Deficient Mice. Mol. Neurobiol. 2017, 54, 7235–7250. [Google Scholar] [CrossRef] [PubMed]
- Stateva, S.R.; Salas, V.; Anguita, E.; Benaim, G.; Villalobo, A. Ca2+/Calmodulin and Apo-Calmodulin Both Bind to and Enhance the Tyrosine Kinase Activity of c-Src. PLoS ONE 2015, 10, e0128783. [Google Scholar] [CrossRef] [Green Version]
- Nagasawa, K.; Tarui, T.; Yoshida, S.; Sekiguchi, F.; Matsunami, M.; Ohi, A.; Fukami, K.; Ichida, S.; Nishikawa, H.; Kawabata, A. Hydrogen Sulfide Evokes Neurite Outgrowth and Expression of High-Voltage-Activated Ca2+ Currents in NG108-15 Cells: Involvement of T-Type Ca2+ Channels. J. Neurochem. 2009, 108, 676–684. [Google Scholar] [CrossRef]
- Tarui, T.; Fukami, K.; Nagasawa, K.; Yoshida, S.; Sekiguchi, F.; Kawabata, A. Involvement of Src Kinase in T-Type Calcium Channel-Dependent Neuronal Differentiation of NG108-15 Cells by Hydrogen Sulfide: Src-Dependent Neuronal Differentiation by H2S. J. Neurochem. 2010, 114, 512–519. [Google Scholar] [CrossRef]
- Lee, J.-O.; Yang, H.; Georgescu, M.-M.; Di Cristofano, A.; Maehama, T.; Shi, Y.; Dixon, J.E.; Pandolfi, P.; Pavletich, N.P. Crystal Structure of the PTEN Tumor Suppressor. Cell 1999, 99, 323–334. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-Y.; Chen, J.; He, L.; Stiles, B.L. PTEN: Tumor Suppressor and Metabolic Regulator. Front. Endocrinol. 2018, 9, 338. [Google Scholar] [CrossRef] [Green Version]
- Fusco, N.; Sajjadi, E.; Venetis, K.; Gaudioso, G.; Lopez, G.; Corti, C.; Guerini Rocco, E.; Criscitiello, C.; Malapelle, U.; Invernizzi, M. PTEN Alterations and Their Role in Cancer Management: Are We Making Headway on Precision Medicine? Genes 2020, 11, 719. [Google Scholar] [CrossRef] [PubMed]
- Barceló, C.; Sisó, P.; Maiques, O.; García-Mulero, S.; Sanz-Pamplona, R.; Navaridas, R.; Megino, C.; Felip, I.; Urdanibia, I.; Eritja, N.; et al. T-Type Calcium Channels as Potential Therapeutic Targets in Vemurafenib-Resistant BRAFV600E Melanoma. J. Investig. Dermatol. 2020, 140, 1253–1265. [Google Scholar] [CrossRef] [PubMed]
- Huc, S.; Monteil, A.; Bidaud, I.; Barbara, G.; Chemin, J.; Lory, P. Regulation of T-Type Calcium Channels: Signalling Pathways and Functional Implications. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2009, 1793, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Alonso, J.L.; Bhargava, A.; O’Hara, T.; Glukhov, A.V.; Schobesberger, S.; Bhogal, N.; Sikkel, M.B.; Mansfield, C.; Korchev, Y.E.; Lyon, A.R.; et al. Microdomain-Specific Modulation of L-Type Calcium Channels Leads to Triggered Ventricular Arrhythmia in Heart Failure. Circ. Res. 2016, 119, 944–955. [Google Scholar] [CrossRef] [PubMed]



| T-Type Ca2+ Channel Isoform | Location | Reference |
|---|---|---|
| Cav3.1 | Brain and Peripheral nervous system | [26] |
| Ovary and Placenta | [27] | |
| Heart | [28] | |
| Pancreatic islets and Beta cells | [29,30] | |
| Adrenal medulla | [31] | |
| Cav3.2 | Brain and Peripheral nervous system | [26,32] |
| Heart | [28,33] | |
| Pancreatic islets and Beta cells | [30,34] | |
| Kidney and liver | [35] | |
| Adrenal cortex | [36] | |
| Cav3.3 | Brain and Peripheral nervous system | [26,37] |
| Pancreatic islets | [30,34] | |
| Cardiac Purkinje fibers | [38] |
| T-Type Ca2+ Channel Isoform | Roles | Reference |
|---|---|---|
| Cav3.1 (Encoded by CACNA1G) | CACNA1G as a tumor suppressor gene | [39,40] |
| Cav3.1 gain-of-function mutations are associated with childhood-onset cerebellar atrophy. | [41] | |
| Cav3.1, Cav3.2 & Cav3.3 | Cav3.1, Cav3.2 & Cav3.3 are involved in the cancer cell proliferation across several cancer types, such as oral squamous cell carcinoma, melanoma, ovarian cancer, colorectal cancer, glioblastoma, neuroendocrine tumors, and esophageal cancer cells as shown by the pharmacological blockade. | [10,42,43,44,45,46,47,48] |
| Cav3.2 (Encoded by CACNA1H) | CACNA1H mutations are associated with amyotrophic lateral sclerosis and Primary Aldosteronism | [49,50] |
| Cav3.2 may be a potential differential biomarker for survival and treatment response in specific breast cancer subtypes | [51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, A.; Rahman, G.; Gorelik, J.; Bhargava, A. Voltage-Gated T-Type Calcium Channel Modulation by Kinases and Phosphatases: The Old Ones, the New Ones, and the Missing Ones. Cells 2023, 12, 461. https://doi.org/10.3390/cells12030461
Sharma A, Rahman G, Gorelik J, Bhargava A. Voltage-Gated T-Type Calcium Channel Modulation by Kinases and Phosphatases: The Old Ones, the New Ones, and the Missing Ones. Cells. 2023; 12(3):461. https://doi.org/10.3390/cells12030461
Chicago/Turabian StyleSharma, Ankush, Ghazala Rahman, Julia Gorelik, and Anamika Bhargava. 2023. "Voltage-Gated T-Type Calcium Channel Modulation by Kinases and Phosphatases: The Old Ones, the New Ones, and the Missing Ones" Cells 12, no. 3: 461. https://doi.org/10.3390/cells12030461