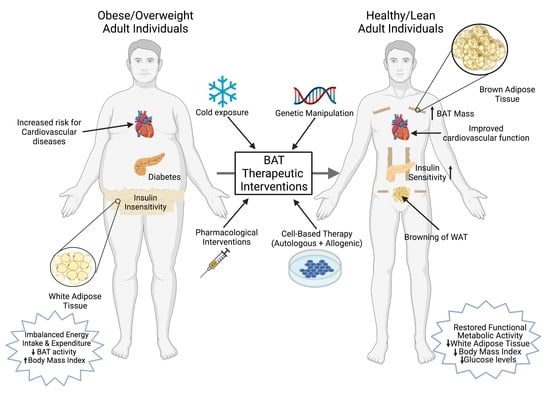
Human Brown Adipose Tissue and Metabolic Health: Potential for Therapeutic Avenues
Abstract
:1. Introduction
2. Imaging Studies for Human Brown Adipose Tissue Detection
3. Sexual Dimorphisms in Rodent and Human Thermogenic Adipose Tissue
4. Aging-Induced Changes in Beige and Brown Adipocytes
5. Molecular Gene Signature of Brown and Beige Adipocytes in Rodents and Humans
6. Cold Exposure and Activation of Sympathetic Nervous System
7. WAT Browning Factors
8. Exercise
9. MicroRNAs in Browning
10. Challenges and Potential Concerns with BAT Activation as a Therapeutic Strategy to Combat Obesity
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, Obesity, and Mortality from Cancer in a Prospectively Studied Cohort of U.S. Adults. N. Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heymsfield, S.B.; Wadden, T.A. Mechanisms, Pathophysiology, and Management of Obesity. N. Engl. J. Med. 2017, 376, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M. Drug Therapy of the Metabolic Syndrome: Minimizing the Emerging Crisis in Polypharmacy. Nat. Rev. Drug. Discov. 2006, 5, 295–309. [Google Scholar] [CrossRef]
- Kajimura, S.; Saito, M. A New Era in Brown Adipose Tissue Biology: Molecular Control of Brown Fat Development and Energy Homeostasis. Annu. Rev. Physiol. 2014, 76, 225–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez, A.; Becerril, S.; Ezquerro, S.; Méndez-Giménez, L.; Frühbeck, G. Crosstalk between Adipokines and Myokines in Fat Browning. Acta Physiol. 2017, 219, 362–381. [Google Scholar] [CrossRef] [PubMed]
- Cypess, A.M.; Lehman, S.; Williams, G.; Tal, I.; Rodman, D.; Goldfine, A.B.; Kuo, F.C.; Palmer, E.L.; Tseng, Y.-H.; Doria, A.; et al. Identification and Importance of Brown Adipose Tissue in Adult Humans. N. Engl. J. Med. 2009, 360, 1509–1517. [Google Scholar] [CrossRef] [Green Version]
- van Marken Lichtenbelt, W.D.; Vanhommerig, J.W.; Smulders, N.M.; Drossaerts, J.M.A.F.L.; Kemerink, G.J.; Bouvy, N.D.; Schrauwen, P.; Teule, G.J.J. Cold-Activated Brown Adipose Tissue in Healthy Men. N. Engl. J. Med. 2009, 360, 1500–1508. [Google Scholar] [CrossRef] [Green Version]
- Virtanen, K.A.; Lidell, M.E.; Orava, J.; Heglind, M.; Westergren, R.; Niemi, T.; Taittonen, M.; Laine, J.; Savisto, N.-J.; Enerbäck, S.; et al. Functional Brown Adipose Tissue in Healthy Adults. N. Engl. J. Med. 2009, 360, 1518–1525. [Google Scholar] [CrossRef]
- Hondares, E.; Iglesias, R.; Giralt, A.; Gonzalez, F.J.; Giralt, M.; Mampel, T.; Villarroya, F. Thermogenic Activation Induces FGF21 Expression and Release in Brown Adipose Tissue. J. Biol. Chem. 2011, 286, 12983–12990. [Google Scholar] [CrossRef] [Green Version]
- Chartoumpekis, D.V.; Habeos, I.G.; Ziros, P.G.; Psyrogiannis, A.I.; Kyriazopoulou, V.E.; Papavassiliou, A.G. Brown Adipose Tissue Responds to Cold and Adrenergic Stimulation by Induction of FGF21. Mol. Med. 2011, 17, 736–740. [Google Scholar] [CrossRef]
- Oka, M.; Kobayashi, N.; Matsumura, K.; Nishio, M.; Nakano, K.; Okamura, T.; Okochi, H.; Minamisawa, T.; Shiba, K.; Saeki, K. New Role for Growth/Differentiation Factor 15 in the Survival of Transplanted Brown Adipose Tissues in Cooperation with Interleukin-6. Cells 2020, 9, 1365. [Google Scholar] [CrossRef] [PubMed]
- Whittle, A.J.; Carobbio, S.; Martins, L.; Slawik, M.; Hondares, E.; Vázquez, M.J.; Morgan, D.; Csikasz, R.I.; Gallego, R.; Rodriguez-Cuenca, S.; et al. BMP8B Increases Brown Adipose Tissue Thermogenesis through Both Central and Peripheral Actions. Cell 2012, 149, 871–885. [Google Scholar] [CrossRef] [Green Version]
- Stanford, K.I.; Lynes, M.D.; Takahashi, H.; Baer, L.A.; Arts, P.J.; May, F.J.; Lehnig, A.C.; Middelbeek, R.J.W.; Richard, J.J.; So, K.; et al. 12,13-DiHOME: An Exercise-Induced Lipokine That Increases Skeletal Muscle Fatty Acid Uptake. Cell Metab. 2018, 27, 1111–1120. [Google Scholar] [CrossRef] [Green Version]
- Leiria, L.O.; Wang, C.-H.; Lynes, M.D.; Yang, K.; Shamsi, F.; Sato, M.; Sugimoto, S.; Chen, E.Y.; Bussberg, V.; Narain, N.R.; et al. 12-Lipoxygenase Regulates Cold Adaptation and Glucose Metabolism by Producing the Omega-3 Lipid 12-HEPE from Brown Fat. Cell Metab. 2019, 30, 768–783. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zheng, Z.; Zhu, X.; Meng, M.; Li, L.; Shen, Y.; Chi, Q.; Wang, D.; Zhang, Z.; Li, C.; et al. Brown Adipose Tissue Transplantation Improves Whole-Body Energy Metabolism. Cell Res. 2013, 23, 851–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanford, K.I.; Middelbeek, R.J.W.; Townsend, K.L.; An, D.; Nygaard, E.B.; Hitchcox, K.M.; Markan, K.R.; Nakano, K.; Hirshman, M.F.; Tseng, Y.-H.; et al. Brown Adipose Tissue Regulates Glucose Homeostasis and Insulin Sensitivity. J. Clin. Investig. 2013, 123, 215–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishio, M.; Yoneshiro, T.; Nakahara, M.; Suzuki, S.; Saeki, K.; Hasegawa, M.; Kawai, Y.; Akutsu, H.; Umezawa, A.; Yasuda, K.; et al. Production of Functional Classical Brown Adipocytes from Human Pluripotent Stem Cells Using Specific Hemopoietin Cocktail without Gene Transfer. Cell Metab. 2012, 16, 394–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becher, T.; Palanisamy, S.; Kramer, D.J.; Eljalby, M.; Marx, S.J.; Wibmer, A.G.; Butler, S.D.; Jiang, C.S.; Vaughan, R.; Schöder, H.; et al. Brown Adipose Tissue Is Associated with Cardiometabolic Health. Nat. Med. 2021, 27, 58–65. [Google Scholar] [CrossRef]
- Dong, M.; Yang, X.; Lim, S.; Cao, Z.; Honek, J.; Lu, H.; Zhang, C.; Seki, T.; Hosaka, K.; Wahlberg, E.; et al. Cold Exposure Promotes Atherosclerotic Plaque Growth and Instability via UCP1-Dependent Lipolysis. Cell Metab. 2013, 18, 118–129. [Google Scholar] [CrossRef] [Green Version]
- Petruzzelli, M.; Wagner, E.F. Mechanisms of Metabolic Dysfunction in Cancer-Associated Cachexia. Genes Dev. 2016, 30, 489–501. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Parveen, M.; Basgen, J.M.; Fazel, S.; Meshesha, M.F.; Thames, E.C.; Moore, B.; Martinez, L.; Howard, C.B.; Vergnes, L.; et al. Increased Expression of Beige/Brown Adipose Markers from Host and Breast Cancer Cells Influence Xenograft Formation in Mice. Mol. Cancer Res. 2016, 14, 78–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heaton, J.M. The Distribution of Brown Adipose Tissue in the Human. J. Anat. 1972, 112, 35–39. [Google Scholar]
- Saito, M.; Okamatsu-Ogura, Y.; Matsushita, M.; Watanabe, K.; Yoneshiro, T.; Nio-Kobayashi, J.; Iwanaga, T.; Miyagawa, M.; Kameya, T.; Nakada, K.; et al. High Incidence of Metabolically Active Brown Adipose Tissue in Healthy Adult Humans: Effects of Cold Exposure and Adiposity. Diabetes 2009, 58, 1526–1531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sampath, S.C.; Sampath, S.C.; Bredella, M.A.; Cypess, A.M.; Torriani, M. Imaging of Brown Adipose Tissue: State of the Art. Radiology 2016, 280, 4–19. [Google Scholar] [CrossRef] [Green Version]
- Ma, S.W.Y.; Foster, D.O. Uptake of Glucose and Release of Fatty Acids and Glycerol by Rat Brown Adipose Tissue in Vivo. Can. J. Physiol. Pharmacol. 1986, 64, 609–614. [Google Scholar] [CrossRef]
- Olsen, J.M.; Sato, M.; Dallner, O.S.; Sandström, A.L.; Pisani, D.F.; Chambard, J.-C.; Amri, E.-Z.; Hutchinson, D.S.; Bengtsson, T. Glucose Uptake in Brown Fat Cells Is Dependent on MTOR Complex 2–Promoted GLUT1 Translocation. J. Cell Biol. 2014, 207, 365–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohade, C.; Osman, M.; Pannu, H.K.; Wahl, R.L. Uptake in Supraclavicular Area Fat (“USA-Fat”): Description on 18F-FDG PET/CT. J. Nucl. Med. 2003, 44, 170–176. [Google Scholar]
- Hany, T.F.; Gharehpapagh, E.; Kamel, E.M.; Buck, A.; Himms-Hagen, J.; von Schulthess, G.K. Brown Adipose Tissue: A Factor to Consider in Symmetrical Tracer Uptake in the Neck and Upper Chest Region. Eur. J. Nucl. Med. 2002, 29, 1393–1398. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.H.; Tovar, J.P.; Pavlova, Z.; Smith, M.L.; Gilsanz, V. Unequivocal Identification of Brown Adipose Tissue in a Human Infant. J. Magn. Reson. Imaging 2012, 35, 938–942. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.H.; Yin, L.; Aggabao, P.C.; Perkins, T.G.; Chia, J.M.; Gilsanz, V. Comparison of Brown and White Adipose Tissues in Infants and Children with Chemical-Shift-Encoded Water-Fat MRI: BAT and WAT in Infants and Children. J. Magn. Reson. Imaging 2013, 38, 885–896. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.H.; Wu, T.-W.; Yin, L.; Kim, M.S.; Chia, J.M.; Perkins, T.G.; Gilsanz, V. MRI Detection of Brown Adipose Tissue with Low Fat Content in Newborns with Hypothermia. Magn. Reson. Imaging 2014, 32, 107–117. [Google Scholar] [CrossRef] [Green Version]
- Iris Chen, Y.-C.; Cypess, A.M.; Chen, Y.-C.; Palmer, M.; Kolodny, G.; Kahn, C.R.; Kwong, K.K. Measurement of Human Brown Adipose Tissue Volume and Activity Using Anatomic MR Imaging and Functional MR Imaging. J. Nucl. Med. 2013, 54, 1584–1587. [Google Scholar] [CrossRef] [Green Version]
- Franz, D.; Weidlich, D.; Freitag, F.; Holzapfel, C.; Drabsch, T.; Baum, T.; Eggers, H.; Witte, A.; Rummeny, E.J.; Hauner, H.; et al. Association of Proton Density Fat Fraction in Adipose Tissue with Imaging-Based and Anthropometric Obesity Markers in Adults. Int. J. Obes. 2018, 42, 175–182. [Google Scholar] [CrossRef] [Green Version]
- Muzik, O.; Mangner, T.J.; Leonard, W.R.; Kumar, A.; Janisse, J.; Granneman, J.G. 15O PET Measurement of Blood Flow and Oxygen Consumption in Cold-Activated Human Brown Fat. J. Nucl. Med. 2013, 54, 523–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frontini, A.; Cinti, S. Distribution and Development of Brown Adipocytes in the Murine and Human Adipose Organ. Cell Metab. 2010, 11, 253–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Law, J.; Chalmers, J.; Morris, D.E.; Robinson, L.; Budge, H.; Symonds, M.E. The Use of Infrared Thermography in the Measurement and Characterization of Brown Adipose Tissue Activation. Temperature 2018, 5, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Hamaoka, T.; Nirengi, S.; Fuse, S.; Amagasa, S.; Kime, R.; Kuroiwa, M.; Endo, T.; Sakane, N.; Matsushita, M.; Saito, M.; et al. Near-Infrared Time-Resolved Spectroscopy for Assessing Brown Adipose Tissue Density in Humans: A Review. Front. Endocrinol. 2020, 11, 261. [Google Scholar] [CrossRef] [PubMed]
- Pfannenberg, C.; Werner, M.K.; Ripkens, S.; Stef, I.; Deckert, A.; Schmadl, M.; Reimold, M.; Haring, H.-U.; Claussen, C.D.; Stefan, N. Impact of Age on the Relationships of Brown Adipose Tissue With Sex and Adiposity in Humans. Diabetes 2010, 59, 1789–1793. [Google Scholar] [CrossRef] [Green Version]
- Ouellet, V.; Routhier-Labadie, A.; Bellemare, W.; Lakhal-Chaieb, L.; Turcotte, E.; Carpentier, A.C.; Richard, D. Outdoor Temperature, Age, Sex, Body Mass Index, and Diabetic Status Determine the Prevalence, Mass, and Glucose-Uptake Activity of 18F-FDG-Detected BAT in Humans. J. Clin. Endocrinol. Metab. 2011, 96, 192–199. [Google Scholar] [CrossRef]
- Fletcher, L.A.; Kim, K.; Leitner, B.P.; Cassimatis, T.M.; O’Mara, A.E.; Johnson, J.W.; Halprin, M.S.; McGehee, S.M.; Brychta, R.J.; Cypess, A.M.; et al. Sexual Dimorphisms in Adult Human Brown Adipose Tissue. Obesity 2020, 28, 241–246. [Google Scholar] [CrossRef]
- Fuse, S.; Nirengi, S.; Amagasa, S.; Homma, T.; Kime, R.; Endo, T.; Sakane, N.; Matsushita, M.; Saito, M.; Yoneshiro, T.; et al. Brown Adipose Tissue Density Measured by Near-Infrared Time-Resolved Spectroscopy in Japanese, across a Wide Age Range. J. Biomed. Opt. 2018, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Yoneshiro, T.; Aita, S.; Matsushita, M.; Okamatsu-Ogura, Y.; Kameya, T.; Kawai, Y.; Miyagawa, M.; Tsujisaki, M.; Saito, M. Age-Related Decrease in Cold-Activated Brown Adipose Tissue and Accumulation of Body Fat in Healthy Humans. Obesity 2011, 19, 1755–1760. [Google Scholar] [CrossRef]
- Keuper, M.; Jastroch, M. The Good and the BAT of Metabolic Sex Differences in Thermogenic Human Adipose Tissue. Mol. Cell. Endocrinol. 2021, 533, 111337. [Google Scholar] [CrossRef] [PubMed]
- Rudnicki, M.; Abdifarkosh, G.; Rezvan, O.; Nwadozi, E.; Roudier, E.; Haas, T.L. Female Mice Have Higher Angiogenesis in Perigonadal Adipose Tissue Than Males in Response to High-Fat Diet. Front. Physiol. 2018, 9, 1452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Cuenca, S.; Monjo, M.; Gianotti, M.; Proenza, A.M.; Roca, P. Expression of Mitochondrial Biogenesis-Signaling Factors in Brown Adipocytes Is Influenced Specifically by 17β-Estradiol, Testosterone, and Progesterone. Am. J. Physiol. -Endocrinol. Metab. 2007, 292, E340–E346. [Google Scholar] [CrossRef]
- Löfgren, P.; Hoffstedt, J.; Rydén, M.; Thörne, A.; Holm, C.; Wahrenberg, H.; Arner, P. Major Gender Differences in the Lipolytic Capacity of Abdominal Subcutaneous Fat Cells in Obesity Observed before and after Long-Term Weight Reduction. J. Clin. Endocrinol. Metab. 2002, 87, 764–771. [Google Scholar] [CrossRef] [Green Version]
- Riis-Vestergaard, M.J.; Richelsen, B.; Bruun, J.M.; Li, W.; Hansen, J.B.; Pedersen, S.B. Beta-1 and Not Beta-3 Adrenergic Receptors May Be the Primary Regulator of Human Brown Adipocyte Metabolism. J. Clin. Endocrinol. Metab. 2020, 105, e994–e1005. [Google Scholar] [CrossRef]
- Kaikaew, K.; van den Beukel, J.C.; Neggers, S.J.C.M.M.; Themmen, A.P.N.; Visser, J.A.; Grefhorst, A. Sex Difference in Cold Perception and Shivering Onset upon Gradual Cold Exposure. J. Therm. Biol. 2018, 77, 137–144. [Google Scholar] [CrossRef]
- Choi, D.K.; Oh, T.S.; Choi, J.-W.; Mukherjee, R.; Wang, X.; Liu, H.; Yun, J.W. Gender Difference in Proteome of Brown Adipose Tissues between Male and Female Rats Exposed to a High Fat Diet. Cell Physiol. Biochem. 2011, 28, 933–948. [Google Scholar] [CrossRef]
- Nookaew, I.; Svensson, P.-A.; Jacobson, P.; Jernås, M.; Taube, M.; Larsson, I.; Andersson-Assarsson, J.C.; Sjöström, L.; Froguel, P.; Walley, A.; et al. Adipose Tissue Resting Energy Expenditure and Expression of Genes Involved in Mitochondrial Function Are Higher in Women than in Men. J. Clin. Endocrinol. Metab. 2013, 98, E370–E378. [Google Scholar] [CrossRef]
- van den Beukel, J.C.; Grefhorst, A.; Hoogduijn, M.J.; Steenbergen, J.; Mastroberardino, P.G.; Dor, F.J.M.F.; Themmen, A.P.N. Women Have More Potential to Induce Browning of Perirenal Adipose Tissue than Men: Browning of Perirenal Fat in Women. Obesity 2015, 23, 1671–1679. [Google Scholar] [CrossRef]
- Norheim, F.; Hasin-Brumshtein, Y.; Vergnes, L.; Chella Krishnan, K.; Pan, C.; Seldin, M.M.; Hui, S.T.; Mehrabian, M.; Zhou, Z.; Gupta, S.; et al. Gene-by-Sex Interactions in Mitochondrial Functions and Cardio-Metabolic Traits. Cell Metab. 2019, 29, 932–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Persichetti, A.; Sciuto, R.; Rea, S.; Basciani, S.; Lubrano, C.; Mariani, S.; Ulisse, S.; Nofroni, I.; Maini, C.L.; Gnessi, L. Prevalence, Mass, and Glucose-Uptake Activity of 18F-FDG-Detected Brown Adipose Tissue in Humans Living in a Temperate Zone of Italy. PLoS ONE 2013, 8, e63391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lecoultre, V.; Ravussin, E. Brown Adipose Tissue and Aging. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Graja, A.; Schulz, T.J. Mechanisms of Aging-Related Impairment of Brown Adipocyte Development and Function. Gerontology 2014, 61, 211–217. [Google Scholar] [CrossRef]
- Rodgers, A.; Sferruzzi-Perri, A.N. Developmental Programming of Offspring Adipose Tissue Biology and Obesity Risk. Int. J. Obes. 2021, 45, 1170–1192. [Google Scholar] [CrossRef]
- Zoico, E.; Rubele, S.; De Caro, A.; Nori, N.; Mazzali, G.; Fantin, F.; Rossi, A.; Zamboni, M. Brown and Beige Adipose Tissue and Aging. Front. Endocrinol. 2019, 10, 368. [Google Scholar] [CrossRef] [Green Version]
- Lenaz, G. Role of Mitochondria in Oxidative Stress and Ageing. Biochim. Biophys. Acta (BBA)-Bioenerg. 1998, 1366, 53–67. [Google Scholar] [CrossRef] [Green Version]
- Bratic, A.; Larsson, N.-G. The Role of Mitochondria in Aging. J. Clin. Investig. 2013, 123, 951–957. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Boström, P.; Sparks, L.M.; Ye, L.; Choi, J.H.; Giang, A.-H.; Khandekar, M.; Virtanen, K.A.; Nuutila, P.; Schaart, G.; et al. Beige Adipocytes Are a Distinct Type of Thermogenic Fat Cell in Mouse and Human. Cell 2012, 150, 366–376. [Google Scholar] [CrossRef] [Green Version]
- Khanh, V.C.; Zulkifli, A.F.; Tokunaga, C.; Yamashita, T.; Hiramatsu, Y.; Ohneda, O. Aging Impairs Beige Adipocyte Differentiation of Mesenchymal Stem Cells via the Reduced Expression of Sirtuin 1. Biochem. Biophys. Res. Commun. 2018, 500, 682–690. [Google Scholar] [CrossRef]
- Nedergaard, J.; Bengtsson, T.; Cannon, B. Unexpected Evidence for Active Brown Adipose Tissue in Adult Humans. Am. J. Physiol. -Endocrinol. Metab. 2007, 293, E444–E452. [Google Scholar] [CrossRef] [PubMed]
- Bahler, L.; Verberne, H.J.; Admiraal, W.M.; Stok, W.J.; Soeters, M.R.; Hoekstra, J.B.; Holleman, F. Differences in Sympathetic Nervous Stimulation of Brown Adipose Tissue Between the Young and Old, and the Lean and Obese. J. Nucl. Med. 2016, 57, 372–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoneshiro, T.; Ogawa, T.; Okamoto, N.; Matsushita, M.; Aita, S.; Kameya, T.; Kawai, Y.; Iwanaga, T.; Saito, M. Impact of UCP1 and Β3AR Gene Polymorphisms on Age-Related Changes in Brown Adipose Tissue and Adiposity in Humans. Int. J. Obes. 2013, 37, 993–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sul, O.; Hyun, H.; Rajasekaran, M.; Suh, J.; Choi, H. Estrogen Enhances Browning in Adipose Tissue by M2 Macrophage Polarization via Heme Oxygenase-1. J. Cell. Physiol. 2021, 236, 1875–1888. [Google Scholar] [CrossRef]
- Fan, W.; Yanase, T.; Nomura, M.; Okabe, T.; Goto, K.; Sato, T.; Kawano, H.; Kato, S.; Nawata, H. Androgen Receptor Null Male Mice Develop Late-Onset Obesity Caused by Decreased Energy Expenditure and Lipolytic Activity but Show Normal Insulin Sensitivity With High Adiponectin Secretion. Diabetes 2005, 54, 1000–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soumano, K. Glucocorticoids Inhibit the Transcriptional Response of the Uncoupling Protein-1 Gene to Adrenergic Stimulation in a Brown Adipose Cell Line. Mol. Cell. Endocrinol. 2000, 165, 7–15. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Takahashi, N.; Yasubuchi, M.; Kim, Y.-I.; Hashizaki, H.; Kim, M.-J.; Sakamoto, T.; Goto, T.; Kawada, T. Triiodothyronine Induces UCP-1 Expression and Mitochondrial Biogenesis in Human Adipocytes. Am. J. Physiol.-Cell Physiol. 2012, 302, C463–C472. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Sánchez, N.; Moreno-Navarrete, J.M.; Contreras, C.; Rial-Pensado, E.; Fernø, J.; Nogueiras, R.; Diéguez, C.; Fernández-Real, J.-M.; López, M. Thyroid Hormones Induce Browning of White Fat. J. Endocrinol. 2017, 232, 351–362. [Google Scholar] [CrossRef] [Green Version]
- Schulz, T.J.; Huang, T.L.; Tran, T.T.; Zhang, H.; Townsend, K.L.; Shadrach, J.L.; Cerletti, M.; McDougall, L.E.; Giorgadze, N.; Tchkonia, T.; et al. Identification of Inducible Brown Adipocyte Progenitors Residing in Skeletal Muscle and White Fat. Proc. Natl. Acad. Sci. USA 2011, 108, 143–148. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-H.; Petkova, A.P.; Mottillo, E.P.; Granneman, J.G. In Vivo Identification of Bipotential Adipocyte Progenitors Recruited by Β3-Adrenoceptor Activation and High-Fat Feeding. Cell Metab. 2012, 15, 480–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berry, R.; Rodeheffer, M.S. Characterization of the Adipocyte Cellular Lineage in Vivo. Nat. Cell Biol. 2013, 15, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Holzman, M.A.; Ryckman, A.; Finkelstein, T.M.; Landry-Truchon, K.; Schindler, K.A.; Bergmann, J.M.; Jeannotte, L.; Mansfield, J.H. HOXA5 Participates in Brown Adipose Tissue and Epaxial Skeletal Muscle Patterning and in Brown Adipocyte Differentiation. Front. Cell Dev. Biol. 2021, 9, 632303. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Wang, G.; Diao, Y.; Long, Y.; Fu, X.; Weng, M.; Zhou, L.; Sun, K.; Cheung, T.H.; Ip, N.Y.; et al. A Molecular Switch Regulating Cell Fate Choice between Muscle Progenitor Cells and Brown Adipocytes. Dev. Cell 2017, 41, 382–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timmons, J.A.; Wennmalm, K.; Larsson, O.; Walden, T.B.; Lassmann, T.; Petrovic, N.; Hamilton, D.L.; Gimeno, R.E.; Wahlestedt, C.; Baar, K.; et al. Myogenic Gene Expression Signature Establishes That Brown and White Adipocytes Originate from Distinct Cell Lineages. Proc. Natl. Acad. Sci. USA 2007, 104, 4401–4406. [Google Scholar] [CrossRef] [Green Version]
- Forner, F.; Kumar, C.; Luber, C.A.; Fromme, T.; Klingenspor, M.; Mann, M. Proteome Differences between Brown and White Fat Mitochondria Reveal Specialized Metabolic Functions. Cell Metab. 2009, 10, 324–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharp, L.Z.; Shinoda, K.; Ohno, H.; Scheel, D.W.; Tomoda, E.; Ruiz, L.; Hu, H.; Wang, L.; Pavlova, Z.; Gilsanz, V.; et al. Human BAT Possesses Molecular Signatures That Resemble Beige/Brite Cells. PLoS ONE 2012, 7, e49452. [Google Scholar] [CrossRef]
- Xue, R.; Lynes, M.D.; Dreyfuss, J.M.; Shamsi, F.; Schulz, T.J.; Zhang, H.; Huang, T.L.; Townsend, K.L.; Li, Y.; Takahashi, H.; et al. Clonal Analyses and Gene Profiling Identify Genetic Biomarkers of the Thermogenic Potential of Human Brown and White Preadipocytes. Nat. Med. 2015, 21, 760–768. [Google Scholar] [CrossRef]
- Seale, P.; Bjork, B.; Yang, W.; Kajimura, S.; Chin, S.; Kuang, S.; Scimè, A.; Devarakonda, S.; Conroe, H.M.; Erdjument-Bromage, H.; et al. PRDM16 Controls a Brown Fat/Skeletal Muscle Switch. Nature 2008, 454, 961–967. [Google Scholar] [CrossRef] [Green Version]
- Lidell, M.E.; Betz, M.J.; Leinhard, O.D.; Heglind, M.; Elander, L.; Slawik, M.; Mussack, T.; Nilsson, D.; Romu, T.; Nuutila, P.; et al. Evidence for Two Types of Brown Adipose Tissue in Humans. Nat. Med. 2013, 19, 631–634. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Xie, H.; Mori, M.A.; Alexander, R.; Yuan, B.; Hattangadi, S.M.; Liu, Q.; Kahn, C.R.; Lodish, H.F. Mir193b–365 Is Essential for Brown Fat Differentiation. Nat. Cell Biol. 2011, 13, 958–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Güller, I.; McNaughton, S.; Crowley, T.; Gilsanz, V.; Kajimura, S.; Watt, M.; Russell, A.P. Comparative Analysis of MicroRNA Expression in Mouse and Human Brown Adipose Tissue. BMC Genom. 2015, 16, 820. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-J.; Cho, H.; Alexander, R.; Patterson, H.C.; Gu, M.; Lo, K.A.; Xu, D.; Goh, V.J.; Nguyen, L.N.; Chai, X.; et al. MicroRNAs Are Required for the Feature Maintenance and Differentiation of Brown Adipocytes. Diabetes 2014, 63, 4045–4056. [Google Scholar] [CrossRef] [Green Version]
- Mori, M.; Nakagami, H.; Rodriguez-Araujo, G.; Nimura, K.; Kaneda, Y. Essential Role for MiR-196a in Brown Adipogenesis of White Fat Progenitor Cells. PLoS Biol. 2012, 10, e1001314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Bi, P.; Shan, T.; Yang, X.; Yin, H.; Wang, Y.-X.; Liu, N.; Rudnicki, M.A.; Kuang, S. MiR-133a Regulates Adipocyte Browning In Vivo. PLoS Genet. 2013, 9, e1003626. [Google Scholar] [CrossRef] [Green Version]
- Svensson, P.-A.; Lindberg, K.; Hoffmann, J.M.; Taube, M.; Pereira, M.J.; Mohsen-Kanson, T.; Hafner, A.-L.; Rizell, M.; Palming, J.; Dani, C.; et al. Characterization of Brown Adipose Tissue in the Human Perirenal Depot: BAT in the Human Perirenal Depot. Obesity 2014, 22, 1830–1837. [Google Scholar] [CrossRef]
- Cypess, A.M.; White, A.P.; Vernochet, C.; Schulz, T.J.; Xue, R.; Sass, C.A.; Huang, T.L.; Roberts-Toler, C.; Weiner, L.S.; Sze, C.; et al. Anatomical Localization, Gene Expression Profiling and Functional Characterization of Adult Human Neck Brown Fat. Nat. Med. 2013, 19, 635–639. [Google Scholar] [CrossRef]
- Zuriaga, M.A.; Fuster, J.J.; Gokce, N.; Walsh, K. Humans and Mice Display Opposing Patterns of “Browning” Gene Expression in Visceral and Subcutaneous White Adipose Tissue Depots. Front. Cardiovasc. Med. 2017, 4, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, P.; Arch, J.R.S.; Ashwell, M. Brown Adipose Tissue in the Parametrial Fat Pad of the Mouse. FEBS Lett. 1984, 167, 10–14. [Google Scholar] [CrossRef] [Green Version]
- Haman, F.; Mantha, O.L.; Cheung, S.S.; DuCharme, M.B.; Taber, M.; Blondin, D.P.; McGarr, G.W.; Hartley, G.L.; Hynes, Z.; Basset, F.A. Oxidative Fuel Selection and Shivering Thermogenesis during a 12- and 24-h Cold-Survival Simulation. J. Appl. Physiol. 2016, 120, 640–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bal, N.C.; Maurya, S.K.; Pani, S.; Sethy, C.; Banerjee, A.; Das, S.; Patnaik, S.; Kundu, C.N. Mild Cold Induced Thermogenesis: Are BAT and Skeletal Muscle Synergistic Partners? Biosci. Rep. 2017, 37, BSR20171087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kern, P.A.; Finlin, B.S.; Zhu, B.; Rasouli, N.; McGehee, R.E.; Westgate, P.M.; Dupont-Versteegden, E.E. The Effects of Temperature and Seasons on Subcutaneous White Adipose Tissue in Humans: Evidence for Thermogenic Gene Induction. J. Clin. Endocrinol. Metab. 2014, 99, E2772–E2779. [Google Scholar] [CrossRef] [PubMed]
- Betz, M.J.; Slawik, M.; Lidell, M.E.; Osswald, A.; Heglind, M.; Nilsson, D.; Lichtenauer, U.D.; Mauracher, B.; Mussack, T.; Beuschlein, F.; et al. Presence of Brown Adipocytes in Retroperitoneal Fat From Patients With Benign Adrenal Tumors: Relationship With Outdoor Temperature. J. Clin. Endocrinol. Metab. 2013, 98, 4097–4104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Lans, A.A.J.J.; Hoeks, J.; Brans, B.; Vijgen, G.H.E.J.; Visser, M.G.W.; Vosselman, M.J.; Hansen, J.; Jörgensen, J.A.; Wu, J.; Mottaghy, F.M.; et al. Cold Acclimation Recruits Human Brown Fat and Increases Nonshivering Thermogenesis. J. Clin. Investig. 2013, 123, 3395–3403. [Google Scholar] [CrossRef] [PubMed]
- Weir, G.; Ramage, L.E.; Akyol, M.; Rhodes, J.K.; Kyle, C.J.; Fletcher, A.M.; Craven, T.H.; Wakelin, S.J.; Drake, A.J.; Gregoriades, M.-L.; et al. Substantial Metabolic Activity of Human Brown Adipose Tissue during Warm Conditions and Cold-Induced Lipolysis of Local Triglycerides. Cell Metab. 2018, 27, 1348–1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouellet, V.; Labbé, S.M.; Blondin, D.P.; Phoenix, S.; Guérin, B.; Haman, F.; Turcotte, E.E.; Richard, D.; Carpentier, A.C. Brown Adipose Tissue Oxidative Metabolism Contributes to Energy Expenditure during Acute Cold Exposure in Humans. J. Clin. Investig. 2012, 122, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Blondin, D.P.; Labbé, S.M.; Noll, C.; Kunach, M.; Phoenix, S.; Guérin, B.; Turcotte, É.E.; Haman, F.; Richard, D.; Carpentier, A.C. Selective Impairment of Glucose but Not Fatty Acid or Oxidative Metabolism in Brown Adipose Tissue of Subjects with Type 2 Diabetes. Diabetes 2015, 64, 2388–2397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blondin, D.P.; Carpentier, A.C. The Role of BAT in Cardiometabolic Disorders and Aging. Best Pract. Res. Clin. Endocrinol. Metab. 2016, 30, 497–513. [Google Scholar] [CrossRef]
- Suchacki, K.J.; Stimson, R.H. Nutritional Regulation of Human Brown Adipose Tissue. Nutrients 2021, 13, 1748. [Google Scholar] [CrossRef]
- Orava, J.; Nuutila, P.; Lidell, M.E.; Oikonen, V.; Noponen, T.; Viljanen, T.; Scheinin, M.; Taittonen, M.; Niemi, T.; Enerbäck, S.; et al. Different Metabolic Responses of Human Brown Adipose Tissue to Activation by Cold and Insulin. Cell Metab. 2011, 14, 272–279. [Google Scholar] [CrossRef] [Green Version]
- Chondronikola, M.; Volpi, E.; Børsheim, E.; Porter, C.; Annamalai, P.; Enerbäck, S.; Lidell, M.E.; Saraf, M.K.; Labbe, S.M.; Hurren, N.M.; et al. Brown Adipose Tissue Improves Whole-Body Glucose Homeostasis and Insulin Sensitivity in Humans. Diabetes 2014, 63, 4089–4099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Himms-Hagen, J.; Cui, J.; Danforth, E.; Taatjes, D.J.; Lang, S.S.; Waters, B.L.; Claus, T.H. Effect of CL-316,243, a Thermogenic Beta 3-Agonist, on Energy Balance and Brown and White Adipose Tissues in Rats. Am. J. Physiol. -Regul. Integr. Comp. Physiol. 1994, 266, R1371–R1382. [Google Scholar] [CrossRef] [PubMed]
- Granneman, J.G.; Li, P.; Zhu, Z.; Lu, Y. Metabolic and Cellular Plasticity in White Adipose Tissue I: Effects of β 3 -Adrenergic Receptor Activation. Am. J. Physiol. -Endocrinol. Metab. 2005, 289, E608–E616. [Google Scholar] [CrossRef] [Green Version]
- Larsen, T.M.; Toubro, S.; van Baak, M.A.; Gottesdiener, K.M.; Larson, P.; Saris, W.H.; Astrup, A. Effect of a 28-d Treatment with L-796568, a Novel Β3-Adrenergic Receptor Agonist, on Energy Expenditure and Body Composition in Obese Men. Am. J. Clin. Nutr. 2002, 76, 780–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weyer, C.; Tataranni, P.A.; Snitker, S.; Danforth, E.; Ravussin, E. Increase in Insulin Action and Fat Oxidation after Treatment with CL 316,243, a Highly Selective Beta3-Adrenoceptor Agonist in Humans. Diabetes 1998, 47, 1555–1561. [Google Scholar] [CrossRef]
- Cypess, A.M.; Weiner, L.S.; Roberts-Toler, C.; Elía, E.F.; Kessler, S.H.; Kahn, P.A.; English, J.; Chatman, K.; Trauger, S.A.; Doria, A.; et al. Activation of Human Brown Adipose Tissue by a Β3-Adrenergic Receptor Agonist. Cell Metab. 2015, 21, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Blondin, D.P.; Nielsen, S.; Kuipers, E.N.; Severinsen, M.C.; Jensen, V.H.; Miard, S.; Jespersen, N.Z.; Kooijman, S.; Boon, M.R.; Fortin, M.; et al. Human Brown Adipocyte Thermogenesis Is Driven by Β2-AR Stimulation. Cell Metab. 2020, 32, 287–300. [Google Scholar] [CrossRef]
- Carey, A.L.; Formosa, M.F.; Van Every, B.; Bertovic, D.; Eikelis, N.; Lambert, G.W.; Kalff, V.; Duffy, S.J.; Cherk, M.H.; Kingwell, B.A. Ephedrine Activates Brown Adipose Tissue in Lean but Not Obese Humans. Diabetologia 2013, 56, 147–155. [Google Scholar] [CrossRef]
- O’Mara, A.E.; Johnson, J.W.; Linderman, J.D.; Brychta, R.J.; McGehee, S.; Fletcher, L.A.; Fink, Y.A.; Kapuria, D.; Cassimatis, T.M.; Kelsey, N.; et al. Chronic Mirabegron Treatment Increases Human Brown Fat, HDL Cholesterol, and Insulin Sensitivity. J. Clin. Investig. 2020, 130, 2209–2219. [Google Scholar] [CrossRef]
- Warner, A.; Mittag, J. Breaking BAT: Can Browning Create a Better White? J. Endocrinol. 2016, 228, R19–R29. [Google Scholar] [CrossRef] [Green Version]
- Kaisanlahti, A.; Glumoff, T. Browning of White Fat: Agents and Implications for Beige Adipose Tissue to Type 2 Diabetes. J. Physiol. Biochem. 2019, 75, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Markan, K.R.; Naber, M.C.; Ameka, M.K.; Anderegg, M.D.; Mangelsdorf, D.J.; Kliewer, S.A.; Mohammadi, M.; Potthoff, M.J. Circulating FGF21 Is Liver Derived and Enhances Glucose Uptake During Refeeding and Overfeeding. Diabetes 2014, 63, 4057–4063. [Google Scholar] [CrossRef] [Green Version]
- Hondares, E.; Gallego-Escuredo, J.M.; Flachs, P.; Frontini, A.; Cereijo, R.; Goday, A.; Perugini, J.; Kopecky, P.; Giralt, M.; Cinti, S.; et al. Fibroblast Growth Factor-21 Is Expressed in Neonatal and Pheochromocytoma-Induced Adult Human Brown Adipose Tissue. Metabolism 2014, 63, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Fisher, f.M.; Kleiner, S.; Douris, N.; Fox, E.C.; Mepani, R.J.; Verdeguer, F.; Wu, J.; Kharitonenkov, A.; Flier, J.S.; Maratos-Flier, E.; et al. FGF21 Regulates PGC-1 and Browning of White Adipose Tissues in Adaptive Thermogenesis. Genes Dev. 2012, 26, 271–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlein, C.; Talukdar, S.; Heine, M.; Fischer, A.W.; Krott, L.M.; Nilsson, S.K.; Brenner, M.B.; Heeren, J.; Scheja, L. FGF21 Lowers Plasma Triglycerides by Accelerating Lipoprotein Catabolism in White and Brown Adipose Tissues. Cell Metab. 2016, 23, 441–453. [Google Scholar] [CrossRef] [Green Version]
- Talukdar, S.; Zhou, Y.; Li, D.; Rossulek, M.; Dong, J.; Somayaji, V.; Weng, Y.; Clark, R.; Lanba, A.; Owen, B.M.; et al. A Long-Acting FGF21 Molecule, PF-05231023, Decreases Body Weight and Improves Lipid Profile in Non-Human Primates and Type 2 Diabetic Subjects. Cell Metab. 2016, 23, 427–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaich, G.; Chien, J.Y.; Fu, H.; Glass, L.C.; Deeg, M.A.; Holland, W.L.; Kharitonenkov, A.; Bumol, T.; Schilske, H.K.; Moller, D.E. The Effects of LY2405319, an FGF21 Analog, in Obese Human Subjects with Type 2 Diabetes. Cell Metab. 2013, 18, 333–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, Y.; Kelton, C.M.L.; Guo, J.J.; Bian, B.; Heaton, P.C. Treatment of Obesity: Pharmacotherapy Trends in the United States from 1999 to 2010: Pharmacotherapy Treatment of Obesity. Obesity 2015, 23, 1721–1728. [Google Scholar] [CrossRef] [Green Version]
- Roberts, L.D.; Boström, P.; O’Sullivan, J.F.; Schinzel, R.T.; Lewis, G.D.; Dejam, A.; Lee, Y.-K.; Palma, M.J.; Calhoun, S.; Georgiadi, A.; et al. β-Aminoisobutyric Acid Induces Browning of White Fat and Hepatic β-Oxidation and Is Inversely Correlated with Cardiometabolic Risk Factors. Cell Metab. 2014, 19, 96–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vegiopoulos, A.; Muller-Decker, K.; Strzoda, D.; Schmitt, I.; Chichelnitskiy, E.; Ostertag, A.; Diaz, M.B.; Rozman, J.; Hrabe de Angelis, M.; Nusing, R.M.; et al. Cyclooxygenase-2 Controls Energy Homeostasis in Mice by de Novo Recruitment of Brown Adipocytes. Science 2010, 328, 1158–1161. [Google Scholar] [CrossRef]
- Qian, S.-W.; Tang, Y.; Li, X.; Liu, Y.; Zhang, Y.-Y.; Huang, H.-Y.; Xue, R.-D.; Yu, H.-Y.; Guo, L.; Gao, H.-D.; et al. BMP4-Mediated Brown Fat-like Changes in White Adipose Tissue Alter Glucose and Energy Homeostasis. Proc. Natl. Acad. Sci. USA 2013, 110, E798–E807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gustafson, B.; Hammarstedt, A.; Hedjazifar, S.; Hoffmann, J.M.; Svensson, P.-A.; Grimsby, J.; Rondinone, C.; Smith, U. BMP4 and BMP Antagonists Regulate Human White and Beige Adipogenesis. Diabetes 2015, 64, 1670–1681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsen, M.; Raschke, S.; Tennagels, N.; Schwahn, U.; Jelenik, T.; Roden, M.; Romacho, T.; Eckel, J. BMP4 and BMP7 Induce the White-to-Brown Transition of Primary Human Adipose Stem Cells. Am. J. Physiol.-Cell Physiol. 2014, 306, C431–C440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braga, M.; Reddy, S.T.; Vergnes, L.; Pervin, S.; Grijalva, V.; Stout, D.; David, J.; Li, X.; Tomasian, V.; Reid, C.B.; et al. Follistatin Promotes Adipocyte Differentiation, Browning, and Energy Metabolism. J. Lipid. Res. 2014, 55, 375–384. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Braga, M.; Reddy, S.T.; Lee, S.-J.; Parveen, M.; Grijalva, V.; Vergnes, L.; Pervin, S. Follistatin Targets Distinct Pathways To Promote Brown Adipocyte Characteristics in Brown and White Adipose Tissues. Endocrinology 2017, 158, 1217–1230. [Google Scholar] [CrossRef] [PubMed]
- Braga, M.; Pervin, S.; Norris, K.; Bhasin, S.; Singh, R. Inhibition of in Vitro and in Vivo Brown Fat Differentiation Program by Myostatin: Brown Fat and Myostatin. Obesity 2013, 21, 1180–1188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.-S.; Kim, E.-S.; Jung, J.-E.; Marciano, D.P.; Jo, A.; Koo, J.Y.; Choi, S.Y.; Yang, Y.R.; Jang, H.-J.; Kim, E.-K.; et al. PPARγ Antagonist Gleevec Improves Insulin Sensitivity and Promotes the Browning of White Adipose Tissue. Diabetes 2016, 65, 829–839. [Google Scholar] [CrossRef] [Green Version]
- Ohno, H.; Shinoda, K.; Spiegelman, B.M.; Kajimura, S. PPARγ Agonists Induce a White-to-Brown Fat Conversion through Stabilization of PRDM16 Protein. Cell Metab. 2012, 15, 395–404. [Google Scholar] [CrossRef] [Green Version]
- Loh, R.K.C.; Formosa, M.F.; Eikelis, N.; Bertovic, D.A.; Anderson, M.J.; Barwood, S.A.; Nanayakkara, S.; Cohen, N.D.; La Gerche, A.; Reutens, A.T.; et al. Pioglitazone Reduces Cold-Induced Brown Fat Glucose Uptake despite Induction of Browning in Cultured Human Adipocytes: A Randomised, Controlled Trial in Humans. Diabetologia 2018, 61, 220–230. [Google Scholar] [CrossRef] [Green Version]
- Pi-Sunyer, X.; Astrup, A.; Fujioka, K.; Greenway, F.; Halpern, A.; Krempf, M.; Lau, D.C.W.; le Roux, C.W.; Violante Ortiz, R.; Jensen, C.B.; et al. A Randomized, Controlled Trial of 3.0 Mg of Liraglutide in Weight Management. N. Engl. J. Med. 2015, 373, 11–22. [Google Scholar] [CrossRef]
- Beiroa, D.; Imbernon, M.; Gallego, R.; Senra, A.; Herranz, D.; Villarroya, F.; Serrano, M.; Ferno, J.; Salvador, J.; Escalada, J.; et al. GLP-1 Agonism Stimulates Brown Adipose Tissue Thermogenesis and Browning Through Hypothalamic AMPK. Diabetes 2014, 63, 3346–3358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teodoro, J.S.; Zouhar, P.; Flachs, P.; Bardova, K.; Janovska, P.; Gomes, A.P.; Duarte, F.V.; Varela, A.T.; Rolo, A.P.; Palmeira, C.M.; et al. Enhancement of Brown Fat Thermogenesis Using Chenodeoxycholic Acid in Mice. Int. J. Obes. 2014, 38, 1027–1034. [Google Scholar] [CrossRef]
- Broeders, E.P.M.; Nascimento, E.B.M.; Havekes, B.; Brans, B.; Roumans, K.H.M.; Tailleux, A.; Schaart, G.; Kouach, M.; Charton, J.; Deprez, B.; et al. The Bile Acid Chenodeoxycholic Acid Increases Human Brown Adipose Tissue Activity. Cell Metab. 2015, 22, 418–426. [Google Scholar] [CrossRef] [Green Version]
- Song, N.-J.; Choi, S.; Rajbhandari, P.; Chang, S.-H.; Kim, S.; Vergnes, L.; Kwon, S.-M.; Yoon, J.-H.; Lee, S.; Ku, J.-M.; et al. Prdm4 Induction by the Small Molecule Butein Promotes White Adipose Tissue Browning. Nat. Chem. Biol. 2016, 12, 479–481. [Google Scholar] [CrossRef]
- Baskaran, P.; Krishnan, V.; Ren, J.; Thyagarajan, B. Capsaicin Induces Browning of White Adipose Tissue and Counters Obesity by Activating TRPV1 Channel-Dependent Mechanisms: TRPV1 Activates Browning of WAT to Counter Obesity. Br. J. Pharmacol. 2016, 173, 2369–2389. [Google Scholar] [CrossRef]
- Yoneshiro, T.; Aita, S.; Matsushita, M.; Kayahara, T.; Kameya, T.; Kawai, Y.; Iwanaga, T.; Saito, M. Recruited Brown Adipose Tissue as an Antiobesity Agent in Humans. J. Clin. Investig. 2013, 123, 3404–3408. [Google Scholar] [CrossRef] [Green Version]
- Yoneshiro, T.; Matsushita, M.; Hibi, M.; Tone, H.; Takeshita, M.; Yasunaga, K.; Katsuragi, Y.; Kameya, T.; Sugie, H.; Saito, M. Tea Catechin and Caffeine Activate Brown Adipose Tissue and Increase Cold-Induced Thermogenic Capacity in Humans. Am. J. Clin. Nutr. 2017, 105, 873–881. [Google Scholar] [CrossRef] [Green Version]
- Carriere, A.; Jeanson, Y.; Berger-Muller, S.; Andre, M.; Chenouard, V.; Arnaud, E.; Barreau, C.; Walther, R.; Galinier, A.; Wdziekonski, B.; et al. Browning of White Adipose Cells by Intermediate Metabolites: An Adaptive Mechanism to Alleviate Redox Pressure. Diabetes 2014, 63, 3253–3265. [Google Scholar] [CrossRef] [Green Version]
- Stanford, K.I.; Middelbeek, R.J.W.; Goodyear, L.J. Exercise Effects on White Adipose Tissue: Beiging and Metabolic Adaptations. Diabetes 2015, 64, 2361–2368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trevellin, E.; Scorzeto, M.; Olivieri, M.; Granzotto, M.; Valerio, A.; Tedesco, L.; Fabris, R.; Serra, R.; Quarta, M.; Reggiani, C.; et al. Exercise Training Induces Mitochondrial Biogenesis and Glucose Uptake in Subcutaneous Adipose Tissue Through ENOS-Dependent Mechanisms. Diabetes 2014, 63, 2800–2811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-Dependent Myokine That Drives Brown-Fat-like Development of White Fat and Thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Lee, P.; Linderman, J.D.; Smith, S.; Brychta, R.J.; Wang, J.; Idelson, C.; Perron, R.M.; Werner, C.D.; Phan, G.Q.; Kammula, U.S.; et al. Irisin and FGF21 Are Cold-Induced Endocrine Activators of Brown Fat Function in Humans. Cell Metab. 2014, 19, 302–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otero-Díaz, B.; Rodríguez-Flores, M.; Sánchez-Muñoz, V.; Monraz-Preciado, F.; Ordoñez-Ortega, S.; Becerril-Elias, V.; Baay-Guzmán, G.; Obando-Monge, R.; García-García, E.; Palacios-González, B.; et al. Exercise Induces White Adipose Tissue Browning Across the Weight Spectrum in Humans. Front. Physiol. 2018, 9, 1781. [Google Scholar] [CrossRef] [PubMed]
- Hecksteden, A.; Wegmann, M.; Steffen, A.; Kraushaar, J.; Morsch, A.; Ruppenthal, S.; Kaestner, L.; Meyer, T. Irisin and Exercise Training in Humans—Results from a Randomized Controlled Training Trial. BMC Med. 2013, 11, 235. [Google Scholar] [CrossRef] [Green Version]
- Pardo, M.; Crujeiras, A.B.; Amil, M.; Aguera, Z.; Jiménez-Murcia, S.; Baños, R.; Botella, C.; de la Torre, R.; Estivill, X.; Fagundo, A.B.; et al. Association of Irisin with Fat Mass, Resting Energy Expenditure, and Daily Activity in Conditions of Extreme Body Mass Index. Int. J. Endocrinol. 2014, 2014, 857270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viitasalo, A.; Ågren, J.; Venäläinen, T.; Pihlajamäki, J.; Jääskeläinen, J.; Korkmaz, A.; Atalay, M.; Lakka, T.A. Association of Plasma Fatty Acid Composition with Plasma Irisin Levels in Normal Weight and Overweight/Obese Children. Pediatric Obes. 2016, 11, 299–305. [Google Scholar] [CrossRef]
- Hansen, J.S.; Pedersen, B.K.; Xu, G.; Lehmann, R.; Weigert, C.; Plomgaard, P. Exercise-Induced Secretion of FGF21 and Follistatin Are Blocked by Pancreatic Clamp and Impaired in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2016, 101, 2816–2825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knudsen, J.G.; Murholm, M.; Carey, A.L.; Biensø, R.S.; Basse, A.L.; Allen, T.L.; Hidalgo, J.; Kingwell, B.A.; Febbraio, M.A.; Hansen, J.B.; et al. Role of IL-6 in Exercise Training- and Cold-Induced UCP1 Expression in Subcutaneous White Adipose Tissue. PLoS ONE 2014, 9, e84910. [Google Scholar] [CrossRef] [Green Version]
- Rao, R.R.; Long, J.Z.; White, J.P.; Svensson, K.J.; Lou, J.; Lokurkar, I.; Jedrychowski, M.P.; Ruas, J.L.; Wrann, C.D.; Lo, J.C.; et al. Meteorin-like Is a Hormone That Regulates Immune-Adipose Interactions to Increase Beige Fat Thermogenesis. Cell 2014, 157, 1279–1291. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.; Choi, E.Y.; Liu, X.; Martin, A.; Wang, C.; Xu, X.; During, M.J. White to Brown Fat Phenotypic Switch Induced by Genetic and Environmental Activation of a Hypothalamic-Adipocyte Axis. Cell Metab. 2011, 14, 324–338. [Google Scholar] [CrossRef] [Green Version]
- Vidal, P.; Stanford, K.I. Exercise-Induced Adaptations to Adipose Tissue Thermogenesis. Front. Endocrinol. 2020, 11, 270. [Google Scholar] [CrossRef]
- Dewal, R.S.; Stanford, K.I. Effects of Exercise on Brown and Beige Adipocytes. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2019, 1864, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Gharanei, S.; Shabir, K.; Brown, J.E.; Weickert, M.O.; Barber, T.M.; Kyrou, I.; Randeva, H.S. Regulatory MicroRNAs in Brown, Brite and White Adipose Tissue. Cells 2020, 9, 2489. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Pan, R.; Pfeifer, A. Regulation of Brown and Beige Fat by MicroRNAs. Pharmacol. Ther. 2017, 170, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Oliverio, M.; Schmidt, E.; Mauer, J.; Baitzel, C.; Hansmeier, N.; Khani, S.; Konieczka, S.; Pradas-Juni, M.; Brodesser, S.; Van, T.-M.; et al. Dicer1–MiR-328–Bace1 Signalling Controls Brown Adipose Tissue Differentiation and Function. Nat. Cell Biol. 2016, 18, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Meakin, P.J.; Harper, A.J.; Hamilton, D.L.; Gallagher, J.; McNeilly, A.D.; Burgess, L.A.; Vaanholt, L.M.; Bannon, K.A.; Latcham, J.; Hussain, I.; et al. Reduction in BACE1 Decreases Body Weight, Protects against Diet-Induced Obesity and Enhances Insulin Sensitivity in Mice. Biochem. J. 2012, 441, 285–296. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Zuo, J.; Zhang, Y.; Xie, Y.; Hu, F.; Chen, L.; Liu, B.; Liu, F. Identification of MiR-106b-93 as a Negative Regulator of Brown Adipocyte Differentiation. Biochem. Biophys. Res. Commun. 2013, 438, 575–580. [Google Scholar] [CrossRef]
- Okamatsu-Ogura, Y.; Matsushita, M.; Bariuan, J.V.; Nagaya, K.; Tsubota, A.; Saito, M. Association of Circulating Exosomal MiR-122 Levels with BAT Activity in Healthy Humans. Sci. Rep. 2019, 9, 13243. [Google Scholar] [CrossRef] [Green Version]
- Karbiener, M.; Pisani, D.F.; Frontini, A.; Oberreiter, L.M.; Lang, E.; Vegiopoulos, A.; Mössenböck, K.; Bernhardt, G.A.; Mayr, T.; Hildner, F.; et al. MicroRNA-26 Family Is Required for Human Adipogenesis and Drives Characteristics of Brown Adipocytes: MicroRNA-26 Family in Human Adipogenesis. Stem. Cells 2014, 32, 1578–1590. [Google Scholar] [CrossRef]
- Zhang, H.; Guan, M.; Townsend, K.L.; Huang, T.L.; An, D.; Yan, X.; Xue, R.; Schulz, T.J.; Winnay, J.; Mori, M.; et al. MicroRNA-455 Regulates Brown Adipogenesis via a Novel HIF1an-AMPK -PGC1α Signaling Network. EMBO Rep. 2015, 16, 1378–1393. [Google Scholar] [CrossRef] [Green Version]
- Giroud, M.; Pisani, D.F.; Karbiener, M.; Barquissau, V.; Ghandour, R.A.; Tews, D.; Fischer-Posovszky, P.; Chambard, J.-C.; Knippschild, U.; Niemi, T.; et al. MiR-125b Affects Mitochondrial Biogenesis and Impairs Brite Adipocyte Formation and Function. Mol. Metab. 2016, 5, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Wang, M.; Xiao, T.; Yin, B.; He, L.; Meng, W.; Dong, M.; Liu, F. MiR-30 Promotes Thermogenesis and the Development of Beige Fat by Targeting RIP140. Diabetes 2015, 64, 2056–2068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fritah, A.; Christian, M.; Parker, M.G. The Metabolic Coregulator RIP140: An Update. Am. J. Physiol. -Endocrinol. Metab. 2010, 299, E335–E340. [Google Scholar] [CrossRef]
- Pan, D.; Mao, C.; Quattrochi, B.; Friedline, R.H.; Zhu, L.J.; Jung, D.Y.; Kim, J.K.; Lewis, B.; Wang, Y.-X. MicroRNA-378 Controls Classical Brown Fat Expansion to Counteract Obesity. Nat. Commun. 2014, 5, 4725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francis, S.H.; Blount, M.A.; Corbin, J.D. Mammalian Cyclic Nucleotide Phosphodiesterases: Molecular Mechanisms and Physiological Functions. Physiol. Rev. 2011, 91, 651–690. [Google Scholar] [CrossRef] [Green Version]
- Fu, X.; Jin, L.; Han, L.; Yuan, Y.; Mu, Q.; Wang, H.; Yang, J.; Ning, G.; Zhou, D.; Zhang, Z. MiR-129-5p Inhibits Adipogenesis through Autophagy and May Be a Potential Biomarker for Obesity. Int. J. Endocrinol. 2019, 2019, 5069578. [Google Scholar] [CrossRef]
- Ge, X.; Sathiakumar, D.; Lua, B.J.G.; Kukreti, H.; Lee, M.; McFarlane, C. Myostatin Signals through MiR-34a to Regulate Fndc5 Expression and Browning of White Adipocytes. Int. J. Obes. 2017, 41, 137–148. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Trajkovski, M. MiR-27 Orchestrates the Transcriptional Regulation of Brown Adipogenesis. Metabolism 2014, 63, 272–282. [Google Scholar] [CrossRef]
- Karkeni, E.; Astier, J.; Tourniaire, F.; El Abed, M.; Romier, B.; Gouranton, E.; Wan, L.; Borel, P.; Salles, J.; Walrand, S.; et al. Obesity-Associated Inflammation Induces MicroRNA-155 Expression in Adipocytes and Adipose Tissue: Outcome on Adipocyte Function. J. Clin. Endocrinol. Metab. 2016, 101, 1615–1626. [Google Scholar] [CrossRef]
- Trayhurn, P.; Douglas, J.B.; McGuckin, M.M. Brown Adipose Tissue Thermogenesis Is ‘Suppressed’ during Lactation in Mice. Nature 1982, 298, 59–60. [Google Scholar] [CrossRef]
- Rothwell, N.J.; Stock, M.J. A Role for Brown Adipose Tissue in Diet-Induced Thermogenesis. Nature 1979, 281, 31–35. [Google Scholar] [CrossRef]
- Osman, F.; Franklyn, J.A.; Daykin, J.; Chowdhary, S.; Holder, R.L.; Sheppard, M.C.; Gammage, M.D. Heart Rate Variability and Turbulence in Hyperthyroidism before, during, and after Treatment. Am. J. Cardiol. 2004, 94, 465–469. [Google Scholar] [CrossRef]
- Scheen, A.J. Sibutramine on Cardiovascular Outcome. Diabetes Care 2011, 34, S114–S119. [Google Scholar] [CrossRef] [Green Version]
- Grundlingh, J.; Dargan, P.I.; El-Zanfaly, M.; Wood, D.M. 2,4-Dinitrophenol (DNP): A Weight Loss Agent with Significant Acute Toxicity and Risk of Death. J. Med. Toxicol. 2011, 7, 205–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langeveld, M.; Tan, C.Y.; Soeters, M.R.; Virtue, S.; Ambler, G.K.; Watson, L.P.E.; Murgatroyd, P.R.; Chatterjee, V.K.; Vidal-Puig, A. Mild Cold Effects on Hunger, Food Intake, Satiety and Skin Temperature in Humans. Endocr. Connect. 2016, 5, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Dulloo, A.G. The Search for Compounds That Stimulate Thermogenesis in Obesity Management: From Pharmaceuticals to Functional Food Ingredients: Thermogenic Bioactive Food Ingredients. Obes. Rev. 2011, 12, 866–883. [Google Scholar] [CrossRef] [PubMed]
- Tsai, V.W.W.; Husaini, Y.; Sainsbury, A.; Brown, D.A.; Breit, S.N. The MIC-1/GDF15-GFRAL Pathway in Energy Homeostasis: Implications for Obesity, Cachexia, and Other Associated Diseases. Cell Metab. 2018, 28, 353–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmers, T.A.; Fishel, M.L.; Bonetto, A. STAT3 in the Systemic Inflammation of Cancer Cachexia. Semin. Cell Dev. Biol. 2016, 54, 28–41. [Google Scholar] [CrossRef] [Green Version]
- Ozguven, S.; Ones, T.; Yilmaz, Y.; Turoglu, H.T.; Imeryuz, N. The Role of Active Brown Adipose Tissue in Human Metabolism. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 355–361. [Google Scholar] [CrossRef]
- Blondin, D.P.; Labbé, S.M.; Tingelstad, H.C.; Noll, C.; Kunach, M.; Phoenix, S.; Guérin, B.; Turcotte, É.E.; Carpentier, A.C.; Richard, D.; et al. Increased Brown Adipose Tissue Oxidative Capacity in Cold-Acclimated Humans. J. Clin. Endocrinol. Metab. 2014, 99, E438–E446. [Google Scholar] [CrossRef] [PubMed]
- Ahfeldt, T.; Schinzel, R.T.; Lee, Y.-K.; Hendrickson, D.; Kaplan, A.; Lum, D.H.; Camahort, R.; Xia, F.; Shay, J.; Rhee, E.P.; et al. Programming Human Pluripotent Stem Cells into White and Brown Adipocytes. Nat. Cell Biol. 2012, 14, 209–219. [Google Scholar] [CrossRef] [Green Version]
- Shankar, K.; Kumar, D.; Gupta, S.; Varshney, S.; Rajan, S.; Srivastava, A.; Gupta, A.; Gupta, A.P.; Vishwakarma, A.L.; Gayen, J.R.; et al. Role of Brown Adipose Tissue in Modulating Adipose Tissue Inflammation and Insulin Resistance in High-Fat Diet Fed Mice. Eur. J. Pharmacol. 2019, 854, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Sohn, J.H.; Kim, J.I.; Jeon, Y.G.; Park, J.; Kim, J.B. Effects of Three Thiazolidinediones on Metabolic Regulation and Cold-Induced Thermogenesis. Mol. Cells 2018, 41, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, C.; Liu, J.; Xie, W.; Xu, W.; Liang, F.; Huang, K.; He, X. Intraperitoneal Administration of Follistatin Promotes Adipocyte Browning in High-Fat Diet-Induced Obese Mice. PLoS ONE 2019, 14, e0220310. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| miRNA | Role in Brown/Beige Fat Development | Target(s) | Species/Reference |
|---|---|---|---|
| miRNA328 | Positive regulation of BAT differentiation | Bace1 | Mouse [155,156] |
| miRNA 193b-365 | Regulation of BAT differentiation | Runx1t1 | Mouse [81] |
| miRNA203 | Promotes BAT adipogenesis | Insig 1, Pdgfr 2 | Mouse [83] |
| miRNA182 | Promotes BAT adipogenesis | Insig 1, Pdgfr 2 | Mouse [83] |
| miRNA129 | Positive regulator of BAT function | Igf2, Egr1 | Human, Mouse [166] |
| miRNA106b | Negative regulator of BAT differentiation | Ppara | Mouse [157] |
| miRNA34a | Negative regulator of BAT and beige adipogenesis | Fgfr1 | Mouse [167] |
| miRNA27b | Negative regulator of BAT and beige adipogenesis | Prdm16, Ppara, Pparg, Creb | Mouse [168] |
| miRNA93b | Negative regulator of BAT differentiation | Ppara | Mouse [157] |
| miRNA196a | Promotes adipose browning | Hoxc8 | Mouse [84] |
| miRNA26a/b | Promotes white and beige adipocyte differentiation | ADAM17, Fbx19 | Human, Mouse [159] |
| miRNA125-5p | Negative regulator of adipose browning and mitochondrial biogenesis | MMP11 | Human [161] |
| miRNA30b/c | Positive regulator of BAT and beige adipocyte development | Rip140 | Mouse [162,163] |
| miRNA455 | Positive regulator of BAT and beige adipose browning | Runx1t1, Necdin, Hif1an | Human, Mouse [160] |
| miRNA378 | Promotes BAT mass and brown adipogenesis but negatively regulates beige adipogenesis | Pde1b | Mouse [164,165] |
| miRNA122 | Negatively regulates BAT activity | CD320, AldoA, BCKDK | Human [158] |
| miRNA133 | Negative regulator of BAT differentiation | Prdm16 | Mouse [85] |
| miRNA155 | Negative regulator of BAT differentiation | C/ebpβ | Mouse, Human [169] |
| Model | Design | Factors Measured | Findings |
|---|---|---|---|
| Human | Tested 17 healthy subjects in 15–16 °C with increasing time to 6 h/day for 10 days with 18F-FDG-PET & abdominal subcutaneous fat biopsy [94] | Glucose uptake in BAT & WAT, energy expenditure, RMR, and NST. | Both females & males in cold exposure: |
| NST , Detectable BAT volume . | |||
| BAT activity , Energy expenditure . | |||
| Human | Single-blinded, placebo-controlled clinical trial. Exposed to 17 °C for 2 h/day for 6 weeks. Subjects with undetectable BAT were placed in cold (n = 12) and control (n = 10) groups. Capsinoid capsules with 9 or 0 mg/day given for 6 weeks. [136] | Body fat content; metabolic activity; EE; 18F-FDG-PET utilized. | After cold exposure: BAT activity in BAT + than in BAT− groups. |
| Beige adipocytes recruited by BAT via cold & capsinoid treatment. | |||
| Capsinoids BAT thermogenesis & recruited BAT. | |||
| Human | A study between BAT & adiposity in 162 healthy volunteers (103 males & 59 females). 18F-FDG-PET/CT scan after 2 h at 19 °C. [42] | BMI, serum leptin, areas of visceral & subcutaneous fat. | 41% of subjects were found with cold-activated BAT. |
| Detectable BAT with age, with BAT found in 50% of 20-year-olds and >10% in 50-year-olds. | |||
| Human | Clinical trial with 15 subjects underwent cold exposure for 120 min at 14 °C. Treated with 200 mg/os mirabegron or placebo in a double blinded trial. [106] | Detected & quantified mirabegron via Agilent 6460 LC-MS/MS triple quadrupole mass spectrometer. | Cold exposure Resting metabolic rate. |
| Mirabegron BAT glucose uptake. | |||
| No correlation between drug- & cold-stimulation to measure BAT mass or activity. | |||
| Human | rospective study analyzed 5907 patients with 18F-FDG PET/CT scanning, but stringent standards analysis of only 100 patients. [179] | Focal FDG uptake, blood glucose, liver fat content, lipid panel (Total, HDL, and LDL cholesterol; ALT; AST; triglycerides). | 8 men and 17 women brown fat uptake [ABAT (+) group]. |
| Total & LDL cholesterol in ABAT (+) vs. ABAT (−). | |||
| Prevalence of NAFLD in ABAT (−) vs. ABAT (+). | |||
| ABAT serum transaminases (ALT & AST). | |||
| Human | 10 lean & 14 overweight men on cold exposure for 2 h. 18F-FDG-PET scan for BAT activity. [7] | BAT activity & volume, skin & core temperature, BMI | BAT activity in obese subjects vs. lean subjects. |
| BAT volume in obese subjects vs. lean subjects. | |||
| Human | 6 non-acclimated men placed in a 10 °C environment for 2 h daily for 4 weeks. Subjected to electromyography and PET with [11C] acetate & [18F] FDG. [180] | Shivering intensity; BAT metabolism; fractional glucose uptake; insulin; triglycerides; T3; T4; ACTH; leptin levels | 1.9-fold in thermogenic rate in cold exposure. |
| Total BAT volume and activity by 45% after 4 weeks. | |||
| Fractional glucose uptake in supraclavicular BAT. | |||
| Mouse | C57Bl/6 mice with FGF21-KO phenotype subjected 5 °C for 72-h period. Extracted brown fat pads and subjected for RNA isolation, immunohistochemistry, and western blot analysis. [114] | Temperature, confluence of BAT in culture, PGC-1a mRNA expression. | Single-dose recombinant FGF21 browning of WAT. |
| Cold exposure & β3-AR stimulation FGF21 in BAT & thermogenically competent WAT depots. | |||
| FGF21 absence impaired adaptive thermogenesis. | |||
| PGC1-α required for FGF21’s function. | |||
| Mouse | Treated hESCs & hiPSCs to induce embryoid bodies and adipogenic differentiation for 21 days. Subcutaneously transplanted adipocytes in Rag2−/−; Il2yc mice. Infused FGF21 or saline pumps transplanted to the interscapular region. [181] | Lipolysis activity; adiponectin & leptin expression; glucose uptake; oxygen consumption | Converted hPSCs MPCs BAT & WAT |
| Doxycycline treatment PPARG2 WAT phenotype. PPARG2-CEBPB-PRDM16 axis programmed BAT. Transplantation of hPSC-derived adipocytes mature functionality. | |||
| Mouse | 6–8 week-old mice fed with HFD for 8 weeks prior to BAT transplantation; no cold exposure. [182] | Body weight, basal glucose levels, and insulin tolerance. | BAT transplantation HFD-induced obesity; Glucose tolerance; Insulin resistance. BAT removal diet-induced obesity & insulin resistance. |
| Mouse | Study with strain-, sex-, and age-matched donor mice, whose BAT transplanted to other mice. Recipient mice fed on HFD, post transplantation for 20 weeks; cold exposure utilized. [15] | Body weight, total body fat (%), body temperature, O2 consumption, mRNA | BAT transplantation HFD-induced weight gain; |
| Total fat; core body temperature; mRNA of FA oxidation-related genes; HFD-induced insulin resistance; HFD-induced hepatic steatosis & obesity. | |||
| Mouse | 12-week-old male C57BL/6 mice as recipient mice fed standard food and were transplanted into the visceral cavity with 0.1 or 0.4 g BAT, or 0.1 g WAT from epididymal fat pad. [16] | Glucose concentration & uptake; food & water intake; CO2 & heat production; plasma lipids, hormones & proteins. | BAT transplantation glucose tolerance & insulin sensitivity; BAT mass metabolic homeostasis. |
| BAT transplantation more favorable circulating lipid and hormonal profile; circulating norepinephrine concentration & 5-fold increase in serum FGF21 levels. | |||
| Mouse | TZDs (Lobe, Rosi, and Pio) tested on cold acclimated 10-week-old C57BLKS/J-Leprdb/Leprdb male mice for 4 weeks. Raw264.7 macrophages & 3T3-cells treated with TZDs. [183] | Blood glucose; Glucose uptake; body weight; serum triglycerides, cholesterol, and FFA. | TZDs promoted adipocyte differentiation. |
| Lobe stimulation >> two TZDs (Rosi & Pio) effect. | |||
| Lobe beige adipocyte formation & proinflammatory signals in leukocytes & adipocytes. | |||
| Mouse | After acclimation, 4- to 6-week-old C57BL/6J male mice put on high a fat diet for 8 weeks. Post-8 weeks, intraperitoneal injection of 8.5 ug/kg of recombinant follistatin (once/day for one week). [184] | Rectal temperature, mice activity; O2 consumption; CO2 production; glucose tolerance. | HFD-induced obesity; insulin sensitivity. |
| subcutaneous fat browning via AMPK-PGC1a-irisin signaling pathway. | |||
| metabolism via insulin pathway in beige adipocytes. | |||
| Mouse | Male and female heterozygous mice used for mouse embryonic fibroblasts. C57BL/6 mice exposed to cold environment for 8 h. BAT harvested and compared to thermoneutral mice. 0.5 ug/uL of FST on brown preadipocytes for BAT differentiation. [124] | Genotyped embryos, gene and protein expressions of browning & metabolism-related genes; Oxygen consumption. | Follistatin cellular respiration. |
| Follistatin secreted by AT in a paracrine manner. | |||
| Follistatin brown adipocyte and thermogenic gene expression in differentiated brown adipocytes and MEF cultures from both WT and FST KO mice. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, R.; Barrios, A.; Dirakvand, G.; Pervin, S. Human Brown Adipose Tissue and Metabolic Health: Potential for Therapeutic Avenues. Cells 2021, 10, 3030. https://doi.org/10.3390/cells10113030
Singh R, Barrios A, Dirakvand G, Pervin S. Human Brown Adipose Tissue and Metabolic Health: Potential for Therapeutic Avenues. Cells. 2021; 10(11):3030. https://doi.org/10.3390/cells10113030
Chicago/Turabian StyleSingh, Rajan, Albert Barrios, Golnaz Dirakvand, and Shehla Pervin. 2021. "Human Brown Adipose Tissue and Metabolic Health: Potential for Therapeutic Avenues" Cells 10, no. 11: 3030. https://doi.org/10.3390/cells10113030