Hypoxia and Extracellular Acidification as Drivers of Melanoma Progression and Drug Resistance
Abstract
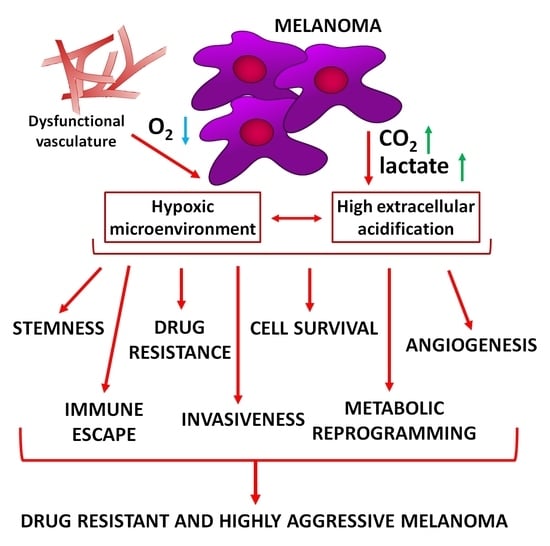
:1. Introduction
2. Hypoxia
2.1. Molecular Basis of Hypoxia
2.2. Role of Hypoxia in Melanoma Progression
2.2.1. Prosurvival Role of Hypoxia
2.2.2. Angiogenesis and Vasculogenic Mimicry Induced by Low Oxygen Levels
2.2.3. Melanoma Plasticity and Invasiveness under Hypoxia
2.2.4. Metabolic Reprogramming Induced by Low Oxygen Level
2.2.5. Hypoxia-Dependent Immune Escape
2.2.6. Reduced Therapy Efficiency and Novel Hypoxia-Targeted Treatment Strategies
3. Acidification
3.1. Molecular Basis of Tumor Microenvironment Acidification
3.2. Transporters Related to pH Regulation
3.3. Acid-Resistant Phenotype of Melanoma Cells
3.4. Epithelial-to-Mesenchymal Transition and Stemness in Acid-Adapted Melanoma Cells
3.5. Extracellular Acidification-Induced Adhesion, Invasion, and Angiogenesis
3.6. Drug Resistance Related to Tumor Niche Acidification
3.7. Immune Escape Mediated by Tumor Niche Acidification
3.8. Therapies Targeting Extracellular Acidification
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miller, A.J.; Mihm, M.C. Melanoma. N. Engl. J. Med. 2006, 355, 51–65. [Google Scholar] [CrossRef]
- Costin, G.E.; Hearing, V.J. Human skin pigmentation: Melanocytes modulate skin color in response to stress. FASEB J. 2007, 21, 976–994. [Google Scholar] [CrossRef]
- Grzywa, T.M.; Paskal, W.; Włodarski, P.K. Intratumor and Intertumor Heterogeneity in Melanoma. Transl. Oncol. 2017, 10, 956–975. [Google Scholar] [CrossRef]
- Ekedahl, H.; Cirenajwis, H.; Harbst, K.; Carneiro, A.; Nielsen, K.; Olsson, H.; Lundgren, L.; Ingvar, C.; Jönsson, G. The clinical significance of BRAF and NRAS mutations in a clinic-based metastatic melanoma cohort. Br. J. Dermatol. 2013, 169, 1049–1055. [Google Scholar] [CrossRef]
- Garbe, C.; Amaral, T.; Peris, K.; Hauschild, A.; Arenberger, P.; Bastholt, L.; Bataille, V.; del Marmol, V.; Dréno, B.; Fargnoli, M.C.; et al. European consensus-based interdisciplinary guideline for melanoma. Part 2: Treatment—Update 2019. Eur. J. Cancer 2020, 126, 159–177. [Google Scholar] [CrossRef] [Green Version]
- Rosell, R.; Karachaliou, N.; Morales-Espinosa, D.; Costa, C.; Molina, M.A.; Sansano, I.; Gasco, A.; Viteri, S.; Massuti, B.; Wei, J.; et al. Adaptive resistance to targeted therapies in cancer. Transl. Lung Cancer Res. 2013, 2, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Mazurkiewicz, J.; Simiczyjew, A.; Dratkiewicz, E.; Ziętek, M.; Matkowski, R.; Nowak, D. Stromal Cells Present in the Melanoma Niche Affect Tumor Invasiveness and Its Resistance to Therapy. Int. J. Mol. Sci. 2021, 22, 529. [Google Scholar] [CrossRef]
- Ruiter, D.; Bogenrieder, T.; Elder, D.; Herlyn, M. Melanoma—Stroma interactions: Structural and functional aspects. Lancet Oncol. 2002, 3, 35–43. [Google Scholar] [CrossRef]
- Gurzu, S.; Beleaua, M.A.; Jung, I. The role of tumor microenvironment in development and progression of malignant melanomas-a systematic review. Rom. J. Morphol. Embryol. 2018, 59, 23–28. [Google Scholar] [PubMed]
- Simiczyjew, A.; Dratkiewicz, E.; Mazurkiewicz, J.; Ziętek, M.; Matkowski, R.; Nowak, D. The Influence of Tumor Microenvironment on Immune Escape of Melanoma. Int. J. Mol. Sci. 2020, 21, 8359. [Google Scholar] [CrossRef] [PubMed]
- Muz, B.; de la Puente, P.; Azab, F.; Azab, A.K. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia 2015, 3, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semenza, G.L. Hypoxia, clonal selection, and the role of HIF-1 in tumor progression. Crit. Rev. Biochem. Mol. Biol. 2000, 35, 71–103. [Google Scholar] [CrossRef] [PubMed]
- Pouysségur, J.; Dayan, F.; Mazure, N.M. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature 2006, 441, 437–443. [Google Scholar] [CrossRef]
- Evans, S.M.; Schrlau, A.E.; Chalian, A.A.; Zhang, P.; Koch, C.J. Oxygen levels in normal and previously irradiated human skin as assessed by EF5 binding. J. Investig. Dermatol. 2006, 126, 2596–2606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaupel, P. Hypoxia and Aggressive Tumor Phenotype: Implications for Therapy and Prognosis. Oncologist 2008, 13, 21–26. [Google Scholar] [CrossRef] [Green Version]
- Lartigau, E.; Randrianarivelo, H.; Avril, M.F.; Margulis, A.; Spatz, A.; Eschwège, F.; Guichard, M. Intratumoral oxygen tension in metastatic melanoma. Melanoma Res. 1997, 7, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lin, D.; Taniguchi, C.M. Hypoxia inducible factor (HIF) in the tumor microenvironment: Friend or foe? Sci. China Life Sci. 2017, 60, 1114–1124. [Google Scholar] [CrossRef]
- Mole, D.R.; Blancher, C.; Copley, R.R.; Pollard, P.J.; Gleadle, J.M.; Ragousis, J.; Ratcliffe, P.J. Genome-wide association of hypoxia-inducible factor (HIF)-1α and HIF-2α DNA binding with expression profiling of hypoxia-inducible transcripts. J. Biol. Chem. 2009, 284, 16767–16775. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.L.; Jiang, B.H.; Rue, E.A.; Semenza, G.L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA 1995, 92, 5510–5514. [Google Scholar] [CrossRef] [Green Version]
- Weidemann, A.; Johnson, R.S. Biology of HIF-1α. Cell Death Differ. 2008, 15, 621–627. [Google Scholar] [CrossRef] [Green Version]
- Semenza, G.L. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu. Rev. Pathol. Mech. Dis. 2014, 9, 47–71. [Google Scholar] [CrossRef] [PubMed]
- Masoud, G.N.; Li, W. HIF-1α pathway: Role, regulation and intervention for cancer therapy. Acta Pharm. Sin. B 2015, 5, 378–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Ignazio, L.; Batie, M.; Rocha, S. Hypoxia and inflammation in cancer, focus on HIF and NF-κB. Biomedicines 2017, 5, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, W.; Wang, Y. Epigenetic regulators: Multifunctional proteins modulating hypoxia-inducible factor-α protein stability and activity. Cell. Mol. Life Sci. 2018, 75, 1043–1056. [Google Scholar] [CrossRef]
- Zhong, H.; De Marzo, A.M.; Laughner, E.; Lim, M.; Hilton, D.A.; Zagzag, D.; Buechler, P.; Isaacs, W.B.; Semenza, G.L.; Jonathan, W. Simons Overexpression of Hypoxia-inducible Factor 1α in Common Human Cancers and Their Metastases. Cancer Res. 1999, 59, 5830–5835. [Google Scholar]
- Mouriaux, F.; Sanschagrin, F.; Diorio, C.; Landreville, S.; Comoz, F.; Petit, E.; Bernaudin, M.; Rousseau, A.P.; Bergeron, D.; Morcos, M. Increased HIF-1α expression correlates with cell proliferation and vascular markers CD31 and VEGF-A in uveal melanoma. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1277–1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mills, C.N.; Joshi, S.S.; Niles, R.M. Expression and function of hypoxia inducible factor-1 alpha in human melanoma under non-hypoxic conditions. Mol. Cancer 2009, 8, 104. [Google Scholar] [CrossRef] [Green Version]
- Kuphal, S.; Winklmeier, A.; Warnecke, C.; Bosserhoff, A.K. Constitutive HIF-1 activity in malignant melanoma. Eur. J. Cancer 2010, 46, 1159–1169. [Google Scholar] [CrossRef]
- Ercin, M.E.; Bozdoğan, Ö.; Çavuşoğlu, T.; Bozdoğan, N.; Atasoy, P.; Koçak, M. Hypoxic gene signature of primary and metastatic melanoma cell lines: Focusing on HIF-1β and NDRG-1. Balkan Med. J. 2020, 37, 15–23. [Google Scholar] [CrossRef]
- Hao, T.; Li, C.X.; Ding, X.Y.; Xing, X.J. MicroRNA-363-3p/p21(Cip1/Waf1) axis is regulated by HIF-2α in mediating stemness of melanoma cells. Neoplasma 2019, 66, 427–436. [Google Scholar] [CrossRef]
- Giatromanolaki, A.; Sivridis, E.; Kouskoukis, C.; Gatter, K.C.; Harris, A.L.; Koukourakis, M.I. Hypoxia-inducible factors 1AE and 2AE are related to vascular endothelial growth factor expression and a poorer prognosis in nodular malignant melanomas of the skin. Melanoma Res. 2003, 13, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Steunou, A.L.; Ducoux-Petit, M.; Lazar, I.; Monsarrat, B.; Erard, M.; Muller, C.; Clottes, E.; Burlet-Schiltz, O.; Nieto, L. Identification of the hypoxia-inducible factor 2α nuclear interactome in melanoma cells reveals master proteins involved in melanoma development. Mol. Cell. Proteom. 2013, 12, 736–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoefflin, R.; Harlander, S.; Schäfer, S.; Metzger, P.; Kuo, F.; Schönenberger, D.; Adlesic, M.; Peighambari, A.; Seidel, P.; Chen, C.Y.; et al. HIF-1α and HIF-2α differently regulate tumour development and inflammation of clear cell renal cell carcinoma in mice. Nat. Commun. 2020, 11, 4111. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, G.; Guo, J.; Chen, X.; Lei, J.; Ze, K.; Dong, L.; Dai, X.; Gao, Y.; Song, D.; et al. Loss of Phd2 cooperates with BRAF V600E to drive melanomagenesis. Nat. Commun. 2018, 9, 5426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greijer, A.E.; Van Der Wall, E. The role of hypoxia inducible factor 1 (HIF-1) in hypoxia induced apoptosis. J. Clin. Pathol. 2004, 57, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Mazar, J.; Qi, F.; Lee, B.; Marchica, J.; Govindarajan, S.; Shelley, J.; Li, J.-L.; Ray, A.; Perera, R.J. MicroRNA 211 Functions as a Metabolic Switch in Human Melanoma Cells. Mol. Cell. Biol. 2016, 36, 1090–1108. [Google Scholar] [CrossRef] [Green Version]
- Maadi, H.; Moshtaghian, A.; Taha, M.F.; Mowla, S.J.; Kazeroonian, A.; Haass, N.K.; Javeri, A. Multimodal tumor suppression by miR-302 cluster in melanoma and colon cancer. Int. J. Biochem. Cell Biol. 2016, 81, 121–132. [Google Scholar] [CrossRef]
- Leszczynska, K.B.; Foskolou, I.P.; Abraham, A.G.; Anbalagan, S.; Tellier, C.; Haider, S.; Span, P.N.; O’Neill, E.E.; Buffa, F.M.; Hammond, E.M. Hypoxia-induced p53 modulates both apoptosis and radiosensitivity via AKT. J. Clin. Investig. 2015, 125, 2385–2398. [Google Scholar] [CrossRef] [Green Version]
- Murphy, M.; Phelps, A.; Swede, H.; Claffey, K. Hypoxia-induced response of cell cycle and apoptosis regulators in melanoma. Int. J. Dermatol. 2012, 51, 1263–1267. [Google Scholar] [CrossRef]
- Warren, C.F.A.; Wong-Brown, M.W.; Bowden, N.A. BCL-2 family isoforms in apoptosis and cancer. Cell Death Dis. 2019, 10, 177. [Google Scholar] [CrossRef] [Green Version]
- Flamant, L.; Notte, A.; Ninane, N.; Raes, M.; Michiels, C. Anti-apoptotic role of HIF-1 and AP-1 in paclitaxel exposed breast cancer cells under hypoxia. Mol. Cancer 2010, 9, 191. [Google Scholar] [CrossRef] [Green Version]
- Boisvert-Adamo, K.; Longmate, W.; Abel, E.V.; Aplin, A.E. Mcl-1 is required for melanoma cell resistance to anoikis. Mol. Cancer Res. 2009, 7, 549–556. [Google Scholar] [CrossRef] [Green Version]
- Bacchi, P.S.; Bloise, A.C.; Bustos, S.O.; Zimmermann, L.; Chammas, R.; Rabbani, S.R. Metabolism under hypoxia in Tm1 murine melanoma cells is affected by the presence of galectin-3, a metabolomics approach. SpringerPlus 2014, 3, 470. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, Y.; Jia, W.; Kidoya, H.; Muramatsu, F.; Tsukada, Y.; Takakura, N. Galectin-3 Inhibits Cancer Metastasis by Negatively Regulating Integrin β3 Expression. Am. J. Pathol. 2019, 189, 900–910. [Google Scholar] [CrossRef]
- Bustos, S.O.; da Silva Pereira, G.J.; de Freitas Saito, R.; Gil, C.D.; Zanatta, D.B.; Smaili, S.S.; Chammas, R. Galectin-3 sensitized melanoma cell lines to vemurafenib (PLX4032) induced cell death through prevention of autophagy. Oncotarget 2018, 9, 14567–14579. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Wang, D.; Li, H. Initiation of premature senescence by Bcl-2 in hypoxic condition. Int. J. Clin. Exp. Pathol. 2014, 7, 2446–2453. [Google Scholar] [PubMed]
- Mo, J.; Sun, B.; Zhao, X.; Gu, Q.; Dong, X.; Liu, Z.; Ma, Y.; Zhao, N.; Tang, R.; Liu, Y.; et al. Hypoxia-induced senescence contributes to the regulation of microenvironment in melanomas. Pathol. Res. Pract. 2013, 209, 640–647. [Google Scholar] [CrossRef]
- Shimizu, T.; Nagane, M.; Suzuki, M.; Yamauchi, A.; Kato, K.; Kawashima, N.; Nemoto, Y.; Maruo, T.; Kawakami, Y.; Yamashita, T. Tumor hypoxia regulates ganglioside GM3 synthase, which contributes to oxidative stress resistance in malignant melanoma. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129723. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Q.; Sun, P.; Go, L.; Koti, V.; Fliman, M.; Paller, A.S. Ganglioside GM3 promotes carcinoma cell proliferation via urokinase plasminogen activator-induced extracellular signal-regulated kinase-independent p70S6 kinase signaling. J. Investig. Dermatol. 2006, 126, 2687–2696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daskalaki, I.; Gkikas, I.; Tavernarakis, N. Hypoxia and selective autophagy in cancer development and therapy. Front. Cell Dev. Biol. 2018, 6, 104. [Google Scholar] [CrossRef] [Green Version]
- Buart, S.; Terry, S.; Noman, M.Z.; Lanoy, E.; Boutros, C.; Fogel, P.; Dessen, P.; Meurice, G.; Gaston-Mathé, Y.; Vielh, P.; et al. Transcriptional response to hypoxic stress in melanoma and prognostic potential of GBE1 and BNIP3. Oncotarget 2017, 8, 108786–108801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giatromanolaki, A.N.; St Charitoudis, G.; Bechrakis, N.E.; Kozobolis, V.P.; Koukourakis, M.I.; Foerster, M.H.; Sivridis, E.L. Autophagy patterns and prognosis in uveal melanomas. Mod. Pathol. 2011, 24, 1036–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuo, T.; Daishaku, S.; Sadzuka, Y. Lactic acid promotes cell survival by blocking autophagy of B16F10 mouse melanoma cells under glucose deprivation and hypoxic conditions. Biol. Pharm. Bull. 2019, 42, 837–839. [Google Scholar] [CrossRef] [Green Version]
- Schito, L. Hypoxia-dependent angiogenesis and lymphangiogenesis in cancer. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2019; Volume 1136, pp. 71–85. [Google Scholar]
- De Palma, M.; Biziato, D.; Petrova, T.V. Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer 2017, 17, 457–474. [Google Scholar] [CrossRef]
- Das, A.M.; Pescatori, M.; Vermeulen, C.E.; Rens, J.A.P.; Seynhaeve, A.L.B.; Koning, G.A.; Eggermont, A.M.M.; ten Hagen, T.L.M. Melanomas prevent endothelial cell death under restrictive culture conditions by signaling through AKT and p38 MAPK/ERK-1/2 cascades. Oncoimmunology 2016, 5, e1219826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klimkiewicz, K.; Weglarczyk, K.; Collet, G.; Paprocka, M.; Guichard, A.; Sarna, M.; Jozkowicz, A.; Dulak, J.; Sarna, T.; Grillon, C.; et al. A 3D model of tumour angiogenic microenvironment to monitor hypoxia effects on cell interactions and cancer stem cell selection. Cancer Lett. 2017, 396, 10–20. [Google Scholar] [CrossRef]
- Spinella, F.; Caprara, V.; Cianfrocca, R.; Rosanò, L.; Di Castro, V.; Garrafa, E.; Natali, P.G.; Bagnato, A. The interplay between hypoxia, endothelial and melanoma cells regulates vascularization and cell motility through endothelin-1 and vascular endothelial growth factor. Carcinogenesis 2014, 35, 840–848. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Goldfinger, L.E. RLIP76 regulates HIF-1 activity, VEGF expression and secretion in tumor cells, and secretome transactivation of endothelial cells. FASEB J. 2014, 28, 4158–4168. [Google Scholar] [CrossRef] [Green Version]
- Yoon, N.A.; Jung, S.J.; Choi, S.H.; Ryu, J.H.; Mani, M.; Lee, U.H.; Vo, M.T.; Jeon, D.Y.; Chung, S.W.; Ju Lee, B.; et al. DRG2 supports the growth of primary tumors and metastases of melanoma by enhancing VEGF-A expression. FEBS J. 2020, 287, 2070–2086. [Google Scholar] [CrossRef]
- Gabellini, C.; De Luca, T.; Trisciuoglio, D.; Desideri, M.; Di Martile, M.; Passeri, D.; Candiloro, A.; Biffoni, M.; Rizzo, M.G.; Orlandi, A.; et al. BH4 domain of bcl-2 protein is required for its proangiogenic function under hypoxic condition. Carcinogenesis 2013, 34, 2558–2567. [Google Scholar] [CrossRef] [Green Version]
- Hu, K.; Babapoor-Farrokhran, S.; Rodrigues, M.; Deshpande, M.; Puchner, B.; Kashiwabuchi, F.; Hassan, S.J.; Asnaghi, L.; Handa, J.T.; Merbs, S.; et al. Hypoxia-inducible factor 1 upregulation of both VEGF and ANGPTL4 is required to promote the angiogenic phenotype in uveal melanoma. Oncotarget 2016, 7, 7816–7828. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Zong, Y.; Gao, Y.; Sun, X.; Zhao, H.; Luo, W.; Jia, S. VEGF Induce Vasculogenic Mimicry of Choroidal Melanoma through the PI3k Signal Pathway. Biomed Res. Int. 2019, 2019, 3909102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ene Nicolae, C.D.; Nicolae, I. Interleukin 8 serum concentration, but not lactate dehydrogenase activity, positively correlates to CD34 antigen in melanoma tumors. J. Immunoass. Immunochem. 2016, 37, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Timani, K.A.; Gyorffy, B.; Liu, Y.; Mohammad, K.S.; He, J.J. Tip110/SART3 regulates IL-8 expression and predicts the clinical outcomes in melanoma. Mol. Cancer 2018, 17, 124. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Li, J.; Koo, J.; Shin, S.S.; Lin, Y.; Jeong, B.S.; Mehnert, J.M.; Chen, S.; Cohen-Sola, K.A.; Goydos, J.S. Activation of the glutamate receptor GRM1 enhances angiogenic signaling to drive melanoma progression. Cancer Res. 2014, 74, 2499–2509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gascard, P.; Tlsty, T.D. Carcinoma-associated fibroblasts: Orchestrating the composition of malignancy. Genes Dev. 2016, 30, 1002–1019. [Google Scholar] [CrossRef] [PubMed]
- Comito, G.; Giannoni, E.; Di Gennaro, P.; Segura, C.P.; Gerlini, G.; Chiarugi, P. Stromal fibroblasts synergize with hypoxic oxidative stress to enhance melanoma aggressiveness. Cancer Lett. 2012, 324, 31–41. [Google Scholar] [CrossRef]
- Arozarena, I.; Wellbrock, C. Phenotype plasticity as enabler of melanoma progression and therapy resistance. Nat. Rev. Cancer 2019, 19, 377–391. [Google Scholar] [CrossRef] [Green Version]
- Levy, C.; Khaled, M.; Fisher, D.E. MITF: Master regulator of melanocyte development and melanoma oncogene. Trends Mol. Med. 2006, 12, 406–414. [Google Scholar] [CrossRef]
- Laugier, F.; Delyon, J.; André, J.; Bensussan, A.; Dumaz, N. Hypoxia and MITF regulate KIT oncogenic properties in melanocytes. Oncogene 2016, 35, 2070–2077. [Google Scholar] [CrossRef]
- Lenggenhager, D.; Curioni-Fontecedro, A.; Storz, M.; Shakhova, O.; Sommer, L.; Widmer, D.S.; Seifert, B.; Moch, H.; Dummer, R.; Mihic-Probst, D. An aggressive hypoxia related subpopulation of melanoma cells is TRP-2 negative. Transl. Oncol. 2014, 7, 206–212. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Barral, A.; Orgaz, J.L.; Gomez, V.; del Peso, L.; Calzada, M.J.; Jiménez, B. Hypoxia negatively regulates antimetastatic PEDF in melanoma cells by a hypoxia inducible factor-independent, autophagy dependent mechanism. PLoS ONE 2012, 7, e32989. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Chen, J.; Wang, X.; He, M.; Zhang, Z.; Cen, Y. Nodal induced by hypoxia exposure contributes to dacarbazine resistance and the maintenance of stemness in melanoma cancer stem-like cells. Oncol. Rep. 2018, 39, 2855–2864. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Zhou, Y. LRIG1 acts as a critical regulator of melanoma cell invasion, migration, and vasculogenic mimicry upon hypoxia by regulating EGFR/ERK-triggered epithelial–mesenchymal transition. Biosci. Rep. 2019, 39, BSR20181165. [Google Scholar] [CrossRef] [Green Version]
- Asnaghi, L.; Lin, M.H.; Lim, K.S.; Lim, K.J.; Tripathy, A.; Wendeborn, M.; Merbs, S.L.; Handa, J.T.; Sodhi, A.; Bar, E.E.; et al. Hypoxia promotes uveal melanoma invasion through enhanced notch and MAPK activation. PLoS ONE 2014, 9, e105372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aster, J.C.; Pear, W.S.; Blacklow, S.C. The Varied Roles of Notch in Cancer. Annu. Rev. Pathol. Mech. Dis. 2017, 12, 245–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Kumar, S.M.; Martin, J.S.; Yang, R.; Xu, X. Snail1 mediates hypoxia-induced melanoma progression. Am. J. Pathol. 2011, 179, 3020–3031. [Google Scholar] [CrossRef] [PubMed]
- Richichi, B.; Comito, G.; Renaudet, O.; Fiore, M.; Marra, A.; Stecca, B.; Pasquato, L.; Chiarugi, P.; Nativi, C. Role of a Preorganized Scaffold Presenting Four Residues of a GM-3 Lactone Mimetic on Melanoma Progression. ACS Med. Chem. Lett. 2016, 7, 28–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bi, J.; Li, P.; Li, C.; He, J.; Wang, Y.; Zhang, H.; Fan, X.; Jia, R.; Ge, S. The SDF-1/CXCR4 chemokine axis in uveal melanoma cell proliferation and migration. Tumor Biol. 2016, 37, 4175–4182. [Google Scholar] [CrossRef]
- Linnskog, R.; Jönsson, G.; Axelsson, L.; Prasad, C.P.; Andersson, T. Interleukin-6 drives melanoma cell motility through p38α-MAPK-dependent up-regulation of WNT5A expression. Mol. Oncol. 2014, 8, 1365–1378. [Google Scholar] [CrossRef]
- Lawson, C.D.; Ridley, A.J. Rho GTPase signaling complexes in cell migration and invasion. J. Cell Biol. 2017, 217, 447–457. [Google Scholar] [CrossRef]
- Tátrai, E.; Bartal, A.; Gacs, A.; Paku, S.; Kenessey, I.; Garay, T.; Hegedus, B.; Molnár, E.; Cserepes, M.T.; Hegedus, Z.; et al. Cell type-dependent HIF1 α-mediated effects of hypoxia on proliferation, migration and metastatic potential of human tumor cells. Oncotarget 2017, 8, 44498–44510. [Google Scholar] [CrossRef]
- Silva, P.; Mendoza, P.; Rivas, S.; Díaz, J.; Moraga, C.; Quest, A.F.G.; Torres, V.A. Hypoxia promotes Rab5 activation, leading to tumor cell migration, invasion and metastasis. Oncotarget 2016, 7, 29548–29562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finger, E.C.; Castellini, L.; Rankin, E.B.; Vilalta, M.; Krieg, A.J.; Jiang, D.; Banh, A.; Zundel, W.; Powell, M.B.; Giaccia, A.J. Hypoxic induction of AKAP12 variant 2 shifts PKA-mediated protein phosphorylation to enhance migration and metastasis of melanoma cells. Proc. Natl. Acad. Sci. USA 2015, 112, 4441–4446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, B.; Zhang, X.; Gao, Y.; Song, J.; Shi, B. Microglial Annexin A3 promoted the development of melanoma via activation of hypoxia-inducible factor-1α/vascular endothelial growth factor signaling pathway. J. Clin. Lab. Anal. 2020, 35, e23622. [Google Scholar] [CrossRef] [PubMed]
- Eble, J.A.; Niland, S. The extracellular matrix in tumor progression and metastasis. Clin. Exp. Metastasis 2019, 36, 171–198. [Google Scholar] [CrossRef] [PubMed]
- Cowden Dahl, K.D.; Robertson, S.E.; Weaver, V.M.; Simon, M.C. Hypoxia-inducible factor regulates αvβ3 integrin cell surface expression. Mol. Biol. Cell 2005, 16, 1901–1912. [Google Scholar] [CrossRef]
- Rezvani, H.R.; Ali, N.; Nissen, L.J.; Harfouche, G.; De Verneuil, H.; Taïeb, A.; Mazurier, F. HIF-1α in epidermis: Oxygen sensing, cutaneous angiogenesis, cancer, and non-cancer disorders. J. Investig. Dermatol. 2011, 131, 1793–1805. [Google Scholar] [CrossRef] [Green Version]
- Chung, H.; Suh, E.K.; Han, I.O.; Oh, E.S. Keratinocyte-derived laminin-332 promotes adhesion and migration in melanocytes and melanoma. J. Biol. Chem. 2011, 286, 13438–13447. [Google Scholar] [CrossRef] [Green Version]
- Prieto-Fernández, E.; Egia-Mendikute, L.; Bosch, A.; García del Río, A.; Jimenez-Lasheras, B.; Antoñana-Vildosola, A.; Lee, S.Y.; Palazon, A. Hypoxia Promotes Syndecan-3 Expression in the Tumor Microenvironment. Front. Immunol. 2020, 11, 2536. [Google Scholar] [CrossRef]
- Silberman, A.; Goldman, O.; Assayag, O.B.; Jacob, A.; Rabinovich, S.; Adler, L.; Lee, J.S.; Keshet, R.; Sarver, A.; Frug, J.; et al. Acid-induced downregulation of ASS1 contributes to the maintenance of intracellular pH in cancer. Cancer Res. 2019, 79, 518–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walbrecq, G.; Lecha, O.; Gaigneaux, A.; Fougeras, M.R.; Philippidou, D.; Margue, C.; Nomigni, M.T.; Bernardin, F.; Dittmar, G.; Behrmann, I.; et al. Hypoxia-induced adaptations of mirnomes and proteomes in melanoma cells and their secreted extracellular vesicles. Cancers 2020, 12, 692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Shi, L.; Zeng, S.; Ma, C.; Xu, W.; Zhang, Z.; Liu, Q.; Zhang, P.; Sun, Y.; Xu, C. Importin-11 overexpression promotes the migration, invasion, and progression of bladder cancer associated with the deregulation of CDKN1A and THBS1. Urol. Oncol. Semin. Orig. Investig. 2018, 36, 311.e1–311.e13. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zhang, L.; Christopher, D.M.; Teng, Z.Q.; Fausett, S.R.; Liu, C.; George, O.L.; Klingensmith, J.; Jin, P.; Zhao, X. RNA-Binding Protein FXR2 Regulates Adult Hippocampal Neurogenesis by Reducing Noggin Expression. Neuron 2011, 70, 924–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majumder, M.; Johnson, R.H.; Palanisamy, V. Fragile X-related protein family: A double-edged sword in neurodevelopmental disorders and cancer. Crit. Rev. Biochem. Mol. Biol. 2020, 55, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Yue, J.; Xiao, M.; Han-Zhang, H.; Wang, Y.V.; Ma, C.; Deng, Z.; Li, Y.; Yu, Y.; Wang, X.; et al. FXR1 regulates transcription and is required for growth of human cancer cells with TP53/FXR2 homozygous deletion. Elife 2017, 6, e26129. [Google Scholar] [CrossRef] [Green Version]
- Ashton, T.M.; Gillies McKenna, W.; Kunz-Schughart, L.A.; Higgins, G.S. Oxidative phosphorylation as an emerging target in cancer therapy. Clin. Cancer Res. 2018, 24, 2482–2490. [Google Scholar] [CrossRef] [Green Version]
- Koch, A.; Ebert, E.V.; Seitz, T.; Dietrich, P.; Berneburg, M.; Bosserhoff, A.; Hellerbrand, C. Characterization of glycolysis-related gene expression in malignant melanoma. Pathol. Res. Pract. 2020, 216, 152752. [Google Scholar] [CrossRef]
- Trojan, S.E.; Piwowar, M.; Ostrowska, B.; Laidler, P.; Kocemba-Pilarczyk, K.A. Analysis of malignant melanoma cell lines exposed to hypoxia reveals the importance of PFKFB4 overexpression for disease progression. Anticancer Res. 2018, 38, 6745–6752. [Google Scholar] [CrossRef]
- Zhuo, M.; Gorgun, F.M.; Tyler, D.S.; Englander, E.W. Hypoxia potentiates the capacity of melanoma cells to evade cisplatin and doxorubicin cytotoxicity via glycolytic shift. FEBS Open Bio. 2020, 10, 789–801. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.; Yim, S.; Park, H. The cancer driver genes IDH1/2, JARID1C/KDM5C, and UTX/KDM6A: Crosstalk between histone demethylation and hypoxic reprogramming in cancer metabolism. Exp. Mol. Med. 2019, 51, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, M.; Reid, M.A.; Lowman, X.H.; Kulkarni, R.P.; Tran, T.Q.; Liu, X.; Yang, Y.; Hernandez-Davies, J.E.; Rosales, K.K.; Li, H.; et al. Regional glutamine deficiency in tumours promotes dedifferentiation through inhibition of histone demethylation. Nat. Cell Biol. 2016, 18, 1090–1101. [Google Scholar] [CrossRef] [PubMed]
- Filipp, F.V.; Scott, D.A.; Ronai, Z.A.; Osterman, A.L.; Smith, J.W. Reverse TCA cycle flux through isocitrate dehydrogenases 1 and 2 is required for lipogenesis in hypoxic melanoma cells. Pigment Cell Melanoma Res. 2012, 25, 375–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louphrasitthiphol, P.; Ledaki, I.; Chauhan, J.; Falletta, P.; Siddaway, R.; Buffa, F.M.; Mole, D.R.; Soga, T.; Goding, C.R. MITF controls the TCA cycle to modulate the melanoma hypoxia response. Pigment Cell Melanoma Res. 2019, 32, 792–808. [Google Scholar] [CrossRef]
- Krzywinska, E.; Stockmann, C. Hypoxia, metabolism and immune cell function. Biomedicines 2018, 6, 56. [Google Scholar] [CrossRef] [Green Version]
- Vito, A.; El-Sayes, N.; Mossman, K. Hypoxia-Driven Immune Escape in the Tumor Microenvironment. Cells 2020, 9, 992. [Google Scholar] [CrossRef]
- Durgeau, A.; Virk, Y.; Corgnac, S.; Mami-Chouaib, F. Recent advances in targeting CD8 T-cell immunity for more effective cancer immunotherapy. Front. Immunol. 2018, 9, 14. [Google Scholar] [CrossRef]
- Manaster, Y.; Shipony, Z.; Hutzler, A.; Kolesnikov, M.; Avivi, C.; Shalmon, B.; Barshack, I.; Besser, M.J.; Feferman, T.; Shakhar, G. Reduced CTL motility and activity in avascular tumor areas. Cancer Immunol. Immunother. 2019, 68, 1287–1301. [Google Scholar] [CrossRef]
- Marijt, K.A.; Sluijter, M.; Blijleven, L.; Tolmeijer, S.H.; Scheeren, F.A.; Van Der Burg, S.H.; Van Hall, T. Metabolic stress in cancer cells induces immune escape through a PI3K-dependent blockade of IFNγreceptor signaling. J. Immunother. Cancer 2019, 7, 152. [Google Scholar] [CrossRef] [Green Version]
- Engel, C.; Brügmann, G.; Lambing, S.; Mühlenbeck, L.H.; Marx, S.; Hagen, C.; Horv, D.; Goldeck, M.; Ludwig, J.; Herzne, A.M.; et al. RIG-I Resists Hypoxia-Induced Immunosuppression and Dedifferentiation. Cancer Immunol. Res. 2017, 5, 455–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatfield, S.M.; Kjaergaard, J.; Lukashev, D.; Belikoff, B.; Schreiber, T.H.; Sethumadhavan, S.; Abbott, R.; Philbrook, P.; Thayer, M.; Shujia, D.; et al. Systemic oxygenation weakens the hypoxia and hypoxia inducible factor 1α-dependent and extracellular adenosine-mediated tumor protection. J. Mol. Med. 2014, 92, 1283–1292. [Google Scholar] [CrossRef]
- Wen, A.Y.; Sakamoto, K.M.; Miller, L.S. The Role of the Transcription Factor CREB in Immune Function. J. Immunol. 2010, 185, 6413–6419. [Google Scholar] [CrossRef] [Green Version]
- Cekic, C.; Day, Y.J.; Sag, D.; Linden, J. Myeloid expression of adenosine a2A receptor suppresses T and NK cell responses in the solid tumor microenvironment. Cancer Res. 2014, 74, 7250–7259. [Google Scholar] [CrossRef] [Green Version]
- Vigano, S.; Alatzoglou, D.; Irving, M.; Ménétrier-Caux, C.; Caux, C.; Romero, P.; Coukos, G. Targeting adenosine in cancer immunotherapy to enhance T-Cell function. Front. Immunol. 2019, 10, 925. [Google Scholar] [CrossRef] [Green Version]
- Tittarelli, A.; Janji, B.; Van Moer, K.; Noman, M.Z.; Chouaib, S. The selective degradation of synaptic connexin 43 protein by hypoxia-induced autophagy impairs natural killer cell-mediated tumor cell killing. J. Biol. Chem. 2015, 290, 23670–23679. [Google Scholar] [CrossRef] [Green Version]
- Noman, M.Z.; Buart, S.; Romero, P.; Ketari, S.; Janji, B.; Mari, B.; Mami-Chouaib, F.; Chouaib, S. Hypoxia-inducible miR-210 regulates the susceptibility of tumor cells to lysis by cytotoxic T cells. Cancer Res. 2012, 72, 4629–4641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romano, G.; Kwong, L.N. MiRNAs, melanoma and microenvironment: An intricate network. Int. J. Mol. Sci. 2017, 18, 2354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kajiwara, T.; Tanaka, T.; Kukita, K.; Kutomi, G.; Saito, K.; Okuya, K.; Takaya, A.; Kochin, V.; Kanaseki, T.; Tsukahara, T.; et al. Hypoxia augments MHC class I antigen presentation via facilitation of ERO1-α-mediated oxidative folding in murine tumor cells. Eur. J. Immunol. 2016, 46, 2842–2851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gropper, Y.; Feferman, T.; Shalit, T.; Salame, T.M.; Porat, Z.; Shakhar, G. Culturing CTLs under Hypoxic Conditions Enhances Their Cytolysis and Improves Their Anti-tumor Function. Cell Rep. 2017, 20, 2547–2555. [Google Scholar] [CrossRef] [PubMed]
- Calvani, M.; Bruno, G.; Dal Monte, M.; Nassini, R.; Fontani, F.; Casini, A.; Cavallini, L.; Becatti, M.; Bianchini, F.; De Logu, F.; et al. β3-Adrenoceptor as a potential immuno-suppressor agent in melanoma. Br. J. Pharmacol. 2019, 176, 2509–2524. [Google Scholar] [CrossRef]
- Bronkhorst, I.H.G.; Jehs, T.M.L.; Dijkgraaf, E.M.; Luyten, G.P.M.; Van Der Velden, P.A.; Van Der Burg, S.H.; Jager, M.J. Effect of hypoxic stress on migration and characteristics of monocytes in uveal melanoma. JAMA Ophthalmol. 2014, 132, 614–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campillo, N.; Falcones, B.; Otero, J.; Colina, R.; Gozal, D.; Navajas, D.; Farré, R.; Almendros, I. Differential oxygenation in tumor microenvironment modulates macrophage and cancer cell crosstalk: Novel experimental settingand proof of concept. Front. Oncol. 2019, 9, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huber, R.; Meier, B.; Otsuka, A.; Fenini, G.; Satoh, T.; Gehrke, S.; Widmer, D.; Levesque, M.P.; Mangana, J.; Kerl, K.; et al. Tumour hypoxia promotes melanoma growth and metastasis via High Mobility Group Box-1 and M2-like macrophages. Sci. Rep. 2016, 6, 29914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.B.; Zhao, Z.B.; Liu, Q.Z.; Hu, T.D.; Long, J.; Yan, K.; Lian, Z.X. FoxO1 is a regulator of MHC-II expression and anti-tumor effect of tumor-associated macrophages. Oncogene 2018, 37, 1192–1204. [Google Scholar] [CrossRef] [PubMed]
- Gaustad, J.-V.; Simonsen, T.G.; Andersen, L.M.K.; Rofstad, E.K. Vascular abnormalities and development of hypoxia in microscopic melanoma xenografts. J. Transl. Med. 2017, 15, 241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, W.L.; Huang, Q.; Liu, X.; Zimmerman, M.; Li, F.; Li, C.Y. Molecular mechanisms involved in tumor repopulation after radiotherapy. Transl. Cancer Res. 2013, 2, 442–448. [Google Scholar] [CrossRef]
- Wittgen, H.G.M.; Van Kempen, L.C.L.T. Reactive oxygen species in melanoma and its therapeutic implications. Melanoma Res. 2007, 17, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Wilson, W.R. Exploiting tumour hypoxia in cancer treatment. Nat. Rev. Cancer 2004, 4, 437–447. [Google Scholar] [CrossRef]
- Jour, G.; Ivan, D.; Aung, P.P. Angiogenesis in melanoma: An update with a focus on current targeted therapies. J. Clin. Pathol. 2016, 69, 472–483. [Google Scholar] [CrossRef]
- Collet, G.; Lamerant-Fayel, N.; Tertil, M.; El Hafny-Rahbi, B.; Stepniewski, J.; Guichard, A.; Foucault-Collet, A.; Klimkiewicz, K.; Petoud, S.; Matejuk, A.; et al. Hypoxia-regulated overexpression of soluble VEGFR2 controls angiogenesis and inhibits tumor growth. Mol. Cancer Ther. 2013, 13, 165–178. [Google Scholar] [CrossRef] [Green Version]
- Roda, J.M.; Sumner, L.A.; Evans, R.; Phillips, G.S.; Marsh, C.B.; Eubank, T.D. Hypoxia-Inducible Factor-2α Regulates GM-CSF–Derived Soluble Vascular Endothelial Growth Factor Receptor 1 Production from Macrophages and Inhibits Tumor Growth and Angiogenesis. J. Immunol. 2011, 187, 1970–1976. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, V.; Maharjan, S.; Kim, K.; Kim, N.J.; Son, J.; Lee, K.; Choi, H.J.; Rho, S.S.; Ahn, S.; Won, M.H.; et al. Direct endothelial junction restoration results in significant tumor vascular normalization and metastasis inhibition in mice. Oncotarget 2014, 5, 2761–2777. [Google Scholar] [CrossRef] [Green Version]
- Martí-Díaz, R.; Montenegro, M.F.; Cabezas-Herrera, J.; Goding, C.R.; Rodríguez-López, J.N.; Sánchez-del-Campo, L. Acriflavine, a Potent Inhibitor of HIF-1α, Disturbs Glucose Metabolism and Suppresses ATF4-Protective Pathways in Melanoma under Non-Hypoxic Conditions. Cancers 2020, 13, 102. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Jiang, H.; Li, Z.; Yafei, Z.; Liu, Y.; Zhou, S.; Xiao, Y.; Xie, C.; Zhou, F.; Zhou, Y. 2-Methoxyestradiol enhances radiosensitivity in radioresistant melanoma MDA-MB-435R cells by regulating glycolysis via HIF-1α/PDK1 axis. Int. J. Oncol. 2017, 50, 1531–1540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patra, K.; Jana, S.; Sarkar, A.; Mandal, D.P.; Bhattacharjee, S. The inhibition of hypoxia-induced angiogenesis and metastasis by cinnamaldehyde is mediated by decreasing HIF-1α protein synthesis via PI3K/Akt pathway. BioFactors 2019, 45, 401–415. [Google Scholar] [CrossRef] [PubMed]
- Scharping, N.E.; Menk, A.V.; Whetstone, R.D.; Zeng, X.; Delgoffe, G.M. Efficacy of PD-1 blockade is potentiated by metformin-induced reduction of tumor hypoxia. Cancer Immunol. Res. 2017, 5, 9–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, M.S.; Yin, X.Y.; Qin, B.; Xuan, S.Y.; Yuan, X.L.; Yin, H.; Zhu, C.; Li, X.; Yang, J.; Du, Y.Z.; et al. Inhibiting Hypoxia and Chemotherapy-Induced Cancer Cell Metastasis under a Valid Therapeutic Effect by an Assistance of Biomimetic Oxygen Delivery. Mol. Pharm. 2019, 16, 4530–4541. [Google Scholar] [CrossRef]
- Hei, Y.; Teng, B.; Zeng, Z.; Zhang, S.; Li, Q.; Pan, J.; Luo, Z.; Xiong, C.; Wei, S. Multifunctional immunoliposomes combining catalase and PD-L1 antibodies overcome tumor hypoxia and enhance immunotherapeutic effects against melanoma. Int. J. Nanomed. 2020, 15, 1677–1691. [Google Scholar] [CrossRef] [Green Version]
- Zou, M.-Z.; Liu, W.-L.; Li, C.-X.; Zheng, D.-W.; Zeng, J.-Y.; Gao, F.; Ye, J.-J.; Zhang, X.-Z. A Multifunctional Biomimetic Nanoplatform for Relieving Hypoxia to Enhance Chemotherapy and Inhibit the PD-1/PD-L1 Axis. Small 2018, 14, 1801120. [Google Scholar] [CrossRef]
- Zhou, J.; Geng, S.; Ye, W.; Wang, Q.; Lou, R.; Yin, Q.; Du, B.; Yao, H. ROS-boosted photodynamic therapy against metastatic melanoma by inhibiting the activity of antioxidase and oxygen-producing nano-dopants. Pharmacol. Res. 2020, 158, 104885. [Google Scholar] [CrossRef]
- Bagati, A.; Hutcherson, T.C.; Koch, Z.; Pechette, J.; Dianat, H.; Higley, C.; Chiu, L.; Song, Y.; Shah, J.; Chazen, E.; et al. Novel combination therapy for melanoma induces apoptosis via a gap junction positive feedback mechanism. Oncotarget 2020, 11, 3443–3458. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Sun, J.D.; Wang, J.; Ahluwalia, D.; Baker, A.F.; Cranmer, L.D.; Ferraro, D.; Wang, Y.; Duan, J.X.; Ammons, W.S.; et al. TH-302, a hypoxia-activated prodrug with broad in vivo preclinical combination therapy efficacy: Optimization of dosing regimens and schedules. Cancer Chemother. Pharmacol. 2012, 69, 1487–1498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Liu, X.; Cao, W.; Hua, Z.-C. Tumor-specifically hypoxia-induced therapy of SPRY1/2 displayed differential therapeutic efficacy for melanoma. Am. J. Cancer Res. 2015, 5, 792–801. [Google Scholar] [PubMed]
- Feng, X.; He, P.; Zeng, C.; Li, Y.H.; Das, S.K.; Li, B.; Yang, H.F.; Du, Y. Novel insights into the role of Clostridium novyi-NT related combination bacteriolytic therapy in solid tumors. Oncol. Lett. 2021, 21, 110. [Google Scholar] [CrossRef]
- Yura, Y.; Chong, B.S.H.; Johnson, R.D.; Watanabe, Y.; Tsukahara, Y.; Ferran, B.; Murdoch, C.E.; Behring, J.B.; McComb, M.E.; Costello, C.E.; et al. Endothelial cell-specific redox gene modulation inhibits angiogenesis but promotes B16F0 tumor growth in mice. FASEB J. 2019, 33, 14147–14158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, L.; You, S.; Zhang, Q.; Osuka, S.; Devi, N.S.; Kaluz, S.; Ferguson, J.H.; Yang, H.; Chen, G.; Wang, B.; et al. Arylsulfonamide 64B inhibits hypoxia/HIF-induced expression of c-Met and CXCR4 and reduces primary tumor growth and metastasis of uveal melanoma. Clin. Cancer Res. 2019, 25, 2206–2218. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Guo, X.; Luo, L.; Wu, Z.; Luo, Z.; Jiang, M.; Zhang, J.; Qin, B.; Shi, Y.; Lou, Y.; et al. Extremely Effective Chemoradiotherapy by Inducing Immunogenic Cell Death and Radio-Triggered Drug Release under Hypoxia Alleviation. ACS Appl. Mater. Interfaces 2019, 11, 46536–46547. [Google Scholar] [CrossRef]
- Liu, S.; Tetzlaff, M.T.; Wang, T.; Chen, X.; Yang, R.; Kumar, S.M.; Vultur, A.; Li, P.; Martin, J.S.; Herlyn, M.; et al. Hypoxia-activated prodrug enhances therapeutic effect of sunitinib in melanoma. Oncotarget 2017, 8, 115140–115152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated pH: A perfect storm for cancer progression. Nat. Rev. Cancer 2011, 11, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Böhme, I.; Bosserhoff, A.K. Acidic tumor microenvironment in human melanoma. Pigment. Cell Melanoma Res. 2016, 29, 508–523. [Google Scholar] [CrossRef]
- Tannock, I.F.; Rotin, D. Acid pH in Tumors and Its Potential for Therapeutic Exploitation. Cancer Res. 1989, 49, 4373–4384. [Google Scholar]
- Gatenby, R.A.; Gillies, R.J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 2004, 4, 891–899. [Google Scholar] [CrossRef]
- Koch, A.; Schwab, A. Cutaneous pH landscape as a facilitator of melanoma initiation and progression. Acta Physiol. 2019, 225, e13105. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Ozawa, S.; Miyamoto, C.; Maehata, Y.; Suzuki, A.; Maeda, T.; Baba, Y. Acidic extracellular microenvironment and cancer. Cancer Cell Int. 2013, 13, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Liu, G.; Wang, R. The intercellular metabolic interplay between tumor and immune cells. Front. Immunol. 2014, 5, 358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, S.R.; Erickson, L.A.; Ichetovkin, I.; Knauer, D.J.; Markovic, S.N. Circulating serologic and molecular biomarkers in malignant melanoma. Mayo Clin. Proc. 2011, 86, 981–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hulikova, A.; Black, N.; Hsia, L.T.; Wilding, J.; Bodmer, W.F.; Swietach, P. Stromal uptake and transmission of acid is a pathway for venting cancer cell-generated acid. Proc. Natl. Acad. Sci. USA 2016, 113, E5344–E5353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Shu, S.; Yang, Y.; Allen, C.L.; Maguire, O.; Minderman, H.; Sen, A.; Ciesielski, M.J.; Collins, K.A.; Bush, P.J.; Singh, P.; et al. Metabolic reprogramming of stromal fibroblasts by melanoma exosome microRNA favours a pre-metastatic microenvironment. Sci. Rep. 2018, 8, 12905. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Huo, Y.; Wang, D.; Tai, Y.; Li, J.; Pang, D.; Zhang, Y.; Zhao, W.; Du, N.; Huang, Y. MiR-216a-5p/Hexokinase 2 axis regulates uveal melanoma growth through modulation of Warburg effect. Biochem. Biophys. Res. Commun. 2018, 501, 885–892. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, X.; Yan, Z.; Li, C.; Zhao, H.; Ma, L.; Zhang, D.; Liu, J.; Liu, Z.; Du, N.; et al. miR-489-3p/SIX1 Axis Regulates Melanoma Proliferation and Glycolytic Potential. Mol. Ther. Oncolytics 2020, 16, 30–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Zhao, H.; Yang, J.; Ma, Y.; Liu, Z.; Li, C.; Wang, T.; Yan, Z.; Du, N. MiR-150-5p regulates melanoma proliferation, invasion and metastasis via SIX1-mediated Warburg Effect. Biochem. Biophys. Res. Commun. 2019, 515, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Vahle, A.K.; Domikowsky, B.; Schwöppe, C.; Krähling, H.; Mally, S.; Schäfers, M.; Hermann, S.; Shahin, V.; Haier, J.; Schwab, A.; et al. Extracellular matrix composition and interstitial pH modulate NHE1-mediated melanoma cell motility. Int. J. Oncol. 2014, 44, 78–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karki, P.; Li, X.; Schrama, D.; Fliegel, L. B-Raf associates with and activates the NHE1 isoform of the Na +/H+ exchanger. J. Biol. Chem. 2011, 286, 13096–13105. [Google Scholar] [CrossRef] [Green Version]
- Ferretta, A.; Maida, I.; Guida, S.; Azzariti, A.; Porcelli, L.; Tommasi, S.; Zanna, P.; Cocco, T.; Guida, M.; Guida, G. New insight into the role of metabolic reprogramming in melanoma cells harboring BRAF mutations. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 2710–2718. [Google Scholar] [CrossRef] [PubMed]
- Wahl, M.L.; Owen, J.A.; Burd, R.; Herlands, R.A.; Nogami, S.S.; Rodeck, U.; Berd, D.; Leeper, D.B.; Owen, C.S. Regulation of intracellular pH in human melanoma: Potential therapeutic implications. Mol. Cancer Ther. 2002, 1, 617–628. [Google Scholar]
- Böhme, I.; Bosserhoff, A. Extracellular acidosis triggers a senescence-like phenotype in human melanoma cells. Pigment. Cell Melanoma Res. 2020, 33, 41–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero-Garcia, S.; Moreno-Altamirano, M.M.B.; Prado-Garcia, H.; Sánchez-García, F.J. Lactate contribution to the tumor microenvironment: Mechanisms, effects on immune cells and therapeutic relevance. Front. Immunol. 2016, 7, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rofstad, E.K.; Mathiesen, B.; Kindem, K.; Galappathi, K. Acidic extracellular pH promotes experimental metastasis of human melanoma cells in athymic nude mice. Cancer Res. 2006, 66, 6699–6707. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Zaguilán, R.; Seftor, E.A.; Seftor, R.E.B.; Chu, Y.W.; Gillies, R.J.; Hendrix, M.J.C. Acidic pH enhances the invasive behavior of human melanoma cells. Clin. Exp. Metastasis 1996, 14, 176–186. [Google Scholar] [CrossRef]
- Pinheiro, C.; Miranda-Gonçalves, V.; Longatto-Filho, A.; Vicente, A.L.S.A.; Berardinelli, G.N.; Scapulatempo-Neto, C.; Costa, R.F.A.; Viana, C.R.; Reis, R.M.; Baltazar, F.; et al. The metabolic microenvironment of melanomas: Prognostic value of MCT1 and MCT4. Cell Cycle 2016, 15, 1462–1470. [Google Scholar] [CrossRef] [Green Version]
- Goda, N.; Kanai, M. Hypoxia-inducible factors and their roles in energy metabolism. Int. J. Hematol. 2012, 95, 457–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wykoff, C.C.; Beasley, N.J.P.; Watson, P.H.; Turner, K.J.; Pastorek, J.; Sibtain, A.; Wilson, G.D.; Turley, H.; Talks, K.L.; Maxwell, P.H.; et al. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res. 2000, 60, 7075–7083. [Google Scholar] [PubMed]
- Brahimi-Horn, M.C.; Pouysségur, J. Hypoxia in cancer cell metabolism and pH regulation. Essays Biochem. 2007, 43, 165–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chafe, S.C.; McDonald, P.C.; Saberi, S.; Nemirovsky, O.; Venkateswaran, G.; Burugu, S.; Gao, D.; Delaidelli, A.; Kyle, A.H.; Baker, J.H.E.; et al. Targeting hypoxia-induced carbonic anhydrase IX enhances immune-checkpoint blockade locally and systemically. Cancer Immunol. Res. 2019, 7, 1064–1078. [Google Scholar] [CrossRef] [Green Version]
- Giuntini, G.; Monaci, S.; Cau, Y.; Mori, M.; Naldini, A.; Carraro, F. Inhibition of Melanoma Cell Migration and Invasion Targeting the Hypoxic Tumor Associated CAXII. Cancers 2020, 12, 3018. [Google Scholar] [CrossRef] [PubMed]
- Andreucci, E.; Peppicelli, S.; Carta, F.; Brisotto, G.; Biscontin, E.; Ruzzolini, J.; Bianchini, F.; Biagioni, A.; Supuran, C.T.; Calorini, L. Carbonic anhydrase IX inhibition affects viability of cancer cells adapted to extracellular acidosis. J. Mol. Med. 2017, 95, 1341–1353. [Google Scholar] [CrossRef]
- Moellering, R.E.; Black, K.C.; Krishnamurty, C.; Baggett, B.K.; Stafford, P.; Rain, M.; Gatenby, R.A.; Gillies, R.J. Acid treatment of melanoma cells selects for invasive phenotypes. Clin. Exp. Metastasis 2008, 25, 411–425. [Google Scholar] [CrossRef]
- Marino, M.L.; Pellegrini, P.; Di Lernia, G.; Djavaheri-Mergny, M.; Brnjic, S.; Zhang, X.; Hägg, M.; Linder, S.; Fais, S.; Codogno, P.; et al. Autophagy is a protective mechanism for human melanoma cells under acidic stress. J. Biol. Chem. 2012, 287, 30664–30676. [Google Scholar] [CrossRef] [Green Version]
- Matsuo, T.; Sadzuka, Y. Extracellular acidification by lactic acid suppresses glucose deprivation-induced cell death and autophagy in B16 melanoma cells. Biochem. Biophys. Res. Commun. 2018, 496, 1357–1361. [Google Scholar] [CrossRef]
- Peppicelli, S.; Bianchini, F.; Torre, E.; Calorini, L. Contribution of acidic melanoma cells undergoing epithelial-to-mesenchymal transition to aggressiveness of non-acidic melanoma cells. Clin. Exp. Metastasis 2014, 31, 423–433. [Google Scholar] [CrossRef]
- Andreucci, E.; Peppicelli, S.; Ruzzolini, J.; Bianchini, F.; Biagioni, A.; Papucci, L.; Magnelli, L.; Mazzanti, B.; Stecca, B.; Calorini, L. The acidic tumor microenvironment drives a stem-like phenotype in melanoma cells. J. Mol. Med. 2020, 98, 1431–1446. [Google Scholar] [CrossRef] [PubMed]
- Krähling, H.; Mally, S.; Eble, J.A.; Noël, J.; Schwab, A.; Stock, C. The glycocalyx maintains a cell surface pH nanoenvironment crucial for integrin-mediated migration of human melanoma cells. Pflugers Arch. Eur. J. Physiol. 2009, 458, 1069–1083. [Google Scholar] [CrossRef] [PubMed]
- Paradise, R.K.; Lauffenburger, D.A.; van Vliet, K.J. Acidic extracellular pH promotes activation of integrin αvβ3. PLoS ONE 2011, 6, e15746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stock, C.; Gassner, B.; Hauck, C.R.; Arnold, H.; Mally, S.; Eble, J.A.; Dieterich, P.; Schwab, A. Migration of human melanoma cells depends on extracellular pH and Na+/H+ exchange. J. Physiol. 2005, 567, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Serpa, J. Tumor Microenvironment; Siemann, D.W., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; ISBN 9780470749968. [Google Scholar]
- Ludwig, F.T.; Schwab, A.; Stock, C. The Na+/H+-exchanger (NHE1) generates pH nanodomains at focal adhesions. J. Cell. Physiol. 2013, 228, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Hofschroër, V.; Koch, K.A.; Ludwig, F.T.; Friedl, P.; Oberleithner, H.; Stock, C.; Schwab, A. Extracellular protonation modulates cell-cell interaction mechanics and tissue invasion in human melanoma cells. Sci. Rep. 2017, 7, 42369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stüwe, L.; Müller, M.; Fabian, A.; Waning, J.; Mally, S.; Noël, J.; Schwab, A.; Stock, C. pH dependence of melanoma cell migration: Protons extruded by NHE1 dominate protons of the bulk solution. J. Physiol. 2007, 585, 351–360. [Google Scholar] [CrossRef]
- Estrella, V.; Chen, T.; Lloyd, M.; Wojtkowiak, J.; Cornnell, H.H.; Ibrahim-Hashim, A.; Bailey, K.; Balagurunathan, Y.; Rothberg, J.M.; Sloane, B.F.; et al. Acidity generated by the tumor microenvironment drives local invasion. Cancer Res. 2013, 73, 1524–1535. [Google Scholar] [CrossRef] [Green Version]
- Kato, Y.; Lambert, C.A.; Colige, A.C.; Mineur, P.; Noël, A.; Frankenne, F.; Foidart, J.M.; Baba, M.; Hata, R.I.; Miyazaki, K.; et al. Acidic extracellular pH induces matrix metalloproteinase-9 expression in mouse metastatic melanoma cells through the phospholipase D-mitogen-activated protein kinase signaling. J. Biol. Chem. 2005, 280, 10938–10944. [Google Scholar] [CrossRef] [Green Version]
- Sonveaux, P.; Copetti, T.; de Saedeleer, C.J.; Végran, F.; Verrax, J.; Kennedy, K.M.; Moon, E.J.; Dhup, S.; Danhier, P.; Frérart, F.; et al. Targeting the lactate transporter MCT1 in endothelial cells inhibits lactate-induced HIF-1 activation and tumor angiogenesis. PLoS ONE 2012, 7, e33418. [Google Scholar] [CrossRef]
- Mahoney, B.P.; Raghunand, N.; Baggett, B.; Gillies, R.J. Tumor acidity, ion trapping and chemotherapeutics: I. Acid pH affects the distribution of chemotherapeutic agents in vitro. Biochem. Pharmacol. 2003, 66, 1207–1218. [Google Scholar] [CrossRef]
- Ruzzolini, J.; Peppicelli, S.; Andreucci, E.; Bianchini, F.; Margheri, F.; Laurenzana, A.; Fibbi, G.; Pimpinelli, N.; Calorini, L. Everolimus selectively targets vemurafenib resistant BRAFV600E melanoma cells adapted to low pH. Cancer Lett. 2017, 408, 43–54. [Google Scholar] [CrossRef]
- Leikam, C.; Hufnagel, A.L.; Otto, C.; Murphy, D.J.; Mühling, B.; Kneitz, S.; Nanda, I.; Schmid, M.; Wagner, T.U.; Haferkamp, S.; et al. In vitro evidence for senescent multinucleated melanocytes as a source for tumor-initiating cells. Cell Death Dis. 2015, 6, e1711. [Google Scholar] [CrossRef] [Green Version]
- Mccarty, M.F.; Whitaker, J. Manipulating tumor acidification as a cancer treatment strategy. Altern. Med. Rev. 2010, 15, 264–272. [Google Scholar]
- Lardner, A. The effects of extracellular pH on immune function. J. Leukoc. Biol. 2001, 69, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Hoffmann, P.; Voelkl, S.; Meidenbauer, N.; Ammer, J.; Edinger, M.; Gottfried, E.; Schwarz, S.; Rothe, G.; Hoves, S.; et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood 2007, 109, 3812–3819. [Google Scholar] [CrossRef]
- Feder-Mengus, C.; Ghosh, S.; Weber, W.P.; Wyler, S.; Zajac, P.; Terracciano, L.; Oertli, D.; Heberer, M.; Martin, I.; Spagnoli, G.C.; et al. Multiple mechanisms underlie defective recognition of melanoma cells cultured in three-dimensional architectures by antigen-specific cytotoxic T lymphocytes. Br. J. Cancer 2007, 96, 1072–1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohn, T.; Rapp, S.; Luther, N.; Klein, M.; Bruehl, T.J.; Kojima, N.; Aranda Lopez, P.; Hahlbrock, J.; Muth, S.; Endo, S.; et al. Tumor immunoevasion via acidosis-dependent induction of regulatory tumor-associated macrophages. Nat. Immunol. 2018, 19, 1319–1329. [Google Scholar] [CrossRef]
- Pilon-Thomas, S.; Kodumudi, K.N.; El-Kenawi, A.E.; Russell, S.; Weber, A.M.; Luddy, K.; Damaghi, M.; Wojtkowiak, J.W.; Mulé, J.J.; Ibrahim-Hashim, A.; et al. Neutralization of tumor acidity improves antitumor responses to immunotherapy. Cancer Res. 2016, 76, 1381–1390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uhl, F.M.; Chen, S.; O’Sullivan, D.; Edwards-Hicks, J.; Richter, G.; Haring, E.; Andrieux, G.; Halbach, S.; Apostolova, P.; Büscher, J.; et al. Metabolic reprogramming of donor T cells enhances graft-versus-leukemia effects in mice and humans. Sci. Transl. Med. 2020, 12, eabb8969. [Google Scholar] [CrossRef] [PubMed]
- Gullotti, E.; Yeo, Y. Extracellularly activated nanocarriers: A new paradigm of tumor targeted drug delivery. Mol. Pharm. 2009, 6, 1041–1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Milito, A.; Canese, R.; Marino, M.L.; Borghi, M.; Iero, M.; Villa, A.; Venturi, G.; Lozupone, F.; Iessi, E.; Logozzi, M.; et al. PH-dependent antitumor activity of proton pump inhibitors against human melanoma is mediated by inhibition of tumor acidity. Int. J. Cancer 2010, 127, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Luciani, F.; Spada, M.; De Milito, A.; Molinari, A.; Rivoltini, L.; Montinaro, A.; Marra, M.; Lugini, L.; Logozzi, M.; Lozupone, F.; et al. Effect of proton pump inhibitor pretreatment on resistance of solid tumors to cytotoxic drugs. J. Natl. Cancer Inst. 2004, 96, 1702–1713. [Google Scholar] [CrossRef] [PubMed]


| The Therapeutic Approach | Treatment Description | Results and Utilized Research Model |
|---|---|---|
| Indirect targeting (angiogenesis) | Anti-VEGF antibodies and multikinase inhibitors | Phase I/II of clinical trials for novel drug combinations are still ongoing. Monotherapies were not effective, while combination treatment with chemotherapy induced short-term response [130]. |
| sVEGFR (vector or induced by GM-CSF) | Reduction of tumor angiogenesis, growth, and metastasis was observed in vitro and using murine melanoma model [131,132]. | |
| Promotion of endothelial gap junctions (Sac-1004) | In murine melanoma model, vasculature normalization, inhibition of metastasis and EMT, reduction of cancer stem-like cells population was noted [133]. | |
| Direct targeting (HIF-1α inhibitors) | Acriflavine | Inhibitors blocked the metabolism and proliferation of melanoma cells in vitro [134]. |
| 2-methoxyestradiol | Drug resensitized radioresistant cells and partially suppressed their glycolytic state [135]. | |
| Cinnamaldehyde | Treatment reduced the invasiveness of melanoma cells in vitro and in vivo [136]. | |
| Arylsulfonamide (64B) | The compound decreased the growth and metastasis of murine uveal melanoma [8]. | |
| Direct targeting (alleviation of hypoxic conditions) | Metformin (inhibition of oxygen consumption) | Improvement of anti-PD-L1 therapy was observed in murine melanoma model [137]. |
| Liposomes with hemoglobin and doxorubicin, followed by radiotherapy | Treatment efficiently blocked migration in vitro and inhibited tumor growth in mice [138]. | |
| Multimodal platforms (CAT@aPDL1-SSL, mZDC, Au@MTM-HA) | Treated mice exhibited improved tumor infiltration by cytotoxic T cells, prolonged survival, and reduced metastasis rate [139,140,141]. | |
| Direct targeting (oxygen-sensitive treatment) | Prodrugs activated in hypoxia (tirapazamine, TH-302) | Combination therapy resulted in effective tumor size reduction in mice [142,143]. |
| Bacteria as drug delivery vectors (S. typhimurium) or oncolytic inducers (C. novyi-NT) | Bacteria-delivered vector reduced the proliferation of melanoma cells, while the oncolytic strain efficiently targeted solid tumors [144,145]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dratkiewicz, E.; Simiczyjew, A.; Mazurkiewicz, J.; Ziętek, M.; Matkowski, R.; Nowak, D. Hypoxia and Extracellular Acidification as Drivers of Melanoma Progression and Drug Resistance. Cells 2021, 10, 862. https://doi.org/10.3390/cells10040862
Dratkiewicz E, Simiczyjew A, Mazurkiewicz J, Ziętek M, Matkowski R, Nowak D. Hypoxia and Extracellular Acidification as Drivers of Melanoma Progression and Drug Resistance. Cells. 2021; 10(4):862. https://doi.org/10.3390/cells10040862
Chicago/Turabian StyleDratkiewicz, Ewelina, Aleksandra Simiczyjew, Justyna Mazurkiewicz, Marcin Ziętek, Rafał Matkowski, and Dorota Nowak. 2021. "Hypoxia and Extracellular Acidification as Drivers of Melanoma Progression and Drug Resistance" Cells 10, no. 4: 862. https://doi.org/10.3390/cells10040862