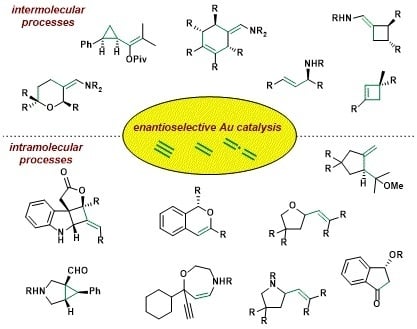
Chiral N-heterocyclic Carbene Gold Complexes: Synthesis and Applications in Catalysis
Abstract
:1. Introduction
2. Mono-N-Heterocyclic Gold(I) Complexes
2.1. Cyclic C2-Symmetric Gold(I) Complexes
2.2. Cyclic Non-C2-Symmetric Gold(I) Complexes
2.3. Acyclic Gold(I) Complexes
2.4. Bis-NHC Gold(I) Complexes
2.4.1. Cyclic Bis-NHC Gold(I) Complexes
2.4.2. Acyclic Bis-NHC Gold(I) Complexes
2.5. Applications of Chiral N-Heterocyclic CarbeneG(I) Complexes in Asymmetric Catalysis
2.5.1. Intramolecular Reactions
Alkylidene Cyclopentane Derivatives
Chromane and Isochromane Derivatives
Furan, Pyrrolidine, and Indanone Derivatives
Complex Fused Cyclobutane Derivatives
Diynamide Desymmetrization
2.5.2. Intermolecular Reaction
Cyclopropanation
Hydrogenolysis
[4+2] and Related Cycloadditions
Hydroamination and Hydroazidation of Allenes
3. Chiral N-Heterocyclic Gold(III) Complexes
3.1. The Synthesis of Gold(III) Complexes
3.2. Applications of Chiral N-Heterocyclic Carbene Gold(III) Complexes in Asymmetric Catalysis
4. Summary and Outlook
Funding
Acknowledgments
Conflicts of Interest
References
- Hashmi, A.S.K. Gold-Catalyzed Organic Reactions. Chem. Rev. 2007, 107, 3180–3211. [Google Scholar] [CrossRef] [PubMed]
- Arcadi, A. Alternative Synthetic Methods through New Developments in Catalysis by Gold. Chem. Rev. 2008, 108, 3266–3325. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Leyva-Pérez, A.; Sabater, M.J. Gold-Catalyzed Carbon−Heteroatom Bond-Forming Reactions. Chem. Rev. 2011, 111, 1657–1712. [Google Scholar] [CrossRef] [PubMed]
- Dorel, R.; Echavarren, A.M. Gold(I)-Catalyzed Activation of Alkynes for the Construction of Molecular Complexity. Chem. Rev. 2015, 115, 9028–9072. [Google Scholar] [CrossRef] [Green Version]
- Pflästerer, D.; Hashmi, A.S.K. Gold catalysis in total synthesis–recent achievements. Chem. Soc. Rev. 2016, 45, 1331–1367. [Google Scholar] [CrossRef]
- Asiri, A.M.; Hashmi, A.S.K. Gold-catalysed reactions of diynes. Chem. Soc. Rev. 2016, 45, 4471–4503. [Google Scholar] [CrossRef]
- Merino, E.; ASalvador, A.; Nevado, C. Gold-Mediated Reactions. In Science of Synthesis; Snyder, S.A., Ed.; Georg Thieme Verlag: Stuttgart, Germany; New York, NY, USA, 2016; pp. 535–576. [Google Scholar]
- Zidan, M.; Rohe, S.; McCallum, T.; Barriault, L. Recent advances in mono and binuclear gold photoredox catalysis. Catal. Sci. Technol. 2018, 8, 6019–6028. [Google Scholar] [CrossRef]
- Ma, B.; Liu, L.; Zhang, J. Gold-Catalyzed Site-Selective C−H Bond Functionalization with Diazo Compounds. Asian J. Org. Chem. 2018, 7, 2015–2025. [Google Scholar] [CrossRef]
- Mascareñas, J.L.; Varela, I.; López, F. Allenes and Derivatives in Gold(I)- and Platinum(II)-Catalyzed Formal Cycloadditions. Acc. Chem. Res. 2019, 52, 465–479. [Google Scholar] [CrossRef] [Green Version]
- Praveen, C. Carbophilic activation of π-systems via gold coordination: Towards regioselective access of intermolecular addition products. Coord. Chem. Rev. 2019, 392, 1–34. [Google Scholar] [CrossRef]
- Wang, Y.-M.; Lackner, A.D.; Toste, F.D. Development of Catalysts and Ligands for Enantioselective Gold Catalysis. Acc. Chem. Res. 2014, 47, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Gu, P.; Xu, Q.; Shi, M. Development and outlook of chiral carbene–gold(I) complexes catalyzed asymmetric reactions. Tetrahedron Lett. 2014, 55, 577–584. [Google Scholar] [CrossRef]
- Inamdar, S.M.; Konala, A.; Patil, N.T. When gold meets chiral Brønsted acid catalysts: Extending the boundaries of enantioselective gold catalysis. Chem. Commun. 2014, 50, 15124–15135. [Google Scholar] [CrossRef] [PubMed]
- Toullec, P.Y.; Pradal, A.; Michelet, V. Recent Developments in Asymmetric Catalysis. In Gold Catalysis an Homogenoues Approach; Toste, F.D., Michelet, V., Eds.; Imperial College Press: London, UK, 2014; pp. 448–493. [Google Scholar]
- Qian, D.; Zhang, J. Gold-catalyzed cyclopropanation reactions using a carbenoid precursor toolbox. Chem. Soc. Rev. 2015, 44, 677–698. [Google Scholar] [CrossRef]
- Zi, W.; Dean Toste, F. Recent advances in enantioselective gold catalysis. Chem. Soc. Rev. 2016, 45, 4567–4589. [Google Scholar] [CrossRef]
- Fu, W.; Tang, W. Chiral Monophosphorus Ligands for Asymmetric Catalytic Reactions. ACS Catal. 2016, 6, 4814–4858. [Google Scholar] [CrossRef]
- Zheng, Y.; Zi, W. Transition-metal catalyzed enantioselective hydrofunctionalization of alkynes. Tetrahedron Lett. 2018, 59, 2205–2213. [Google Scholar] [CrossRef]
- Brill, M.; Nolan, S.P. Chiral Carbophilic Gold Lewis Acid Complexes in Enantioselective Catalysis. In Chiral Lewis Acids; Mikami, K., Ed.; Springer International Publishing: Cham, Vietnam, 2018; pp. 51–90. [Google Scholar]
- Shen, W.-B.; Tang, X.-T. Recent advances in catalytic asymmetric intermolecular oxidation of alkynes. Org. Biomol. Chem. 2019, 17, 7106–7113. [Google Scholar] [CrossRef]
- Gimeno, M.C.; Laguna, A. Three-and Four-Coordinate Gold(I) Complexes. Chem. Rev. 1997, 97, 511–522. [Google Scholar] [CrossRef]
- Fürstner, A.; Davies, P.W. Catalytic Carbophilic Activation: Catalysis by Platinum and Gold π Acids. Angew. Chem. Int. Ed. 2007, 46, 3410–3449. [Google Scholar] [CrossRef]
- Gorin, D.J.; Toste, F.D. Relativistic effects in homogeneous gold catalysis. Nature 2007, 446, 395. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, A.S.K. Homogeneous Gold Catalysis Beyond Assumptions and Proposals—Characterized Intermediates. Angew. Chem. Int. Ed. 2010, 49, 5232–5241. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Xu, Y.; Shi, S.-L. A Bulky Chiral N-Heterocyclic Carbene Palladium Catalyst Enables Highly Enantioselective Suzuki–Miyaura Cross-Coupling Reactions for the Synthesis of Biaryl Atropisomers. J. Am. Chem. Soc. 2019, 141, 14938–14945. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.-j.; Wang, F.; Shi, M. Synthesis of Chiral Bis(N-heterocyclic carbene) Palladium and Rhodium Complexes with 1,1′-Biphenyl Scaffold and Their Application in Asymmetric Catalysis. Organometallics 2009, 28, 4416–4420. [Google Scholar] [CrossRef]
- Bonnet, L.G.; Douthwaite, R.E.; Kariuki, B.M. Synthesis of New Chiral N-Heterocyclic Carbene−Imine Ligands and Their Application to an Asymmetric Allylic Alkylation Reaction. Organometallics 2003, 22, 4187–4189. [Google Scholar] [CrossRef]
- Benhamou, L.; Besnard, C.; Kündig, E.P. Chiral PEPPSI Complexes: Synthesis, Characterization, and Application in Asymmetric Suzuki–Miyaura Coupling Reactions. Organometallics 2014, 33, 260–266. [Google Scholar] [CrossRef]
- Van Veldhuizen, J.J.; Gillingham, D.G.; Garber, S.B.; Kataoka, O.; Hoveyda, A.H. Chiral Ru-Based Complexes for Asymmetric Olefin Metathesis: Enhancement of Catalyst Activity through Steric and Electronic Modifications. J. Am. Chem. Soc. 2003, 125, 12502–12508. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, C.; del Pozo, J.; Torker, S.; Hoveyda, A.H. Ru-Based Catechothiolate Complexes Bearing an Unsaturated NHC Ligand: Effective Cross-Metathesis Catalysts for Synthesis of (Z)-α,β-Unsaturated Esters, Carboxylic Acids, and Primary, Secondary, and Weinreb Amides. J. Am. Chem. Soc. 2019, 141, 7137–7146. [Google Scholar] [CrossRef]
- Dumas, A.; Tarrieu, R.; Vives, T.; Roisnel, T.; Dorcet, V.; Baslé, O.; Mauduit, M. A Versatile and Highly Z-Selective Olefin Metathesis Ruthenium Catalyst Based on a Readily Accessible N-Heterocyclic Carbene. ACS Catal. 2018, 8, 3257–3262. [Google Scholar] [CrossRef]
- Paul, D.; Beiring, B.; Plois, M.; Ortega, N.; Kock, S.; Schlüns, D.; Neugebauer, J.; Wolf, R.; Glorius, F. A Cyclometalated Ruthenium-NHC Precatalyst for the Asymmetric Hydrogenation of (Hetero)arenes and Its Activation Pathway. Organometallics 2016, 35, 3641–3646. [Google Scholar] [CrossRef]
- Larsen, A.O.; Leu, W.; Oberhuber, C.N.; Campbell, J.E.; Hoveyda, A.H. Bidentate NHC-Based Chiral Ligands for Efficient Cu-Catalyzed Enantioselective Allylic Alkylations: Structure and Activity of an Air-Stable Chiral Cu Complex. J. Am. Chem. Soc. 2004, 126, 11130–11131. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Jung, B.; Torker, S.; Hoveyda, A.H. N-Heterocyclic Carbene–Copper-Catalyzed Group-, Site-, and Enantioselective Allylic Substitution with a Readily Accessible Propargyl(pinacolato)boron Reagent: Utility in Stereoselective Synthesis and Mechanistic Attributes. J. Am. Chem. Soc. 2015, 137, 8948–8964. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Jung, B.; Hoveyda, A.H. Catalytic Enantioselective Protoboration of Disubstituted Allenes. Access to Alkenylboron Compounds in High Enantiomeric Purity. Org. Lett. 2014, 16, 4658–4661. [Google Scholar] [CrossRef] [PubMed]
- Tarrieu, R.; Dumas, A.; Thongpaen, J.; Vives, T.; Roisnel, T.; Dorcet, V.; Crévisy, C.; Baslé, O.; Mauduit, M. Readily Accessible Unsymmetrical Unsaturated 2,6-Diisopropylphenyl N-Heterocyclic Carbene Ligands. Applications in Enantioselective Catalysis. J. Org. Chem. 2017, 82, 1880–1887. [Google Scholar] [CrossRef]
- Drissi-Amraoui, S.; Morin, M.S.T.; Crévisy, C.; Baslé, O.; Marcia de Figueiredo, R.; Mauduit, M.; Campagne, J.-M. Copper-Catalyzed Asymmetric Conjugate Addition of Dimethylzinc to Acyl-N-methylimidazole Michael Acceptors: A Powerful Synthetic Platform. Angew. Chem. Int. Ed. 2015, 54, 11830–11834. [Google Scholar] [CrossRef]
- Jahier-Diallo, C.; Morin, M.S.T.; Queval, P.; Rouen, M.; Artur, I.; Querard, P.; Toupet, L.; Crévisy, C.; Baslé, O.; Mauduit, M. Multicomponent Synthesis of Chiral Bidentate Unsymmetrical Unsaturated N-Heterocyclic Carbenes: Copper-Catalyzed Asymmetric C—C Bond Formation. Chem.–Eur. J. 2015, 21, 993–997. [Google Scholar] [CrossRef]
- Park, J.K.; Lackey, H.H.; Rexford, M.D.; Kovnir, K.; Shatruk, M.; McQuade, D.T. A Chiral 6-Membered N-Heterocyclic Carbene Copper(I) Complex That Induces High Stereoselectivity. Org. Lett. 2010, 12, 5008–5011. [Google Scholar] [CrossRef]
- Selim, K.B.; Nakanishi, H.; Matsumoto, Y.; Yamamoto, Y.; Yamada, K.-I.; Tomioka, K. Chiral N-Heterocyclic Carbene−Copper(I)-Catalyzed Asymmetric Allylic Arylation of Aliphatic Allylic Bromides: Steric and Electronic Effects on γ-Selectivity. J. Org. Chem. 2011, 76, 1398–1408. [Google Scholar] [CrossRef]
- Selim, K.B.; Matsumoto, Y.; Yamada, K.-I.; Tomioka, K. Efficient Chiral N-Heterocyclic Carbene/Copper(I)-Catalyzed Asymmetric Allylic Arylation with Aryl Grignard Reagents. Angew. Chem. Int. Ed. 2009, 48, 8733–8735. [Google Scholar] [CrossRef]
- Benhamou, L.; Chardon, E.; Lavigne, G.; Bellemin-Laponnaz, S.; César, V. Synthetic Routes to N-Heterocyclic Carbene Precursors. Chem. Rev. 2011, 111, 2705–2733. [Google Scholar] [CrossRef]
- Czerwiński, P.J.; Michalak, M. Synthetic Approaches to Chiral Non-C2-symmetric N-Heterocyclic Carbene Precursors. Synthesis 2019, 51, 1689–1714. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Horibe, T.; Jacobsen, C.B.; Toste, F.D. Stable gold(III) catalysts by oxidative addition of a carbon–carbon bond. Nature 2015, 517, 449. [Google Scholar] [CrossRef] [PubMed]
- Basova, T.V.; Hassan, A.; Morozova, N.B. Chemistry of gold(I, III) complexes with organic ligands as potential MOCVD precursors for fabrication of thin metallic films and nanoparticles. Coord. Chem. Rev. 2019, 380, 58–82. [Google Scholar] [CrossRef]
- Bohan, P.T.; Toste, F.D. Well-Defined Chiral Gold(III) Complex Catalyzed Direct Enantioconvergent Kinetic Resolution of 1,5-Enynes. J. Am. Chem. Soc. 2017, 139, 11016–11019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Frémont, P.; Singh, R.; Stevens, E.D.; Petersen, J.L.; Nolan, S.P. Synthesis, Characterization and Reactivity of N-Heterocyclic Carbene Gold(III) Complexes. Organometallics 2007, 26, 1376–1385. [Google Scholar] [CrossRef]
- Hashmi, A.S.K.; Blanco, M.C.; Fischer, D.; Bats, J.W. Gold Catalysis: Evidence for the In-situ Reduction of Gold(III) During the Cyclization of Allenyl Carbinols. Eur. J. Org. Chem. 2006, 2006, 1387–1389. [Google Scholar] [CrossRef]
- Wolf, W.J.; Winston, M.S.; Toste, F.D. Exceptionally fast carbon–carbon bond reductive elimination from gold(III). Nat. Chem. 2013, 6, 159. [Google Scholar] [CrossRef]
- Wang, H.M.J.; Lin, I.J.B. Facile Synthesis of Silver(I)−Carbene Complexes. Useful Carbene Transfer Agents. Organometallics 1998, 17, 972–975. [Google Scholar] [CrossRef]
- Furst, M.R.L.; Cazin, C.S.J. Copper N-heterocyclic carbene (NHC) complexes as carbene transfer reagents. Chem. Commun. 2010, 46, 6924–6925. [Google Scholar] [CrossRef]
- Lin, J.C.Y.; Huang, R.T.W.; Lee, C.S.; Bhattacharyya, A.; Hwang, W.S.; Lin, I.J.B. Coinage Metal−N-Heterocyclic Carbene Complexes. Chem. Rev. 2009, 109, 3561–3598. [Google Scholar] [CrossRef]
- de Frémont, P.; Scott, N.M.; Stevens, E.D.; Nolan, S.P. Synthesis and Structural Characterization of N-Heterocyclic Carbene Gold(I) Complexes. Organometallics 2005, 24, 2411–2418. [Google Scholar] [CrossRef]
- Zhu, S.; Liang, R.; Chen, L.; Wang, C.; Ren, Y.; Jiang, H. A direct and practical approach for the synthesis of Au(I)-NHC complexes from commercially available imidazolium salts and Au(III) salts. Tetrahedron Lett. 2012, 53, 815–818. [Google Scholar] [CrossRef]
- Fèvre, M.; Pinaud, J.; Leteneur, A.; Gnanou, Y.; Vignolle, J.; Taton, D.; Miqueu, K.; Sotiropoulos, J.-M. Imidazol(in)ium Hydrogen Carbonates as a Genuine Source of N-Heterocyclic Carbenes (NHCs): Applications to the Facile Preparation of NHC Metal Complexes and to NHC-Organocatalyzed Molecular and Macromolecular Syntheses. J. Am. Chem. Soc. 2012, 134, 6776–6784. [Google Scholar] [CrossRef] [PubMed]
- Visbal, R.; Laguna, A.; Gimeno, M.C. Simple and efficient synthesis of [MCI(NHC)] (M = Au, Ag) complexes. Chem. Commun. 2013, 49, 5642–5644. [Google Scholar] [CrossRef] [PubMed]
- Collado, A.; Gomez-Suarez, A.; Martin, A.R.; Slawin, A.M.Z.; Nolan, S.P. Straightforward synthesis of [Au(NHC)X] (NHC = N-heterocyclic carbene, X = Cl, Br, I) complexes. Chem. Commun. 2013, 49, 5541–5543. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.; Gimeno, M.C. An efficient and sustainable synthesis of NHC gold complexes. Chem. Commun. 2016, 52, 9664–9667. [Google Scholar] [CrossRef]
- Zhukhovitskiy, A.V.; Kobylianskii, I.J.; Wu, C.-Y.; Toste, F.D. Migratory Insertion of Carbenes into Au(III)–C Bonds. J. Am. Chem. Soc. 2018, 140, 466–474. [Google Scholar] [CrossRef]
- Gaillard, S.; Slawin, A.M.Z.; Bonura, A.T.; Stevens, E.D.; Nolan, S.P. Synthetic and Structural Studies of [AuCl3(NHC)] Complexes. Organometallics 2010, 29, 394–402. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Selim, K.B.; Nakanishi, H.; Yamada, K.-i.; Yamamoto, Y.; Tomioka, K. Chiral carbene approach to gold-catalyzed asymmetric cyclization of 1,6-enynes. Tetrahedron Lett. 2010, 51, 404–406. [Google Scholar] [CrossRef]
- Glorius, F.; Altenhoff, G.; Goddard, R.; Lehmann, C. Oxazolines as chiral building blocks for imidazolium salts and N-heterocyclic carbene ligands. Chem. Commun. 2002, 22, 2704–2705. [Google Scholar] [CrossRef]
- Kündig, E.P.; Seidel, T.M.; Jia, Y.-X.; Bernardinelli, G. Bulky Chiral Carbene Ligands and Their Application in the Palladium-Catalyzed Asymmetric Intramolecular α-Arylation of Amides. Angew. Chem. Int. Ed. 2007, 46, 8484–8487. [Google Scholar] [CrossRef] [PubMed]
- Gung, B.W.; Bailey, L.N.; Craft, D.T.; Barnes, C.L.; Kirschbaum, K. Preparation and Characterization of Two New N-Heterocyclic Carbene Gold(I) Complexes and Comparison of Their Catalytic Activity to Au(IPr)Cl. Organometallics 2010, 29, 3450–3456. [Google Scholar] [CrossRef]
- Gung, B.W.; Holmes, M.R.; Jones, C.A.; Ma, R.; Barnes, C.L. Structure–enantioselectivity correlation in NHC–Au(I) catalysis for 1,6-enynecyclizations. Tetrahedron Lett. 2016, 57, 3912–3915. [Google Scholar] [CrossRef]
- Holmes, M.R.; Manganaro, J.F.; Barnes, C.L.; Gung, B.W. Synthesis and characterization of novel chiral [(NHC)Au(I)Cl] complexes: Featuring ortho-biphenyl substituents. J. Organomet. Chem. 2015, 795, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Cai, R.; Sharma, S.; Jirak, J.; Thummanapelli, S.K.; Akhmedov, N.G.; Zhang, H.; Liu, X.; Petersen, J.L.; Shi, X. “Silver Effect” in Gold(I) Catalysis: An Overlooked Important Factor. J. Am. Chem. Soc. 2012, 134, 9012–9019. [Google Scholar] [CrossRef]
- Liu, C.; Shen, H.-Q.; Chen, M.-W.; Zhou, Y.-G. C2-Symmetric Hindered “Sandwich” Chiral N-Heterocyclic Carbene Precursors and Their Transition Metal Complexes: Expedient Syntheses, Structural Authentication, and Catalytic Properties. Organometallics 2018, 37, 3756–3769. [Google Scholar] [CrossRef]
- Poater, A.; Cosenza, B.; Correa, A.; Giudice, S.; Ragone, F.; Scarano, V.; Cavallo, L. SambVca: A Web Application for the Calculation of the Buried Volume of N-Heterocyclic Carbene Ligands. Eur. J. Inorg. Chem. 2009, 13, 1759–1766. [Google Scholar] [CrossRef]
- Falivene, L.; Credendino, R.; Poater, A.; Petta, A.; Serra, L.; Oliva, R.; Scarano, V.; Cavallo, L. SambVca 2. A Web Tool for Analyzing Catalytic Pockets with Topographic Steric Maps. Organometallics 2016, 35, 2286–2293. [Google Scholar] [CrossRef] [Green Version]
- Wilckens, K.; Lentz, D.; Czekelius, C. Synthesis of Gold Complexes Bearing Sterically Highly Encumbered, Chiral Carbene Ligands. Organometallics 2011, 30, 1287–1290. [Google Scholar] [CrossRef]
- Baskakov, D.; Herrmann, W.A.; Herdtweck, E.; Hoffmann, S.D. Chiral N-Heterocyclic Carbenes with Restricted Flexibility in Asymmetric Catalysis. Organometallics 2007, 26, 626–632. [Google Scholar] [CrossRef]
- Ye, R.; Zhukhovitskiy, A.V.; Kazantsev, R.V.; Fakra, S.C.; Wickemeyer, B.B.; Toste, F.D.; Somorjai, G.A. Supported Au Nanoparticles with N-Heterocyclic Carbene Ligands as Active and Stable Heterogeneous Catalysts for Lactonization. J. Am. Chem. Soc. 2018, 140, 4144–4149. [Google Scholar] [CrossRef] [PubMed]
- Queval, P.; Jahier, C.; Rouen, M.; Artur, I.; Legeay, J.-C.; Falivene, L.; Toupet, L.; Crévisy, C.; Cavallo, L.; Baslé, O.; et al. Multicomponent Synthesis of Unsymmetrical Unsaturated N-Heterocyclic Carbene Precursors and Their Related Transition-Metal Complexes. Angew. Chem. Int. Ed. 2013, 52, 14103–14107. [Google Scholar] [CrossRef] [PubMed]
- Usui, K.; Yoshida, T.; Nakada, M. Research on the design, synthesis, and catalytic activity of chiral N-heterocyclic carbene ligand–metal complexes. Tetrahedron Asymmetry 2016, 27, 107–113. [Google Scholar] [CrossRef]
- Ooi, T.; Kameda, M.; Maruoka, K. Design of N-Spiro C2-Symmetric Chiral Quaternary Ammonium Bromides as Novel Chiral Phase-Transfer Catalysts: Synthesis and Application to Practical Asymmetric Synthesis of α-Amino Acids. J. Am. Chem. Soc. 2003, 125, 5139–5151. [Google Scholar] [CrossRef] [PubMed]
- Okitsu, N.; Yoshida, T.; Usui, K.; Nakada, M. Synthesis of a New Chiral C2-Symmetric NHC-AuCl Complex. Heterocycles 2016, 92, 720–732. [Google Scholar] [CrossRef]
- Liu, L.-J.; Wang, F.; Wang, W.; Zhao, M.-X.; Shi, M. Synthesis of chiral mono(N-heterocyclic carbene) palladium and gold complexes with a 1,1’-biphenyl scaffold and their applications in catalysis. Beilstein J. Org. Chem. 2011, 7, 555–564. [Google Scholar] [CrossRef]
- Wang, W.; Yang, J.; Wang, F.; Shi, M. Axially Chiral N-Heterocyclic Carbene Gold(I) Complex Catalyzed Asymmetric Cycloisomerization of 1,6-Enynes. Organometallics 2011, 30, 3859–3869. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, R.; Wang, W.; Zhang, Z.; Shi, M. Axially chiral N-heterocyclic carbene gold(I) complex catalyzed asymmetric Friedel–Crafts/cyclization reaction of nitrogen-tethered 1,6-enynes with indole derivatives. Tetrahedron Asymmetry 2011, 22, 2029–2038. [Google Scholar] [CrossRef]
- Sun, Y.-W.; Xu, Q.; Shi, M. Synthesis of axially chiral gold complexes and their applications in asymmetric catalyses. Beilstein J. Org. Chem. 2013, 9, 2224–2232. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Li, S.; Qu, M.; Zhao, M.-X.; Liu, L.-J.; Shi, M. Synthesis of axially chiral oxazoline–carbene ligands with an N-naphthyl framework and a study of their coordination with AuCl·SMe2. Beilstein J. Org. Chem. 2012, 8, 726–731. [Google Scholar] [CrossRef]
- Francos, J.; Grande-Carmona, F.; Faustino, H.; Iglesias-Sigüenza, J.; Díez, E.; Alonso, I.; Fernández, R.; Lassaletta, J.M.; López, F.; Mascareñas, J.L. Axially Chiral Triazoloisoquinolin-3-ylidene Ligands in Gold(I)-Catalyzed Asymmetric Intermolecular (4 + 2) Cycloadditions of Allenamides and Dienes. J. Am. Chem. Soc. 2012, 134, 14322–14325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grande-Carmona, F.; Iglesias-Sigüenza, J.; Álvarez, E.; Díez, E.; Fernández, R.; Lassaletta, J.M. Synthesis and Characterization of Axially Chiral Imidazoisoquinolin-2-ylidene Silver and Gold Complexes. Organometallics 2015, 34, 5073–5080. [Google Scholar] [CrossRef]
- Varela, I.; Faustino, H.; Díez, E.; Iglesias-Sigüenza, J.; Grande-Carmona, F.; Fernández, R.; Lassaletta, J.M.; Mascareñas, J.L.; López, F. Gold(I)-Catalyzed Enantioselective [2 + 2 + 2] Cycloadditions: An Expedient Entry to Enantioenriched Tetrahydropyran Scaffolds. ACS Catal. 2017, 7, 2397–2402. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, J.; Su, X.; Wang, X.; Song, S.; Shi, M. Synthesis of Various Saturated and Unsaturated N-Heterocyclic Carbene Precursors by Triflic Anhydride Mediated Intramolecular Cyclization. Chem. Asian J. 2013, 8, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Espina, M.; Rivilla, I.; Conde, A.; Díaz-Requejo, M.M.; Pérez, P.J.; Álvarez, E.; Fernández, R.; Lassaletta, J.M. Chiral, Sterically Demanding N-Heterocyclic Carbenes Fused into a Heterobiaryl Skeleton: Design, Synthesis, and Structural Analysis. Organometallics 2015, 34, 1328–1338. [Google Scholar] [CrossRef]
- Available online: https://www.molnac.unisa.it/OMtools/sambvca2.0/ (accessed on 29 June 2007).
- Zhang, J.-Q.; Liu, Y.; Wang, X.-W.; Zhang, L. Synthesis of Chiral Bifunctional NHC Ligands and Survey of Their Utilities in Asymmetric Gold Catalysis. Organometallics 2019. [Google Scholar] [CrossRef]
- Guitet, M.; Zhang, P.; Marcelo, F.; Tugny, C.; Jiménez-Barbero, J.; Buriez, O.; Amatore, C.; Mouriès-Mansuy, V.; Goddard, J.-P.; Fensterbank, L.; et al. NHC-Capped Cyclodextrins (ICyDs): Insulated Metal Complexes, Commutable Multicoordination Sphere, and Cavity-Dependent Catalysis. Angew. Chem. Int. Ed. 2013, 52, 7213–7218. [Google Scholar] [CrossRef]
- Handa, S.; Slaughter, L.M. Enantioselective Alkynylbenzaldehyde Cyclizations Catalyzed by Chiral Gold(I) Acyclic Diaminocarbene Complexes Containing Weak Au–Arene Interactions. Angew. Chem. Int. Ed. 2012, 51, 2912–2915. [Google Scholar] [CrossRef]
- Bartolomé, C.; García-Cuadrado, D.; Ramiro, Z.; Espinet, P. Synthesis and Catalytic Activity of Gold Chiral Nitrogen Acyclic Carbenes and Gold Hydrogen Bonded Heterocyclic Carbenes in Cyclopropanation of Vinyl Arenes and in Intramolecular Hydroalkoxylation of Allenes. Inorg. Chem. 2010, 49, 9758–9764. [Google Scholar] [CrossRef]
- Khrakovsky, D.A.; Tao, C.; Johnson, M.W.; Thornbury, R.T.; Shevick, S.L.; Toste, F.D. Enantioselective, Stereodivergent Hydroazidation and Hydroamination of Allenes Catalyzed by Acyclic Diaminocarbene (ADC) Gold(I) Complexes. Angew. Chem. Int. Ed. 2016, 55, 6079–6083. [Google Scholar] [CrossRef] [Green Version]
- Niemeyer, Z.L.; Pindi, S.; Khrakovsky, D.A.; Kuzniewski, C.N.; Hong, C.M.; Joyce, L.A.; Sigman, M.S.; Toste, F.D. Parameterization of Acyclic Diaminocarbene Ligands Applied to a Gold(I)-Catalyzed Enantioselective Tandem Rearrangement/Cyclization. J. Am. Chem. Soc. 2017, 139, 12943–12946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Göker, V.; Kohl, S.R.; Rominger, F.; Meyer-Eppler, G.; Volbach, L.; Schnakenburg, G.; Lützen, A.; Hashmi, A.S.K. Chiral [2.2] paracyclophane-based NAC- and NHC-gold(I) complexes. J. Organomet. Chem. 2015, 795, 45–52. [Google Scholar] [CrossRef]
- Arnanz, A.; González-Arellano, C.; Juan, A.; Villaverde, G.; Corma, A.; Iglesias, M.; Sánchez, F. New chiral ligands bearing two N-heterocyclic carbene moieties at a dioxolane backbone. Gold, palladium and rhodium complexes as enantioselective catalysts. Chem. Commun. 2010, 46, 3001–3003. [Google Scholar] [CrossRef] [PubMed]
- Villaverde, G.; Corma, A.; Iglesias, M.; Sánchez, F. Chiral NHC-Complexes with Dioxolane Backbone Heterogenized on MCM-41. Catalytic Activity. ChemCatChem 2011, 3, 1320–1328. [Google Scholar] [CrossRef]
- Mahule, S.R.; Gangwar, M.K.; Vishnoi, P. Neutral Binuclear Ag(I) and Au(I) N-Heterocyclic Carbene Complexes of Axially Chiral and Racemic Scaffolds: Synthesis and Characterization. ChemistrySelect 2018, 3, 4023–4026. [Google Scholar] [CrossRef]
- Schmidbaur, H.; Schier, A. Aurophilic interactions as a subject of current research: An up-date. Chem. Soc. Rev. 2012, 41, 370–412. [Google Scholar] [CrossRef]
- Visbal, R.; Gimeno, M.C. N-heterocyclic carbene metal complexes: Photoluminescence and applications. Chem. Soc. Rev. 2014, 43, 3551–3574. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, Y.-C.; Jin, R.; Chen, B.-L.; Chen, Z. Synthesis of Axially Chiral 1,2,3-Triazol-5-ylidene–Au(I) Complex and Its Application in Enantioselective [2 + 2] Cycloaddition of Alleneamides with Alkenes. Organometallics 2018, 37, 3196–3209. [Google Scholar] [CrossRef]
- Wang, Y.-M.; Kuzniewski, C.N.; Rauniyar, V.; Hoong, C.; Toste, F.D. Chiral (Acyclic Diaminocarbene)Gold(I)-Catalyzed Dynamic Kinetic Asymmetric Transformation of Propargyl Esters. J. Am. Chem. Soc. 2011, 133, 12972–12975. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, D.; Buzas, A.K.; Besnard, C.; Kündig, E.P. Chiral N-Heterocyclic Carbene Gold Complexes: Synthesis, Properties, and Application in Asymmetric Catalysis. Organometallics 2012, 31, 8348–8354. [Google Scholar] [CrossRef]
- Muñoz, M.P.; Adrio, J.; Carretero, J.C.; Echavarren, A.M. Ligand Effects in Gold- and Platinum-Catalyzed Cyclization of Enynes: Chiral Gold Complexes for Enantioselective Alkoxycyclization. Organometallics 2005, 24, 1293–1300. [Google Scholar] [CrossRef]
- Yamada, K.-I.; Matsumoto, Y.; Selim, K.B.; Yamamoto, Y.; Tomioka, K. Steric tuning of C2-symmetric chiral N-heterocyclic carbene in gold-catalyzed asymmetric cyclization of 1,6-enynes. Tetrahedron 2012, 68, 4159–4165. [Google Scholar] [CrossRef]
- Charruault, L.; Michelet, V.; Taras, R.; Gladiali, S.; Genêt, J.-P. Functionalized carbo- and heterocycles via Pt-catalyzed asymmetric alkoxycyclization of 1,6-enynes. Chem. Commun. 2004, 7, 850–851. [Google Scholar] [CrossRef] [PubMed]
- Pradal, A.; Chao, C.-M.; Vitale, M.R.; Toullec, P.Y.; Michelet, V. Asymmetric Au-catalyzed domino cyclization/nucleophile addition reactions of enynes in the presence of water, methanol and electron-rich aromatic derivatives. Tetrahedron 2011, 67, 4371–4377. [Google Scholar] [CrossRef]
- Dell’Acqua, M.; Castano, B.; Cecchini, C.; Pedrazzini, T.; Pirovano, V.; Rossi, E.; Caselli, A.; Abbiati, G. Mild Regiospecific Synthesis of 1-Alkoxy-isochromenes Catalyzed by Well-Defined [Silver(I)(Pyridine-Containing Ligand)] Complexes. J. Org. Chem. 2014, 79, 3494–3505. [Google Scholar] [CrossRef]
- Godet, T.; Vaxelaire, C.; Michel, C.; Milet, A.; Belmont, P. Silver versus Gold Catalysis in Tandem Reactions of Carbonyl Functions onto Alkynes: A Versatile Access to Furoquinoline and Pyranoquinoline Cores. Chem. Eur. J. 2007, 13, 5632–5641. [Google Scholar] [CrossRef]
- Patil, N.T.; Yamamoto, Y. Synthesis of Cyclic Alkenyl Ethers via Intramolecular Cyclization of O-Alkynylbenzaldehydes. Importance of Combination between CuI Catalyst and DMF. J. Org. Chem. 2004, 69, 5139–5142. [Google Scholar] [CrossRef]
- Asao, N.; Nogami, T.; Takahashi, K.; Yamamoto, Y. Pd(II) Acts Simultaneously as a Lewis Acid and as a Transition-Metal Catalyst: Synthesis of Cyclic Alkenyl Ethers from Acetylenic Aldehydes. J. Am. Chem. Soc. 2002, 124, 764–765. [Google Scholar] [CrossRef]
- Aikawa, K.; Kojima, M.; Mikami, K. Synergistic Effect: Hydroalkoxylation of Allenes through Combination of Enantiopure BIPHEP-Gold Complexes and Chiral Anions. Adv. Synth. Catal. 2010, 352, 3131–3135. [Google Scholar] [CrossRef]
- Wang, Z.; Nicolini, C.; Hervieu, C.; Wong, Y.-F.; Zanoni, G.; Zhang, L. Remote Cooperative Group Strategy Enables Ligands for Accelerative Asymmetric Gold Catalysis. J. Am. Chem. Soc. 2017, 139, 16064–16067. [Google Scholar] [CrossRef]
- Michon, C.; Abadie, M.-A.; Medina, F.; Agbossou-Niedercorn, F. Mononuclear gold catalysts for the asymmetric intramolecular hydroamination of alkenes. Catal. Today 2014, 235, 2–13. [Google Scholar] [CrossRef]
- Mukherjee, P.; Widenhoefer, R.A. Gold(I)-Catalyzed Enantioselective Intramolecular Dehydrative Amination of Allylic Alcohols with Carbamates. Angew. Chem. Int. Ed. 2012, 51, 1405–1407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arbour, J.L.; Rzepa, H.S.; Contreras-García, J.; Adrio, L.A.; Barreiro, E.M.; Hii, K.K. Silver-Catalysed Enantioselective Addition of O—H and N—H Bonds to Allenes: A New Model for Stereoselectivity Based on Noncovalent Interactions. Chem. Eur. J. 2012, 18, 11317–11324. [Google Scholar] [CrossRef] [PubMed]
- Michon, C.; Medina, F.; Abadie, M.-A.; Agbossou-Niedercorn, F. Asymmetric Intramolecular Hydroamination of Allenes using Mononuclear Gold Catalysts. Organometallics 2013, 32, 5589–5600. [Google Scholar] [CrossRef]
- Abadie, M.-A.; Trivelli, X.; Medina, F.; Capet, F.; Roussel, P.; Agbossou-Niedercorn, F.; Michon, C. Asymmetric Intramolecular Hydroamination of Alkenes in Mild and Wet Conditions—Structure and Reactivity of Cationic Binuclear Gold(I) Catalysts. ChemCatChem 2014, 6, 2235–2239. [Google Scholar] [CrossRef]
- Abadie, M.-A.; Trivelli, X.; Medina, F.; Duhal, N.; Kouach, M.; Linden, B.; Génin, E.; Vandewalle, M.; Capet, F.; Roussel, P.; et al. Gold(I)-Catalysed Asymmetric Hydroamination of Alkenes: A Silver- and Solvent-Dependent Enantiodivergent Reaction. Chem. Eur. J. 2017, 23, 10777–10788. [Google Scholar] [CrossRef]
- Chao, C.-M.; Genin, E.; Toullec, P.Y.; Genêt, J.-P.; Michelet, V. Towards asymmetric Au-catalyzed hydroxy- and alkoxycyclization of 1,6-enynes. J. Organomet. Chem. 2009, 694, 538–545. [Google Scholar] [CrossRef]
- Zi, W.; Toste, F.D. Gold(I)-Catalyzed Enantioselective Carboalkoxylation of Alkynes. J. Am. Chem. Soc. 2013, 135, 12600–12603. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.-J.; Cui, J.-F.; Yang, B.; Ning, Y.; Lai, N.C.-H.; Wong, M.-K. Chiral Cyclometalated Oxazoline Gold(III) Complex-Catalyzed Asymmetric Carboalkoxylation of Alkynes. Org. Lett. 2019, 21, 6289–6294. [Google Scholar] [CrossRef]
- Zhang, L. Tandem Au-Catalyzed 3,3-Rearrangement−[2 + 2] Cycloadditions of Propargylic Esters: Expeditious Access to Highly Functionalized 2,3-Indoline-Fused Cyclobutanes. J. Am. Chem. Soc. 2005, 127, 16804–16805. [Google Scholar] [CrossRef]
- Wilckens, K.; Uhlemann, M.; Czekelius, C. Gold-Catalyzed endo-Cyclizations of 1,4-Diynes to Seven-Membered Ring Heterocycles. Chem. Eur. J. 2009, 15, 13323–13326. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.J.; Gorin, D.J.; Staben, S.T.; Toste, F.D. Gold(I)-Catalyzed Stereoselective Olefin Cyclopropanation. J. Am. Chem. Soc. 2005, 127, 18002–18003. [Google Scholar] [CrossRef] [PubMed]
- Pirovano, V.; Borri, M.; Abbiati, G.; Rizzato, S.; Rossi, E. Gold(I)-Catalyzed Enantioselective Synthesis of Tetrahydrocarbazoles through Dearomative [4+2] Cycloadditions of 3/2-Substituted 2/3-Vinylindoles. Adv. Synth. Catal. 2017, 359, 1912–1918. [Google Scholar] [CrossRef]
- Faustino, H.; López, F.; Castedo, L.; Mascareñas, J.L. Gold(i)-catalyzed intermolecular (4 + 2) cycloaddition of allenamides and acyclic dienes. Chem. Sci. 2011, 2, 633–637. [Google Scholar] [CrossRef]
- Hu, H.; Wang, Y.; Qian, D.; Zhang, Z.-M.; Liu, L.; Zhang, J. Enantioselective gold-catalyzed intermolecular [2 + 2]-cycloadditions of 3-styrylindoles with N-allenyl oxazolidinone. Org. Chem. Front. 2016, 3, 759–763. [Google Scholar] [CrossRef]
- García-Morales, C.; Ranieri, B.; Escofet, I.; López-Suarez, L.; Obradors, C.; Konovalov, A.I.; Echavarren, A.M. Enantioselective Synthesis of Cyclobutenes by Intermolecular [2 + 2] Cycloaddition with Non-C2 Symmetric Digold Catalysts. J. Am. Chem. Soc. 2017, 139, 13628–13631. [Google Scholar] [CrossRef]
- Hurtado-Rodrigo, C.; Hoehne, S.; Muñoz, M.P. A new gold-catalysed azidation of allenes. Chem. Commun. 2014, 50, 1494–1496. [Google Scholar] [CrossRef]
- Usón, R.; Vicente, J.; Cirac, J.A.; Chicote, M.T. Synthesis and reactivity of dibenzometallole complexes of gold(III) and platinum(II). J. Organomet. Chem. 1980, 198, 105–112. [Google Scholar] [CrossRef]
- Nishina, N.; Yamamoto, Y. Gold-catalyzed hydrofunctionalization of allenes with nitrogen and oxygen nucleophiles and its mechanistic insight. Tetrahedron 2009, 65, 1799–1808. [Google Scholar] [CrossRef]



















































© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalak, M.; Kośnik, W. Chiral N-heterocyclic Carbene Gold Complexes: Synthesis and Applications in Catalysis. Catalysts 2019, 9, 890. https://doi.org/10.3390/catal9110890
Michalak M, Kośnik W. Chiral N-heterocyclic Carbene Gold Complexes: Synthesis and Applications in Catalysis. Catalysts. 2019; 9(11):890. https://doi.org/10.3390/catal9110890
Chicago/Turabian StyleMichalak, Michał, and Wioletta Kośnik. 2019. "Chiral N-heterocyclic Carbene Gold Complexes: Synthesis and Applications in Catalysis" Catalysts 9, no. 11: 890. https://doi.org/10.3390/catal9110890