Palladium-Nickel Electrocatalysts on Nitrogen-Doped Reduced Graphene Oxide Nanosheets for Direct Hydrazine/Hydrogen Peroxide Fuel Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physical Characterization of Nanocatalysts
2.2. Half-Cell Measurements
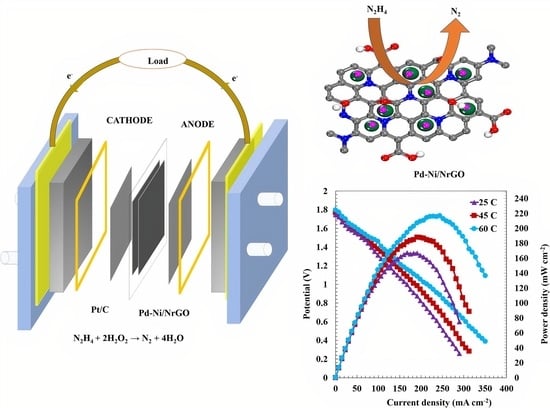
2.3. Single-Cell Tests
3. Experimental
3.1. Materials
3.2. Methods
3.3. Physical and Electrochemical Characterization
3.4. DHzHPFC Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Andrew, M.R.; Gressler, W.J.; Johnson, J.K.; Short, R.T.; Williams, K.R. Engineering aspects of hydrazine-air fuel-cell power systems. J. Appl. Electrochem. 1972, 2, 327–336. [Google Scholar] [CrossRef]
- Tamura, K.; Kahara, T. Exhaust Gas Compositions and Fuel Efficiencies of Hydrazine–Air Fuel Cells. J. Electrochem. Soc. 1976, 123, 776–780. [Google Scholar] [CrossRef]
- Akbar, K.; Kim, J.H.; Lee, Z.; Kim, M.; Yi, Y.; Chun, S.-H. Superaerophobic graphene nano-hills for direct hydrazine fuel cells. NPG Asia Mater. 2017, 9, e378. [Google Scholar] [CrossRef]
- Abdolmaleki, M.; Ahadzadeh, I.; Goudarziafshar, H. Direct hydrazine-hydrogen peroxide fuel cell using carbon supported Co@Au core-shell nanocatalyst. Int. J. Hydrog. Energ. 2017, 42, 15623–15631. [Google Scholar] [CrossRef]
- Silva, T.L.; Cazetta, A.L.; Zhang, T.; Koh, K.; Silva, R.; Asefa, T.; Almeida, V.C. Nanoporous Heteroatom-Doped Carbons Derived from Cotton Waste: Efficient Hydrazine Oxidation Electrocatalysts. ACS Appl. Energ. Mater. 2019, 2, 2313–2323. [Google Scholar] [CrossRef]
- Mohammad, A.; Ehtisham Khan, M.; Alarifi, I.M.; Cho, M.H.; Yoon, T. A sensitive electrochemical detection of hydrazine based on SnO2/CeO2 nanostructured oxide. Microchem. J. 2021, 171, 106784. [Google Scholar] [CrossRef]
- Blomen, L.J.M.J.; Mugerwa, M.N. Fuel Cell Systems; Springer: Boston, MA, USA, 1993; ISBN 0-306-44158-6. [Google Scholar]
- Amirfakhri, S.J.; Meunier, J.-L.; Berk, D. A comprehensive study of the kinetics of hydrogen peroxide reduction reaction by rotating disk electrode. Electrochim. Acta 2013, 114, 551–559. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Shi, S.; Yin, H.-M. CuPd Alloy Oxide Nanobelts as Electrocatalyst towards Hydrazine Oxidation. ChemElectroChem 2019, 6, 1514–1519. [Google Scholar] [CrossRef]
- Wei, J.; Wang, X.; Wang, Y.; Guo, J.; He, P.; Yang, S.; Li, N.; Pei, F.; Wang, Y. Carbon-Supported Au Hollow Nanospheres as Anode Catalysts for Direct Borohydride−Hydrogen Peroxide Fuel Cells. Energ. Fuels 2009, 23, 4037–4041. [Google Scholar] [CrossRef]
- Cao, D.; Chen, D.; Lan, J.; Wang, G. An alkaline direct NaBH4–H2O2 fuel cell with high power density. J. Power Sources 2009, 190, 346–350. [Google Scholar] [CrossRef]
- Khilari, S.; Pradhan, D. MnFe2O4@nitrogen-doped reduced graphene oxide nanohybrid: An efficient bifunctional electrocatalyst for anodic hydrazine oxidation and cathodic oxygen reduction. Catal. Sci. Technol. 2017, 7, 5920–5931. [Google Scholar] [CrossRef]
- Hosseini, M.G.; Mahmoodi, R.; Abdolmaleki, M. High performance direct hydrazine-hydrogen peroxide fuel cell using reduced graphene oxide supported Ni@M (M = Pt, Pd, Ru) nanoparticles as novel anodic electrocatalysts. New J. Chem. 2018, 42, 12222–12233. [Google Scholar] [CrossRef]
- Sapner, V.S.; Chavan, P.P.; Munde, A.V.; Sayyad, U.S.; Sathe, B.R. Heteroatom (N, O, and S)-Based Biomolecule-Functionalized Graphene Oxide: A Bifunctional Electrocatalyst for Enhancing Hydrazine Oxidation and Oxygen Reduction Reactions. Energ. Fuels 2021, 35, 6823–6834. [Google Scholar] [CrossRef]
- Zabielaitė, A.; Balčiūnaitė, A.; Šimkūnaitė, D.; Lichušina, S.; Stalnionienė, I.; Šimkūnaitė-Stanynienė, B.; Naruškevičius, L.; Tamašauskaitė-Tamašiūnaitė, L.; Norkus, E.; Selskis, A.; et al. High Performance Direct N2H4-H2O2 Fuel Cell Using Fiber-Shaped Co Decorated with Pt Crystallites as Anode Electrocatalysts. J. Electrochem. Soc. 2019, 167, 054502. [Google Scholar] [CrossRef]
- Nagai, T.; Kojima, T.; Takeichi, N.; Asahi, M.; Yamazaki, S.-i.; Siroma, Z.; Fujiwara, N.; Ioroi, T. Performance of Perovskite-Type Oxides for Oxygen Reduction Reaction in Direct Hydrazine Fuel Cell. J. Electrochem. Soc. 2020, 167, 164506. [Google Scholar] [CrossRef]
- Milikić, J.; Oliveira, R.C.P.; Tapia, A.; Santos, D.M.F.; Zdolšek, N.; Trtić-Petrović, T.; Vraneš, M.; Šljukić, B. Ionic Liquid-Derived Carbon-Supported Metal Electrocatalysts as Anodes in Direct Borohydride-Peroxide Fuel Cells. Catalysts 2021, 11, 632. [Google Scholar] [CrossRef]
- Bortoloti, F.; Angelo, A.C.D. Ordered PtSn/C Electrocatalyst: A High Performance Material for the Borohydride Electrooxidation Reaction. Catalysts 2017, 7, 198. [Google Scholar] [CrossRef] [Green Version]
- Yin, W.X.; Li, Z.P.; Zhu, J.K.; Qin, H.Y. Effects of NaOH addition on performance of the direct hydrazine fuel cell. J. Power Sources 2008, 182, 520–523. [Google Scholar] [CrossRef]
- Lao, S.J.; Qin, H.Y.; Ye, L.Q.; Liu, B.H.; Li, Z.P. A development of direct hydrazine/hydrogen peroxide fuel cell. J. Power Sources 2010, 195, 4135–4138. [Google Scholar] [CrossRef]
- Yan, X.; Meng, F.; Xie, Y.; Liu, J.; Ding, Y. Direct N2H4/H2O2 Fuel Cells Powered by Nanoporous Gold Leaves. Sci. Rep. 2012, 2, 941. [Google Scholar] [CrossRef]
- Huang, R.; Wen, Y.-H.; Shao, G.-F.; Sun, S.-G. Insight into the Melting Behavior of Au–Pt Core–Shell Nanoparticles from Atomistic Simulations. J. Phys. Chem. C 2013, 117, 4278–4286. [Google Scholar] [CrossRef]
- Gholinejad, M.; Khosravi, F.; Afrasi, M.; Sansano, J.M.; Nájera, C. Applications of bimetallic PdCu catalysts. Catal. Sci. Technol. 2021, 11, 2652–2702. [Google Scholar] [CrossRef]
- Ma, J.; Sahai, Y.; Buchheit, R.G. Direct borohydride fuel cell using Ni-based composite anodes. J. Power Sources 2010, 195, 4709–4713. [Google Scholar] [CrossRef]
- Hosseini, M.G.; Mahmoodi, R. Ni@M (M = Pt, Pd and Ru) core@shell nanoparticles on a Vulcan XC-72R support with superior catalytic activity toward borohydride oxidation: Electrochemical and fuel cell studies. New J. Chem. 2017, 41, 13408–13417. [Google Scholar] [CrossRef]
- Smith, A.T.; LaChance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Chen, Y.; Tian, Y.; Qiu, Y.; Liu, Z.; He, H.; Li, B.; Cao, H. Synthesis and superior cathode performance of sandwiched LiMn2O4@rGO nanocomposites for lithium-ion batteries. Mater. Today Adv. 2019, 1, 100001. [Google Scholar] [CrossRef]
- Lei, Y.; Lu, J.; Luo, X.; Wu, T.; Du, P.; Zhang, X.; Ren, Y.; Wen, J.; Miller, D.J.; Miller, J.T.; et al. Synthesis of porous carbon supported palladium nanoparticle catalysts by atomic layer deposition: Application for rechargeable lithium-O2 battery. Nano Lett. 2013, 13, 4182–4189. [Google Scholar] [CrossRef]
- Taqi-uddeen Safian, M.; Umar, K.; Mohamad Ibrahim, M.N. Synthesis and scalability of graphene and its derivatives: A journey towards sustainable and commercial material. J. Clean. Prod. 2021, 318, 128603. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Mohamad Ibrahim, M.N.; Umar, K.; Bhawani, S.A.; Khan, A.; Asiri, A.M.; Rizwan Khan, M.; Azam, M.; Al Ammari, A.M. Cellulose Derived Graphene/Polyaniline Nanocomposite Anode for Energy Generation and Bioremediation of Toxic Metals via Benthic Microbial Fuel Cells. Polymers 2021, 13, 135. [Google Scholar] [CrossRef]
- Lu, Z.-J.; Bao, S.-J.; Gou, Y.-T.; Cai, C.-J.; Ji, C.-C.; Xu, M.-W.; Song, J.; Wang, R. Nitrogen-doped reduced-graphene oxide as an efficient metal-free electrocatalyst for oxygen reduction in fuel cells. RSC Adv. 2013, 3, 3990–3995. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, J.; Yang, C.; Yang, Y.; Zhang, Y.; Yang, F.; Ma, R.; Yang, L.; He, H.; Huang, H. Pd nanocrystals anchored on 3D hybrid architectures constructed from nitrogen-doped graphene and low-defect carbon nanotube as high-performance multifunctional electrocatalysts for formic acid and methanol oxidation. Mater. Today Energ. 2020, 16, 100409. [Google Scholar] [CrossRef]
- Yang, H.; Ko, Y.; Lee, W.; Züttel, A.; Kim, W. Nitrogen-doped carbon black supported Pt–M (M = Pd, Fe, Ni) alloy catalysts for oxygen reduction reaction in proton exchange membrane fuel cell. Mater. Today Energ. 2019, 13, 374–381. [Google Scholar] [CrossRef]
- Guo, R.; An, N.; An, S.; Zhang, J.; Chou, K.; Guan, L.; Tian, X. One-Step Preparation of Nitrogen-Doped Platinum-Based Catalysts for Electrocatalytic Oxidation of Ethanol. Catalysts 2021, 11, 1264. [Google Scholar] [CrossRef]
- Wu, G.; Santandreu, A.; Kellogg, W.; Gupta, S.; Ogoke, O.; Zhang, H.; Wang, H.-L.; Dai, L. Carbon nanocomposite catalysts for oxygen reduction and evolution reactions: From nitrogen doping to transition-metal addition. Nano Energ. 2016, 29, 83–110. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Neyerlin, K.; Olson, T.S.; Pylypenko, S.; Bult, J.; Dinh, H.N.; Gennett, T.; Shao, Z.; O’Hayre, R. Enhancement of Pt and Pt-alloy fuel cell catalyst activity and durability via nitrogen-modified carbon supports. Energ. Environ. Sci. 2010, 3, 1437–1446. [Google Scholar] [CrossRef]
- Schauermann, S.; Nilius, N.; Shaikhutdinov, S.; Freund, H.-J. Nanoparticles for Heterogeneous Catalysis: New Mechanistic Insights. Acc. Chem. Res. 2013, 46, 1673–1681. [Google Scholar] [CrossRef]
- Wilde, M.; Fukutani, K.; Naschitzki, M.; Freund, H.J. Hydrogen absorption in oxide-supported palladium nanocrystals. Phys. Rev. B 2008, 77, 113412. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, M.G.; Daneshvari-Esfahlan, V.; Wolf, S.; Hacker, V. Novel Bimetallic Pd–X (X = Ni, Co) Nanoparticles Assembled on N-Doped Reduced Graphene Oxide as an Anode Catalyst for Highly Efficient Direct Sodium Borohydride–Hydrogen Peroxide Fuel Cells. ACS Appl. Energ. Mater. 2021, 4, 6025–6039. [Google Scholar] [CrossRef]
- Kumar, N.A.; Choi, H.-J.; Shin, Y.R.; Chang, D.W.; Dai, L.; Baek, J.-B. Polyaniline-Grafted Reduced Graphene Oxide for Efficient Electrochemical Supercapacitors. ACS Nano 2012, 6, 1715–1723. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, J.; Chen, H.; Wang, R.; Li, D.; Qu, J.; Dai, L. Nitrogen-Doped Graphene Foams as Metal-Free Counter Electrodes in High-Performance Dye-Sensitized Solar Cells. Angew. Chem. Int. Ed. 2012, 51, 12124–12127. [Google Scholar] [CrossRef]
- Mu, X.; Yuan, B.; Feng, X.; Qiu, S.; Song, L.; Hu, Y. The effect of doped heteroatoms (nitrogen, boron, phosphorus) on inhibition thermal oxidation of reduced graphene oxide. RSC Adv. 2016, 6, 105021–105029. [Google Scholar] [CrossRef]
- Kumar, M.P.; Raju, M.M.; Arunchander, A.; Selvaraj, S.; Kalita, G.; Narayanan, T.N.; Sahu, A.K.; Pattanayak, D.K. Nitrogen Doped Graphene as Metal Free Electrocatalyst for Efficient Oxygen Reduction Reaction in Alkaline Media and Its Application in Anion Exchange Membrane Fuel Cells. J. Electrochem. Soc. 2016, 163, F848–F855. [Google Scholar] [CrossRef]
- Soo, L.T.; Loh, K.S.; Mohamad, A.B.; Daud, W.R.W.; Wong, W.Y. Effect of nitrogen precursors on the electrochemical performance of nitrogen-doped reduced graphene oxide towards oxygen reduction reaction. J. Alloy. Compd. 2016, 677, 112–120. [Google Scholar] [CrossRef]
- Fuge, G.M.; Rennick, C.J.; Pearce, S.R.J.; May, P.W.; Ashfold, M.N.R. Structural characterisation of CNx thin films deposited by pulsed laser ablation. Diam. Relat. Mater. 2003, 12, 1049–1054. [Google Scholar] [CrossRef]
- Jang, J.W.; Lee, C.E.; Lyu, S.C.; Lee, T.J.; Lee, C.J. Structural study of nitrogen-doping effects in bamboo-shaped multiwalled carbon nanotubes. Appl. Phys. Lett. 2004, 84, 2877–2879. [Google Scholar] [CrossRef]
- Gopalsamy, K.; Balamurugan, J.; Thanh, T.D.; Kim, N.H.; Hui, D.; Lee, J.H. Surfactant-free synthesis of NiPd nanoalloy/graphene bifunctional nanocomposite for fuel cell. Compos. Part B Eng. 2017, 114, 319–327. [Google Scholar] [CrossRef]
- Zhang, J.-W.; Zhang, B.; Zhang, X. Enhanced catalytic activity of ternary NiCoPd nanocatalyst dispersed on carbon nanotubes toward methanol oxidation reaction in alkaline media. J. Solid State Electrochem. 2017, 21, 447–453. [Google Scholar] [CrossRef]
- Wang, J.; Bao, J.; Zhou, Y.; Zhang, Y.; Sun, B.; Wang, M.; Sheng, X.; Liu, W.; Luo, C.; Xue, Y.; et al. Dopamine-assisted synthesis of rGO@NiPd@NC sandwich structure for highly efficient hydrogen evolution reaction. J. Solid State Electrochem. 2020, 24, 137–144. [Google Scholar] [CrossRef]
- Shviro, M.; Polani, S.; Dunin-Borkowski, R.E.; Zitoun, D. Bifunctional Electrocatalysis on Pd-Ni Core-Shell Nanoparticles for Hydrogen Oxidation Reaction in Alkaline Medium. Adv. Mater. Interfaces 2018, 5, 1701666. [Google Scholar] [CrossRef]
- Mageed, A.K.; Radiah, D.; Salmiaton, A.; Izhar, S.; Razak, M.A.; Yusoff, H.M.; Yasin, F.; Kamarudin, S. Preparation and Characterization of Nitrogen Doped Reduced Graphene Oxide Sheet. Int. J. Appl. Chem. 2016, 12, 104–108. [Google Scholar]
- Liu, S.; Huang, S. Theoretical insights into the activation of O2 by Pt single atom and Pt4 nanocluster on functionalized graphene support: Critical role of Pt positive polarized charges. Carbon 2017, 115, 11–17. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Asadollahi, S. Exploring different reaction mechanisms for oxidation of CO over a single Pd atom incorporated nitrogen-doped graphene: A DFT study. Appl. Surf. Sci. 2019, 463, 526–534. [Google Scholar] [CrossRef]
- Trafela, S.; Zavasnik, J.; Sturm, S.; Rozman, K.Z. Formation of a Ni(OH)2/NiOOH active redox couple on nickel nanowires for formaldehyde detection in alkaline media. Electrochim. Acta 2019, 309, 346–353. [Google Scholar] [CrossRef]
- Medway, S.L.; Lucas, C.A.; Kowal, A.; Nichols, R.J.; Johnson, D. In situ studies of the oxidation of nickel electrodes in alkaline solution. J. Electroanal. Chem. 2006, 587, 172–181. [Google Scholar] [CrossRef]
- El-Shafei, A.A. Electrocatalytic oxidation of methanol at a nickel hydroxide/glassy carbon modified electrode in alkaline medium. J. Electroanal. Chem. 1999, 471, 89–95. [Google Scholar] [CrossRef]
- Dong, H.; Feng, R.; Ai, X.; Cao, Y.; Yang, H.; Cha, C. Electrooxidation Mechanisms and Discharge Characteristics of Borohydride on Different Catalytic Metal Surfaces. J. Phys. Chem. B 2005, 109, 10896–10901. [Google Scholar] [CrossRef]
- Santos, D.M.F.; Sequeira, C.A.C. Cyclic voltammetry investigation of borohydride oxidation at a gold electrode. Electrochim. Acta 2010, 55, 6775–6781. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, R.L. Electrochemical Methods: Fundamentals and Applications; Wiley: New York, NY, USA, 1980. [Google Scholar]
- Denuault, G.; Mirkin, M.V.; Bard, A.J. Direct determination of diffusion coefficients by chronoamperometry at microdisk electrodes. J. Electroanal. Chem. Interfacial Electrochem. 1991, 308, 27–38. [Google Scholar] [CrossRef]
- Ding, W.; Wu, M.; Liang, M.; Ni, H.; Li, Y. Sensitive Hydrazine Electrochemical Biosensor Based on a Porous Chitosan–Carbon Nanofiber Nanocomposite Modified Electrode. Anal. Lett. 2015, 48, 1551–1569. [Google Scholar] [CrossRef]
- Vats, T.; Dutt, S.; Kumar, R.; Siril, P.F. Facile synthesis of pristine graphene-palladium nanocomposites with extraordinary catalytic activities using swollen liquid crystals. Sci. Rep. 2016, 6, 33053. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, M.G.; Momeni, M.M.; Faraji, M. Highly Active Nickel Nanoparticles Supported on TiO2 Nanotube Electrodes for Methanol Electrooxidation. Electroanalysis 2010, 22, 2620–2625. [Google Scholar] [CrossRef]
- Wu, H.J.; Wang, C.; Liu, Z.X.; Mao, Z.Q. Influence of operation conditions on direct NaBH4/H2O2 fuel cell performance. Int. J. Hydrog. Energ. 2010, 35, 2648–2651. [Google Scholar] [CrossRef]
- Liu, B.H.; Li, Z.P.; Arai, K.; Suda, S. Performance improvement of a micro borohydride fuel cell operating at ambient conditions. Electrochim. Acta 2005, 50, 3719–3725. [Google Scholar] [CrossRef]
- Duteanu, N.; Vlachogiannopoulos, G.; Shivhare, M.R.; Yu, E.H.; Scott, K. A parametric study of a platinum ruthenium anode in a direct borohydride fuel cell. J. Appl. Electrochem. 2007, 37, 1085–1091. [Google Scholar] [CrossRef]
- Crisafulli, R.; De Barros, V.V.S.; Rodrigues De Oliveira, F.E.; De Araújo Rocha, T.; Zignani, S.; Spadaro, L.; Palella, A.; Dias, J.A.; Linares, J.J. On the promotional effect of Cu on Pt for hydrazine electrooxidation in alkaline medium. Appl. Catal. B Environ. 2018, 236, 36–44. [Google Scholar] [CrossRef]
- Feng, G.; Kuang, Y.; Li, P.; Han, N.; Sun, M.; Zhang, G.; Sun, X. Single Crystalline Ultrathin Nickel–Cobalt Alloy Nanosheets Array for Direct Hydrazine Fuel Cells. Adv. Sci. 2017, 4, 1600179. [Google Scholar] [CrossRef]
- Paganin, V.A.; Ticianelli, E.A.; Gonzalez, E.R. Development and electrochemical studies of gas diffusion electrodes for polymer electrolyte fuel cells. J. Appl. Electrochem. 1996, 26, 297–304. [Google Scholar] [CrossRef]
- Deng, J.; Li, X.; Imhanria, S.; Chen, K.; Deng, X.; Wang, W. Molybdenum carbide-nitrogen doped carbon composites as effective non-precious electrocatalyst for direct hydrazine fuel cell. Electrochim. Acta 2021, 384, 138417. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Prasanna, M.; Cho, E.A.; Lim, T.H.; Oh, I.H. Effects of MEA fabrication method on durability of polymer electrolyte membrane fuel cells. Electrochim. Acta 2008, 53, 5434–5441. [Google Scholar] [CrossRef]











| Catalyst Type | ECSA (m2 g−1) | ip (A g−1) | Onset Potential (V vs. MOE) |
|---|---|---|---|
| Pd/NrGO | 63.67 | 6821.22 | −0.40 |
| Pd–Ni/NrGO | 166.38 | 12,360.50 | −0.45 |
| N2H4 Concentration (mol L−1) | Potential (V vs. MOE) | Temperature (°C) | Rct (Ω cm2) |
|---|---|---|---|
| 0.02 | −0.5 | 25 | 92.76 |
| 0.06 | −0.5 | 25 | 31.27 |
| 0.1 | −0.5 | 25 | 22.03 |
| 0.1 | −0.5 | 45 | 17.12 |
| 0.1 | −0.9 | 25 | 4422 |
| FC Condition | MPD/(mW cm−2) | ||
|---|---|---|---|
| [N2H4]/(mol L−1) | [H2O2]/(mol L−1) | T/(°C) | |
| 2.0 | 0.5 | 25 | 101.93 |
| 2.0 | 1.0 | 25 | 120.50 |
| 2.0 | 2.0 | 25 | 152.74 |
| 2.0 | 3.0 | 25 | 130.25 |
| 0.5 | 2.0 | 25 | 136.72 |
| 1.0 | 2.0 | 25 | 167.38 |
| 1.0 | 2.0 | 45 | 187.87 |
| 1.0 | 2.0 | 60 | 216.71 |
| Anode | Cathode | Membrane | Anolyte | Catholyte | Temperature (℃) | Maximum Power Density (mW cm−2) | Ref. |
|---|---|---|---|---|---|---|---|
| Pt53Cu47/C (0.5 mg cm−2) | Pt/C (20 wt.%) (1.0 mg cm−2) | Tokuyama | KOH 1.0 M + N2H4 1.0 M | O2 flow rate: 30 SCCM | 80 | 56.1 | [67] |
| Ni0.6Co0.4 nanosheets (1.4 mg cm−2) | Pt/C (40.0 wt.%) | Nafion 115 | KOH 4.0 M + N2H4 20.0 wt% | H2O2 20.0% + H2SO4 0.5 M | 80 | 107.1 | [68] |
| Pd/CNT (1.0 mg cm−2) | Pt/C (0.25 mg cm−2) | Nafion 117 | NaOH 1.0 M + N2H4 2.0 M | O2 flow rate: 150.0 mL min−1 | 60 | 110 | [69] |
| Co–Au/C (1.0 mg cm−2) | Au/C (1.0 mg cm−2) | Nafion 117 | NaOH 2.0 M + N2H4 2.0 M | H2O2 2.0 M + H2SO4 0.5 M | 60 | 122.8 | [4] |
| MoCx–NC (1.0 mg cm−2) | Pt/C (1.0 mg cm−2) | KOH-doped PBI | KOH 6.0 M + N2H4 0.5 M | O2 Flux: 0.2 slpm | 80 | 158.26 | [70] |
| Ni–Pd/rGO (1.0 mg cm−2) | Pt/C (0.5 mg cm−2) | Nafion 117 | NaOH 2.0 M + N2H4 1.0 M | H2O2 2.0 M + H2SO4 0.5 M | 60 | 204.8 | [13] |
| Pd–Ni/NrGO (1.0 mg cm−2) | Pt/C (0.5 mg cm−2) | Nafion 117 | N2H4 1.0 M + NaOH 2.0 M | H2O2 2.0 M + H2SO4 0.5 M | 25 60 | 187.87 216.71 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosseini, M.G.; Daneshvari-Esfahlan, V.; Aghajani, H.; Wolf, S.; Hacker, V. Palladium-Nickel Electrocatalysts on Nitrogen-Doped Reduced Graphene Oxide Nanosheets for Direct Hydrazine/Hydrogen Peroxide Fuel Cells. Catalysts 2021, 11, 1372. https://doi.org/10.3390/catal11111372
Hosseini MG, Daneshvari-Esfahlan V, Aghajani H, Wolf S, Hacker V. Palladium-Nickel Electrocatalysts on Nitrogen-Doped Reduced Graphene Oxide Nanosheets for Direct Hydrazine/Hydrogen Peroxide Fuel Cells. Catalysts. 2021; 11(11):1372. https://doi.org/10.3390/catal11111372
Chicago/Turabian StyleHosseini, Mir Ghasem, Vahid Daneshvari-Esfahlan, Hossein Aghajani, Sigrid Wolf, and Viktor Hacker. 2021. "Palladium-Nickel Electrocatalysts on Nitrogen-Doped Reduced Graphene Oxide Nanosheets for Direct Hydrazine/Hydrogen Peroxide Fuel Cells" Catalysts 11, no. 11: 1372. https://doi.org/10.3390/catal11111372