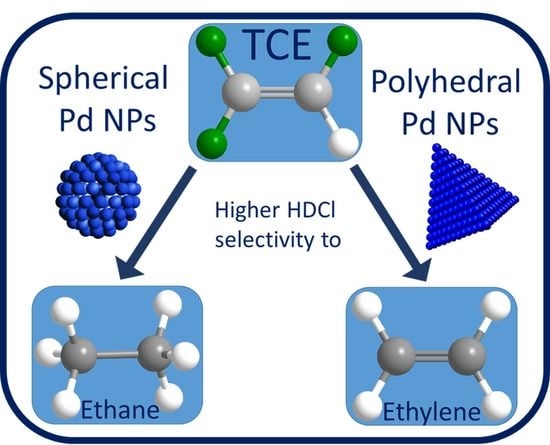
The Effect of Shape-Controlled Pt and Pd Nanoparticles on Selective Catalytic Hydrodechlorination of Trichloroethylene
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of NPs by (HR-)TEM
2.1.1. (HR-)TEM of Cubic (c) Pt NPs
2.1.2. (HR-)TEM of Cuboctahedral (co) Pt NPs
2.1.3. (HR-)TEM of Spherical (s) Pd NPs
2.1.4. (HR-)TEM of Mixed-Shape (m) Pd NPs
2.2. X-ray Diffraction (XRD) of the NPs
2.3. Surface Properties of the NPs Examined by FTIR of CO Adsorption at Room Temperature
2.4. Catalytic Application of Pt and Pd NPs for TCE Hydrodechlorination
3. Materials and Methods
3.1. Materials
3.2. Synthesis of the NPs
3.2.1. Synthesis of Cubic (c) Pt NPs and Deposition on ZrO2
3.2.2. Synthesis of Cuboctahedral (co) Pt NPs and Deposition on ZrO2
3.2.3. Synthesis of Spherical (s) Pd NPs and Deposition on ZrO2
3.2.4. Synthesis of the Mixed-Shaped (m) Pd NPs and Deposition on ZrO2
3.3. Characterization
3.3.1. Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES) Analysis of the Pt and Pd NPs
3.3.2. High-Resolution Transmission Electron Microscopy (HR-TEM)
3.3.3. X-ray Diffraction (XRD)
3.3.4. Fourier Transform Infrared Spectroscopy (FTIR)
3.4. Catalytic HDCl Reaction of TCE
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Delpire, L.; Scarano, L. Environmental Protection Agency; 740-R1-4002; EPA: Washington, DC, USA, 2014; p. 212. [Google Scholar]
- Weidlich, T.; Kamenická, B.; Melánová, K.; Čičmancová, V.; Komersova, A.; Čermák, J. Hydrodechlorination of Different Chloroaromatic Compounds at Room Temperature and Ambient Pressure—Differences in Reactivity of Cu- and Ni Based Al Alloys in an Alkaline Aqueous Solution. Catalysts 2020, 10, 994. [Google Scholar] [CrossRef]
- Hahn, T.; Botzenhart, K.; Schweinsberg, F. 2-Toxic Effects of Solvent Exposure. Toxicokinet. Toxicodyn. Toxicol. 2014, 19, 98. [Google Scholar]
- Schrick, B.; Blough, J.L.; Jones, A.D.; Mallouk, T.E. Hydrodechlorination of Trichloroethylene to Hydrocarbons Using Bimetallic Nickel-Iron Nanoparticles. Chem. Mater. 2002, 14, 5140–5147. [Google Scholar] [CrossRef]
- Liu, Y.; Majetich, S.A.; Tilton, R.D.; Sholl, D.S.; Lowry, G.V. TCE Dechlorination Rates, Pathways, and Efficiency of Nanoscale Iron Particles with Different Properties. Environ. Sci. Technol. 2005, 39, 1338–1345. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, K.; Frenzel, H.; Kopinke, F.-D. Hydrodehalogenation of Halogenated Hydrocarbons in Water with Pd Catalysts: Reaction Rates and Surface Competition. Appl. Catal. B Environ. 2006, 63, 161–167. [Google Scholar] [CrossRef]
- Lee, A.F.; Carr, P.A.; Wilson, K. Low temperature 111-trichloroethane dehydrochlorination over Pt catalysts: From model surfaces to the real world. Chem. Commun. 2004, 2774–2775. [Google Scholar] [CrossRef]
- Lee, A.F.; Wilson, K. Sulfate-Enhanced Catalytic Destruction of 1,1,1-Trichlorethane over Pt(111). J. Phys. Chem. B 2006, 110, 907–913. [Google Scholar] [CrossRef]
- Tee, Y.-H.; Bachas, L.; Bhattacharyya, D. Degradation of Trichloroethylene by Iron-Based Bimetallic Nanoparticles. J. Phys. Chem. C 2009, 113, 9454–9464. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Ruiz, C.; Bedia, J.; Andreoli, S.; Eser, S.; Rodríguez, J.J.; Gómez-Sainero, L.M. Selectivity to Olefins in the Hydrodechlorination of Chloroform with Activated Carbon-Supported Palladium Catalysts. Ind. Eng. Chem. Res. 2019, 58, 20592–20600. [Google Scholar] [CrossRef]
- Rabek, J.F.; Rinby, B.; Skowronski, T.A. Photothermal Dehydrochlorination of Poly(vinyl chloride). Macromolecules 1985, 18, 1810–1818. [Google Scholar] [CrossRef]
- Hjertberg, T.; Martinsson, E.; Sorvik, E. Influence of the Dehydrochlorination Rate on the Degradation Mechanism of Poly(vinyl chloride). Macromolecules 1987, 21, 603–609. [Google Scholar] [CrossRef]
- Starnes, W.H.; Ge, X. Mechanism of Autocatalysis in the Thermal Dehydrochlorination of Poly(vinyl chloride). Macromolecules 2004, 37, 352–359. [Google Scholar] [CrossRef]
- Carter, W.P.L.; Luo, D.; Malkina, I.L. Investigation of the Atmospheric Ozone Formation Potential of Trichloroethylene; Halogenated Solvents Industry Alliance: Riverside, CA, USA, 1997; p. 71. [Google Scholar]
- Andersin, J.; Parkkinen, P.; Honkala, K. Pd-catalyzed hydrodehalogenation of chlorinated olefins: Theoretical insights to the reaction mechanism. J. Catal. 2012, 290, 118–125. [Google Scholar] [CrossRef]
- Jugnet, Y.; Bertolini, J.C.; Barbosa, L.A.M.M.; Sautet, P. Vibrational identification of the surface reaction intermediates for the dehalogenation of trichloroethene on PdCu(1 1 0) alloy. Surf. Sci. 2002, 505, 153–162. [Google Scholar] [CrossRef]
- Nutt, M.O.; Heck, K.N.; Alvarez, P.; Wong, M.S. Improved Pd-on-Au bimetallic nanoparticle catalysts for aqueous-phase trichloroethene hydrodechlorination. Appl. Catal. B Environ. 2006, 69, 115–125. [Google Scholar] [CrossRef]
- Yu, H.; Kennedy, E.M.; Uddin, M.A.; Dlugogorski, B.Z. Catalytic hydrodehalogenation of halon 1211 (CBrClF2) over γ-alumina-supported Ni, Pd and Pt catalysts. Catal. Today 2004, 88, 183–194. [Google Scholar] [CrossRef]
- Zhang, Z.C.; Beard, B.C. Genesis of durable catalyst for selective hydrodechlorination of CCl4 to CHCl3. Appl. Catal. A Gen. 1998, 174, 33–39. [Google Scholar] [CrossRef]
- Barbosa, L.A.M.M.; Sautet, P. Trichloroethene Dechlorination Reactions on the PdCu (110) Alloy Surface: A Periodical Density Functional Theory Study of the Mechanism. J. Catal. 2002, 207, 127–138. [Google Scholar] [CrossRef]
- Crampton, A.S.; Rotzer, M.D.; Landman, U.; Heiz, U. Can Support Acidity Predict Sub-Nanometer Catalyst Activity Trends? ACS Catal. 2017, 7, 6738–6744. [Google Scholar] [CrossRef]
- Barrabes, N.; Cornado, D.; Föttinger, K.; Dafinov, A.; Llorca, J.; Medina, F.; Rupprechter, G. Hydrodechlorination of trichloroethylene on noble metal promoted Cu-hydrotalcite-derived catalysts. J. Catal. 2009, 262, 239–246. [Google Scholar] [CrossRef]
- Barrabés, N.; Föttinger, K.; Dafinov, A.; Medina, F.; Rupprechter, G.; Llorca, J.; Sueiras, J.E. Study of Pt–CeO2 interaction and the effect in the selective hydrodechlorination of trichloroethylene. Appl. Catal. B Environ. 2010, 87, 84–91. [Google Scholar] [CrossRef]
- Barrabes, N.; Föttinger, K.; Llorca, J.; Dafinov, A.; Medina, F.; Sa, J.; Hardacre, C.; Rupprechter, G. Pretreatment Effect on Pt/CeO2 Catalyst in the Selective Hydrodechlorination of Trichloroethylene. J. Phys. Chem. C 2010, 114. [Google Scholar] [CrossRef]
- Bonarowskaa, M.; Kaszkura, Z.; Łomota, D.; Rawskib, M.; Karpinski, Z. Effect of gold on catalytic behavior of palladium catalysts in hydrodechlorination of tetrachloromethane. Appl. Catal. B Environ. 2015, 162, 45–56. [Google Scholar] [CrossRef]
- Flid, M.R.; Kartashov, L.M.; Treger, Y.A. Theoretical and Applied Aspects of Hydrodechlorination Processes—Catalysts and Technologies. Catalysts 2020, 10, 216. [Google Scholar] [CrossRef] [Green Version]
- Rupprechter, G.; Somorjai, G.A. Palladium-catalyzed hydrogenation without hydrogen: The hydrodechlorination of chlorofluorocarbons with solid state hydrogen over the palladium (111) crystal surface and its implications. Catal. Lett. 1997, 17–20. [Google Scholar] [CrossRef]
- Ribeiro, F.H.; Gerken, C.A.; Rupprechter, G.; Somorjai, G.A.; Kellner, C.S.; Coulston, G.W.; Manzer, L.E.; Abrams, L. Structure Insensitivity and Effect of Sulfur in the Reaction of Hydrodechlorination of 1,1-Dichlorotetrafluoroethane (CF3–CFCl2) over Pd Catalysts. J. Catal. 1998, 176, 352–357. [Google Scholar] [CrossRef]
- Markova, V.K.; Philbin, J.P.; Zhao, W.; Genest, A.; Silvestre-Albero, J.; Rupprechter, G.; Rösch, N. Catalytic Transformations of 1-Butene over Palladium. A Combined Experimental and Theoretical Study. ACS Catal. 2018, 8, 5675–5685. [Google Scholar] [CrossRef]
- Somorjai, G.A.; Contreras, A.M.; Montano, M.; Rioux, R.M. Clusters, surfaces, and catalysis. Proc. Natl. Acad. Sci. USA 2006, 103, 10577–10583. [Google Scholar] [CrossRef] [Green Version]
- Carlsson, A.F.; Madix, R.J. The dynamics of ethylene adsorption on Pt (111) into di-σ and π-bonded states. J. Chem. Phys. 2001, 115, 8074–8082. [Google Scholar] [CrossRef]
- Díaz, E.; Faba, L.; Ordóñez, S. Effect of carbonaceous supports on the Pd-catalyzed aqueous-phase trichloroethylene hydrodechlorination. Appl. Catal. B Environ. 2011, 104, 415–417. [Google Scholar] [CrossRef]
- Srebowata, A.; Kaminska, I.I.; Gizinski, D.; Wideł, D.; Oszczudłowski, J. Remarkable effect of soft-templating synthesis procedure on catalytic properties of mesoporous carbon supported Ni in hydrodechlorination of trichloroethylene in liquid phase. Catal. Today 2015, 251, 60–65. [Google Scholar] [CrossRef]
- Fernandez-Ruiz, C.; Bedia, J.; Grau, J.M.; Romero, A.C.; Rodríguez, D.; Rodríguez, J.J.; Gómez-Sainero, L.M. Promoting Light Hydrocarbons Yield by Catalytic Hydrodechlorination of Residual Chloromethanes Using Palladium Supported on Zeolite Catalysts. Catalysts 2020, 10, 199. [Google Scholar] [CrossRef] [Green Version]
- Tian, M.; Ma, M.; Xu, B.; Chen, C.; He, C.; Hao, Z.; Albilali, R. Catalytic removal of 1,2-dichloroethane over LaSrMnCoO6/H-ZSM-5 composite: Insights into synergistic effect and pollutant-destruction mechanism. Catal. Sci. Technol. 2018, 8, 4503–4514. [Google Scholar] [CrossRef]
- Wang, X.; Bokhoven, J.A.v.; Palagin, D. Atomically dispersed platinum on low index and stepped ceria surfaces: Phase diagrams and stability analysis. Phys. Chem. Chem. Phys. 2019, 22, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Tereshchenko, A.; Polyakov, V.; Guda, A.; Lastovina, T.; Pimonova, Y.; Bulgakov, A.; Tarasov, A.; Kustov, L.; Butova, V.; Trigub, A.; et al. Ultra-Small Pd Nanoparticles on Ceria as an Advanced Catalyst for CO Oxidation. Catalysts 2019, 9, 385. [Google Scholar] [CrossRef] [Green Version]
- Fujiwara, K.; Okuyama, K.; Pratsinis, S.E. Metal–support interactions in catalysts for environmental remediation. Environ. Sci. Nano 2017, 4, 2076–2092. [Google Scholar] [CrossRef]
- Wang, P.; Chu, Y.; Dong, F.; Shi, X.; Fu, C.; Li, X.; Li, J.; Lv, X.; Jiang, G. Strong pyrrolic-N-Pd interactions boost the electrocatalytic hydrodechlorination reaction on palladium nanoparticles. Nanoscale 2020, 12, 843–850. [Google Scholar] [CrossRef]
- Suchorski, Y.; Kozlov, S.M.; Bespalov, I.; Datler, M.; Vogel, D.; Budinska, Z.; Neyman, K.M.; Rupprechter, G. The role of metal/oxide interfaces for long-range metal particle activation during CO oxidation. Nat. Mater. 2018. [Google Scholar] [CrossRef]
- Cai, C.; Han, S.; Liu, W.; Sun, K.; Qiao, L.; Li, S.; Zu, X. Tuning catalytic performance by controlling reconstruction process in operando condition. Appl. Catal. B: Environ. 2020, 260, 118103. [Google Scholar]
- Todoroki, N.; Tei, H.; Tsurumaki, H.; Miyakawa, T.; Inoue, T.; Wadayama, T. Surface Atomic Arrangement Dependence of Electrochemical CO2 Reduction on Gold: Online Electrochemical Mass Spectrometric Study on Low-Index Au(hkl) Surfaces. ACS Catal. 2019, 9, 1383–1388. [Google Scholar] [CrossRef]
- Bernardo, C.G.P.M.; Gomes, J.A.N.F. The adsorption of ethylene on the (100) surfaces of platinum, palladium and nickel: A DFT study. J. Mol. Struct. 2001, 542, 263–271. [Google Scholar] [CrossRef] [Green Version]
- Baeza, J.A.; Calvo, L.; Gilarranz, M.A.; Mohedano, A.F.; Casas, J.A.; Rodriguez, J.J. Catalytic behavior of size-controlled palladium nanoparticles in the hydrodechlorination of 4-chlorophenol in aqueous phase. J. Catal. 2012, 293, 85–93. [Google Scholar] [CrossRef]
- Zhu, Z.; Barroo, C.d.; Lichtenstein, L.; Eren, B.; Wu, C.H.; Mao, B.; Bocarmé, T.V.d.; Liu, Z.; Kruse, N.; Salmeron, M.; et al. Influence of Step Geometry on the Reconstruction of Stepped Platinum Surfaces under Coadsorption of Ethylene and CO. J. Phys. Chem. Lett. 2014, 5, 2626–2631. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Lian, C.; Hu, S.; Deng, Z.; Gong, J.; Li, M.; Liu, H.; Xing, M.; ZhangZhang, J. Size-dependent activity and selectivity of carbon dioxide photocatalytic reduction over platinum nanoparticles. Nat. Commun. 2018, 9, 1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lykaki, M.; Stefa, S.; Carabineiro, S.A.C.; Pandis, P.K.; Stathopoulos, V.N.; Konsolakis, M. Facet-Dependent Reactivity of Fe2O3/CeO2 Nanocomposites: Effect of Ceria Morphology on CO Oxidation. Catalysts 2019, 9, 371. [Google Scholar] [CrossRef] [Green Version]
- Alayoglu, S.; Aliaga, C.; Sprung, C.; Somorjai, G.A. Size and Shape Dependence on Pt Nanoparticles for the Methylcyclopentane/Hydrogen Ring Opening/Ring Enlargement Reaction. Catal. Lett. 2011, 914–924. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Jing, Q.; Lin, Y. The Influence of Pluronic F-127 Modification on Nano Zero-Valent Iron (NZVI): Sedimentation and Reactivity with 2,4-Dichlorophenol in Water Using Response Surface Methodology. Catalysts 2020, 10, 412. [Google Scholar] [CrossRef] [Green Version]
- Aramendia, M.A.; Borau, V.; Garcia, I.M.; Jimenez, C.; Lafont, F.; Marinas, A.; Marinas, J.M.; Urbano, F.J. Influence of the Reaction Conditions and Catalytic Properties on the Liquid-Phase Hydrodechlorination of Chlorobenzene over Palladium-Supported Catalysts: Activity and Deactivation. J. Catal. 1999, 187, 392–399. [Google Scholar] [CrossRef]
- Yuan, G.; Bai, J.; Gao, B.; Ren, L.; Mei, J.; Zhang, L. The effect of crystal facet (312) exposure intensity of Ni12P5 nanoparticle on its hydrodechlorination catalytic activity. Inorg. Chem. Commun. 2020, 111, 107595. [Google Scholar] [CrossRef]
- Munoz, M.; Ponce, S.; Zhang, G.-R.; Etzold, B.J.M. Size-controlled PtNi nanoparticles as highly efficient catalyst for hydrodechlorination reactions. Appl. Catal. B Environ. 2016, 192, 1–7. [Google Scholar] [CrossRef]
- Ruiza, C.F.; Bedia, J.; Bonal, P.; Rodriguez, J.J.; Gómez-Sainero, L.M. Chloroform conversion into ethane and propane by catalytic hydrodechlorination with Pd supported on activated carbons from lignin. Catal. Sci. Technol. 2018, 8, 3926–3935. [Google Scholar] [CrossRef]
- Lan, X.; Xue, K.; Wang, T. Combined synergetic and steric effects for highly selective hydrogenation of unsaturated aldehyde. J. Catal. 2019, 372, 49–60. [Google Scholar] [CrossRef]
- Rizo, R.; Roldan-Cuenya, B. Shape-Controlled Nanoparticles as Anodic Catalysts in Low-Temperature Fuel Cells. ACS Energy Lett. 2019, 4, 1484–1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arán-Ais, R.M.; Solla-Gullón, J.; Herrero, E.; Feliu, J.M. On the quality and stability of preferentially oriented (100) Pt nanoparticles: An electrochemical insight. J. Electroanal. Chem. 2018, 808, 433–438. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.; Moon, K.; Park, Y.; Park, S.; Park, K.H. Recent Studies on Multifunctional Electrocatalysts for Fuel Cell by Various Nanomaterials. Catalysts 2020, 10, 621. [Google Scholar] [CrossRef]
- Crespo-Quesada, M.; Yarulin, A.; Jin, M.; Xia, Y.; Kiwi-Minsker, L. Structure Sensitivity of Alkynol Hydrogenation on Shape- and Size-Controlled Palladium Nanocrystals: Which Sites Are Most Active and Selective? J. Am. Chem. Soc. 2011, 133, 12787–12794. [Google Scholar] [CrossRef]
- Eom, T.; Kim, W.J.; Lim, H.-K.; Han, M.H.; Han, K.H.; Lee, E.K.; Lebègue, S.; Hwang, Y.J.; Min, B.K.; Kim, H. Cluster Expansion Method for Simulating Realistic Size of Nanoparticle Catalysts with an Application in CO2 Electroreduction. J. Phys. Chem. C 2018, 122, 9245–9254. [Google Scholar] [CrossRef]
- Liu, J.-X.; Filot, I.A.W.; Su, Y.; Zijlstra, B.; Hensen, E.J.M. Optimum Particle Size for Gold-Catalyzed CO Oxidation. Phys. Chem. C 2018, 122, 8327–8340. [Google Scholar] [CrossRef] [Green Version]
- Mehmood, F.; Kara, A.; Rahman, T.S.; Henry, C.R. Comparative study of CO adsorption on flat, stepped, and kinked Au surfaces using density functional theory. Phys. Rev. B 2009, 79, 075422. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Zhang, X.; Wang, Q.; Han, Y.; Fang, Y.; Dong, S. Shape-Control of Pt-Ru Nanocrystals: Tuning Surface Structure for Enhanced Electrocatalytic Methanol Oxidation. J. Am. Chem. Soc. 2017, 140, 1142–1147. [Google Scholar] [CrossRef]
- Bai, S.; Bu, L.; Shao, Q.; Zhu, X.; Huang, X. Multicomponent Pt-based Zigzag Nanowires as Selectivity Controllers for Selective Hydrogenation Reactions. J. Am. Chem. Soc. 2018, 140, 8384–8387. [Google Scholar] [CrossRef]
- Delbecq, F.; Sautet, P. Competitive C=C and C=O Adsorption of α-β-Unsaturated Aldehydes on Pt and Pd Surfaces in Relation with the Selectivity of Hydrogenation Reactions: A Theoretical Approach. J. Catal. 1995, 152, 217–236. [Google Scholar] [CrossRef]
- Wang, C.; Daimon, H.; Lee, Y.; Kim, J.; Sun, S. Synthesis of Monodisperse Pt Nanocubes and Their Enhanced Catalysis for Oxygen Reduction. J. Am. Chem. Soc. 2007, 129, 6974–6975. [Google Scholar] [CrossRef] [PubMed]
- Michel, J.A.; Morris, W.H.; Lukehart, C.M. Synthesis of shaped Pt nanoparticles using common anions or small molecules as shapedirecting agents: Observation of a strong halide or pseudo-halide effect. J. Mater. Chem. A 2015, 3, 2012–2018. [Google Scholar] [CrossRef] [Green Version]
- Long, N.V.; Chien, N.D.; Hayakawa, T.; Matsubara, T.; Ohtaki, M.; Nogami, M. Sharp cubic and octahedral morphologies of poly(vinylpyrrolidone)-stabilised platinum nanoparticles by polyol method in ethylene glycol: Their nucleation, growth and formation mechanisms. J. Exp. Nanosci. 2012, 7, 133–149. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Buonsanti, R. Colloidal Nanocrystals as Heterogeneous Catalysts for Electrochemical CO2 Conversion. Chem. Mater. 2019, 31, 13–25. [Google Scholar] [CrossRef] [Green Version]
- Mantella, V.; Castilla-Amoros, L.; Buonsanti, R. Shaping non-noble metal nanocrystals via colloidal chemistry. R. Soc. Chem. 2020, 1–10. [Google Scholar] [CrossRef]
- Ren, J.; Tilley, R. Preparation, Self-Assembly, and Mechanistic Study of Highly Monodispersed Nanocubes. J. Am. Chem. Soc. 2007, 129, 3287–3291. [Google Scholar] [CrossRef]
- Rupprechter, G.; Freund, H.-J. Adsorbate-induced restructuring and pressure-dependent adsorption on metal nanoparticles studied by electron microscopy and sum frequency generation spectroscopy. Top. Catal. 2001, 14, 1022–5528. [Google Scholar] [CrossRef]
- Penner, S.; Rupprechter, G.; Sauer, H.; Su, D.S.; Tessadri, R.; Podloucky, R.; Schlögl, R.; Hayek, K. Pt/ceria thin film model catalysts after high temperature reduction: A (HR)TEM study. Vacuum 2003, 71, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Penner, S.; Su, D.S.; Rupprechter, G.; Hayek, K.; Schlögl, R. SiO2-supported Pt particles studied by electron microscopy. Mater. Chem. Phys. 2003, 81, 341–344. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Sun, Z.; Tian, D.; Nevirkovets, I.P.; Dou, S.-X. Platinum dendritic nanoparticles with magnetic behavior. J. Appl. Phys. 2014, 116, 033911. [Google Scholar] [CrossRef] [Green Version]
- Lim, B.; Kobayashi, H.; Camargo, P.H.C.; Allard, L.F.; Liu, J.; Xia, Y. New insights into the growth mechanism and surface structure of palladium nanocrystals. Nano Res. 2010, 3, 180–188. [Google Scholar] [CrossRef] [Green Version]
- Rupprechter, G.; Seeber, G.; Hayek, K.; Hofmeister, H. Epitaxial Noble Metal Particles upon Oxidation and Iduction. A Model System for Supported Metal Catalysts. Phys. Status Solidi (a) 1994, 146, 449–459. [Google Scholar] [CrossRef]
- Cheong, S.; Watt, J.D.; Tilley, R.D. Shape control of platinum and palladium nanoparticles for catalysis. Nanoscale 2010, 2, 2045–2053. [Google Scholar] [CrossRef]
- Anic, K.; Föttinger, K.; Wolfbeisser, A.; Bernardi, J.; Li, H.; Rameshan, C.; Rupprechter, G. Surface Spectroscopy on UHV-Grown and Technological Ni–ZrO2 Reforming Catalysts: From UHV to Operando Conditions. Top. Catal. 2016, 59, 1614–1627. [Google Scholar] [CrossRef] [Green Version]
- Long, N.V.; Ohtaki, M.; Nogami, M.; Hien, T.D. Effects of heat treatment and poly(vinylpyrrolidone) (PVP) polymer on electrocatalytic activity of polyhedral Pt nanoparticles towards their methanol oxidation. Colloid Polym. Sci. 2011, 289, 1373–1386. [Google Scholar] [CrossRef]
- Nguyen, V.L.; Ohtaki, M.; Ngo, V.N.; Cao, M.-T.; Nogami, M. Structure and morphology of platinum nanoparticles with critical new issues of low- and high-index facets. Adv. Nat. Sci. Nanosci. Nanotechnol. 2012, 3, 025005. [Google Scholar] [CrossRef] [Green Version]
- Leontyev, I.N.; Kuriganova, A.B.; Leontyev, N.G.; Hennet, L.; Rakhmatullin, A.; Smirnovad, N.V.; Dmitriev, V. Size dependence of the lattice parameters of carbon supported platinum nanoparticles: X-ray diffraction analysis and theoretical considerations. RSC Adv. 2014, 4, 35959–35965. [Google Scholar] [CrossRef]
- Londoño-Restrepo, S.M.; Jeronimo-Cruz, R.; Millán-Malo, B.M.; Rivera-Muñoz, E.M.; Rodriguez-García, M.E. Effect of the Nano Crystal Size on the X-ray Diffraction Patterns of Biogenic Hydroxyapatite from Human, Bovine, and Porcine Bones. Sci. Rep. 2019, 9, 5915. [Google Scholar] [CrossRef]
- Muniz, F.T.L.; Mirand, M.A.R.; Santos, C.M.d.; Sasaki, J.M. The Scherrer equation and the dynamical theory of X-ray diffraction. Acta Crystallogr. A Found. Adv. 2016, 72, 385–390. [Google Scholar] [CrossRef]
- Haghofer, A.; Sonström, P.; Fenske, D.; Föttinger, K.; Schwarz, S.; Bernardi, J.; Al-Shamery, K.; Bäumer, M.; Rupprechter, G. Colloidally Prepared Pt Nanowires versus Impregnated Pt Nanoparticles: Comparison of Adsorption and Reaction Properties. Langmuir 2010, 26, 16330–16338. [Google Scholar] [CrossRef] [PubMed]
- Rupprechter, G.; Unterhalt, H.; Morkel, M.; Galletto, P.; Dellwig, T.; Freund, H.-J. Extending UHV studies to the mbar range: Vibrational SFG spectroscopy of high-pressure CO adsorption on Pt(111) and Pd(111). Vacuum 2003, 83–87. [Google Scholar] [CrossRef]
- Rupprechter, G. Sum Frequency Generation and Polarization–Modulation Infrared Reflection Absorption Spectroscopy of Functioning Model Catalysts from Ultrahigh Vacuum to Ambient Pressure. Adv. Catal. 2007, 133–263. [Google Scholar] [CrossRef]
- Rupprechter, G.; Unterhalt, H.; Morkel, M.; Galletto, P.; Hu, L.; Freund, H.-J. Sum frequency generation vibrational spectroscopy at solid–gas interfaces: CO adsorption on Pd model catalysts at ambient pressure. Surf. Sci. 2002, 502, 109–122. [Google Scholar] [CrossRef]
- Bertarione, S.; Scarano, D.; Zecchina, A.; Johanek, V.; Hoffmann, J.; Schauermann, S.; Frank, M.M.; Libuda, J.; Rupprechter, G.; Freund, H.-J. Surface Reactivity of Pd Nanoparticles Supported on Polycrystalline Substrates as Compared to Thin Film Model Catalysts: Infrared Study of CO Adsorption. J. Phys. Chem. B 2004, 108, 3603–3613. [Google Scholar] [CrossRef]
- Zorn, K.; Giorgio, S.; Halwax, E.; Henry, C.R.; Grönbeck, H.; Rupprechter, G. CO Oxidation on Technological Pd-Al2O3 Catalysts: Oxidation State and Activity. J. Phys. Chem. C 2011, 115, 1103–1111. [Google Scholar] [CrossRef]
- Lear, T.; Marshall, R.; Lopez-Sanchez, J.A.; Jackson, S.D.; Klapötke, T.M.; Bäumer, M.; Rupprechter, G.; Freund, H.-J.; Lennon, D. The application of infrared spectroscopy to probe the surface morphology of alumina-supported palladium catalysts. J. Chem. Phys. 2005, 123, 174706. [Google Scholar] [CrossRef] [Green Version]
- Morkel, M.; Rupprechter, G.; Freund, H.-J. Finite size effects on supported Pd nanoparticles: Interaction of hydrogen with CO and C2H4. Surf. Sci. 2005, 588, L209–L219. [Google Scholar] [CrossRef]
- Lear, T.; Marshall, R.; Gibson, E.K.; Schutt, T.; Klapötke, T.M.; Rupprechter, G.; Freund, H.-J.; Winfielda, J.M.; Lennon, D. A model high surface area alumina-supported palladium catalyst. Phys. Chem. Chem. Phys. 2005, 7, 565–567. [Google Scholar] [CrossRef]
- Föttinger, K.; Emhofer, W.; Lennon, D.; Rupprechter, G. Adsorption and Reaction of CO on (Pd–)Al2O3 and (Pd–)ZrO2: Vibrational Spectroscopy of Carbonate Formation. Top. Catal. 2017, 60, 1722–1734. [Google Scholar] [CrossRef] [Green Version]
- Kaftan, A.; Kollhoff, F.; Nguyen, T.S.; Piccolo, L.; Laurin, M.; Libuda, J. Sensitivity of CO oxidation toward metal oxidation state in ceria-supported catalysts: An operando DRIFTS-MS study. Catal. Sci. Technol. 2016, 6, 818–828. [Google Scholar] [CrossRef] [Green Version]
- Lundwall, M.J.; McClure, S.M.; Goodman, D.W. Probing Terrace and Step Sites on Pt Nanoparticles Using CO and Ethylene. J. Phys. Chem. C 2010, 114, 7904–7912. [Google Scholar] [CrossRef]
- Gunasooriya, G.T.K.K.; Saeys, M. CO Adsorption Site Preference on Platinum: Charge Is the Essence. ACS Catal. 2018, 8, 3770–3774. [Google Scholar] [CrossRef]
- Kitla, A.; Safonova, O.V.; Föttinger, K. Infrared Studies on Bimetallic Copper/Nickel Catalysts Supported on Zirconia and Ceria/Zirconia. Catal Lett. 2013, 143, 517–530. [Google Scholar] [CrossRef] [Green Version]
- Stropp, A.; Termentzidis, K.; Paier, J.; Kresse, G.; Hafner, J. CO adsorption on metal surfaces: A hybrid functional study with plane-wave basis set. Phys. Rev. B 2007, 76. [Google Scholar] [CrossRef] [Green Version]
- Soriaga, M.P.; Chen, X.; Li, D.; Stickney, J.L. Applications of physical methods to inorganic and bioinorganic chemistry. In High Resolution Electron Energy-Loss Spectroscopy; RA Scott, C.L., Ed.; John WILEY: Hoboken, NJ, USA, 2013. [Google Scholar]
- Borodko, Y.; Lee, H.S.; Joo, S.H.; Zhang, Y.; Somorjai, G.A. Spectroscopic Study of the Thermal Degradation of PVP-Capped Rh and Pt Nanoparticles in H2 and O2 Environments. J. Phys. Chem. C 2010, 114, 1117–1126. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Dong, X.; Yu, Y.; Zhang, M. Investigation on the conversion of ethylene to ethylidyne on Pt(100) and Pd(100) using density functional theory. Phys. Chem. Chem. Phys. 2016, 18, 26949–26955. [Google Scholar] [CrossRef]
- Heard, C.J.; Siahrostami, S.; Grönbeck, H. Structural and Energetic Trends of Ethylene Hydrogenation over Transition Metal Surfaces. J. Phys. Chem. C 2016, 120, 995–1003. [Google Scholar] [CrossRef]
- Xu, L.; Stangland, E.E.; Mavrikakis, M. Ethylene versus ethane: A DFT-based selectivity descriptor for efficient catalyst screening. J. Catal. 2018, 362, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Baxter, E.T.; Ha, M.A.; Alexandrova, A.N.; Anderson, S.L. Ethylene Dehydrogenation on Pt, 4,7,8 Clusters on Al2O3: Strong Cluster Size Dependence Linked to Preferred Catalyst Morphologies. ACS Catal. 2017, 7, 3322–3335. [Google Scholar] [CrossRef]
- Gorey, T.J.; Zandkarimi, B.; Li, G.; Baxter, E.T.; Alexandrova, A.N.; Anderson, S.L. Coking-Resistant Sub-Nano Dehydrogenation Catalysts: PtnSnx/SiO2 (n = 4, 7). ACS Catal. 2020, 10, 4543–4558. [Google Scholar] [CrossRef] [Green Version]
- Cassuto, A.; Hugenschmidt, M.B.; Parent, P.; Laffon, C.; Tourillon, H.G. A NEXAFS and UPS study of the adsorption of tetrachloroethylene, trichloroethylene, iso-, cisand trans-dichloroethylene on platinum surfaces at 95 K: Multilayers and monolayers. Surf. Sci. 1994, 310, 390–398. [Google Scholar] [CrossRef]
- Moskaleva, L.V.; Chen, Z.-X.; Aleksandrov, H.A.; Mohammed, A.B.; Sun, Q.; Rosch, N. Ethylene Conversion to Ethylidyne over Pd(111): Revisiting the Mechanism with First-Principles Calculations. J. Phys. Chem. C 2009, 113, 2512–2520. [Google Scholar] [CrossRef]
- Cremer, P.S.; Su, X.; Shen, Y.R.; Somorjai, G.A. Ethylene Hydrogenation on Pt (111) Monitored in Situ at High Pressures Using Sum Frequency Generation. J. Am. Chem. Soc. 1996, 118, 2942–2949. [Google Scholar] [CrossRef]
- Xu, L.; Bhandari, S.; Chen, I.; Glasgow, J.; Mavrikakis, M. Chloroform Hydrodechlorination on Palladium Surfaces: A Comparative DFT Study on Pd (111), Pd (100), and Pd (211). Top. Catal. 2020, 63, 762–776. [Google Scholar] [CrossRef]
- Posada-Borbón, A.; Heard, C.J.; Grönbeck, H. Cluster Size Effects in Ethylene Hydrogenation over Palladium. J. Phys. Chem. C 2017, 121, 10870–10875. [Google Scholar] [CrossRef]
- Roling, L.T.; Choksi, T.S.; Pedersen, F.A. A coordination-based model for transition metal alloy nanoparticles. Nanoscale 2019, 11, 4438–4452. [Google Scholar] [CrossRef]
- Gao, F.; Goodman, D.W. Pd-Au bimetallic catalysts: Understanding alloy effects from planar models and (supported) nanoparticles. Chem. Soc. Rev. 2012, 41, 8009–8020. [Google Scholar] [CrossRef]
- Pei, G.; Liu, X.; Wang, A.; Lee, A.F.; Isaacs, M.A.; Li, L.; Pan, X.; Yang, X.; Wang, X.; Tai, Z.; et al. Ag Alloyed Pd Single-Atom Catalysts for Efficient Selective Hydrogenation of Acetylene to Ethylene in Excess Ethylene. ACS Catal. 2015, 5, 3717–3725. [Google Scholar] [CrossRef]
- Han, Y.; Sun, J.; Fu, H.; Qu, X.; Wan, H.; Xu, Z.; Zheng, S. Highly selective hydrodechlorination of 1,2-dichloroethane to ethylene over Ag-Pd/ZrO2 catalysts with trace Pd. Appl. Catal. A Gen. 2016, 519, 1–6. [Google Scholar] [CrossRef]
- Han, Y.; Gu, G.; Sun, J.; Wang, W.; Wan, H.; Xu, Z.; Zheng, S. Selective hydrodechlorination of 1, 2-dichloroethane to ethylene over Pd-Ag/Al2O3 catalysts prepared by surface reduction. Appl. Surf. Sci. 2015, 355, 183–190. [Google Scholar] [CrossRef]
- Lee, A.F.; Carr, P.; Wilson, K. Direct Observation of Extremely Low Temperature Catalytic Dehydrochlorination of 1,1,1-Trichloroethane over Platinum. J. Phys. Chem. B 2004, 108, 14811–14814. [Google Scholar] [CrossRef]
- Abdollahi, T.; Farmanzadeh, D. Selective hydrogenation of acetylene in the presence of ethylene on palladium nanocluster surfaces: A DFT study. Appl. Surf. Sci. 2018, 433, 513–529. [Google Scholar] [CrossRef]
- Krooswyk, J.D.; Kruppe, C.M.; Trenary, M. In-situ spectroscopic monitoring of the ambient pressure hydrogenation of C2 to ethane on Pt (111). Surf. Sci. 2016, 652, 142–147. [Google Scholar] [CrossRef] [Green Version]
- Sohn, H.; Celik, G.; Gunduz, S.; Dean, S.L.; Painting, E.; Edmiston, P.L.; Ozkan, U.S. Hydrodechlorination of trichloroethylene over Pd supported on swellable organically-modified silica (SOMS). Appl. Catal. B Environ. 2017, 203, 641–653. [Google Scholar] [CrossRef] [Green Version]
- Zaera, F. New advances in the use of infrared absorption spectroscopy for the characterization of heterogeneous catalytic reactions. Chem. Soc. Rev. 2014, 43, 7624–7663. [Google Scholar] [CrossRef]
- Beebe, T.P.; Yates, J.T. An in situ infrared spectroscopic investigation of the role of ethylidyne in the ethylene hydrogenation reaction on palladium/alumina. J. Am. Chem. Soc. 1986, 108, 663–671. [Google Scholar] [CrossRef]
- Kaltchev, M.; Thompson, A.W.; Tysoe, W.T. Reflection-absorption infrared spectroscopy of ethylene on palladium (111) at high pressure. Surf. Sci. 1997, 391, 145–149. [Google Scholar] [CrossRef]
- Usoltsev, O.A.; Pnevskaya, A.Y.; Kamyshova, E.G.; Tereshchenko, A.A.; Skorynina, A.A.; Zhang, W.; Yao, T.; Bugaev, A.L.; Soldatov, A.V. Dehydrogenation of Ethylene on Supported Palladium Nanoparticles: A Double View from Metal and Hydrocarbon Sides. Nanomaterials 2020, 10, 1643. [Google Scholar] [CrossRef]
- Siddique, M.N.; Ahmed, A.; Tripathi, P. Electric transport and enhanced dielectric permittivity in pure and Al doped NiO nanostructures. J. Alloys Compd. 2018, 735, 516–529. [Google Scholar] [CrossRef]
- Cristol, S.; Haller, L. Dehydrochlorination of 1-Trichloro-2-o-chlorophenyl-2-p-chlorophenylethane (o, p-DDT Isomer). J. Am. Chem. Soc. 1945, 67, 2222–2223. [Google Scholar] [CrossRef] [PubMed]
- Cristol, S.J.; Bly, R.S., Jr. Mechanisms of Elimination Reactions. XXIII. Phenyllithium-induced Dehydrochlorination of the Isomeric Chlorodiphenylethene. J. Am. Chem. Soc. 1961, 83, 4027–4032. [Google Scholar] [CrossRef]












Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarıbıyık, O.Y.; Weilach, C.; Serin, S.; Rupprechter, G. The Effect of Shape-Controlled Pt and Pd Nanoparticles on Selective Catalytic Hydrodechlorination of Trichloroethylene. Catalysts 2020, 10, 1314. https://doi.org/10.3390/catal10111314
Sarıbıyık OY, Weilach C, Serin S, Rupprechter G. The Effect of Shape-Controlled Pt and Pd Nanoparticles on Selective Catalytic Hydrodechlorination of Trichloroethylene. Catalysts. 2020; 10(11):1314. https://doi.org/10.3390/catal10111314
Chicago/Turabian StyleSarıbıyık, Oğuz Yunus, Christian Weilach, Selahattin Serin, and Günther Rupprechter. 2020. "The Effect of Shape-Controlled Pt and Pd Nanoparticles on Selective Catalytic Hydrodechlorination of Trichloroethylene" Catalysts 10, no. 11: 1314. https://doi.org/10.3390/catal10111314