Photodynamic Diagnosis and Therapy for Peritoneal Carcinomatosis: Emerging Perspectives
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Peritoneal Carcinomatosis: Origins, Occurrence, Diagnosis and Treatment
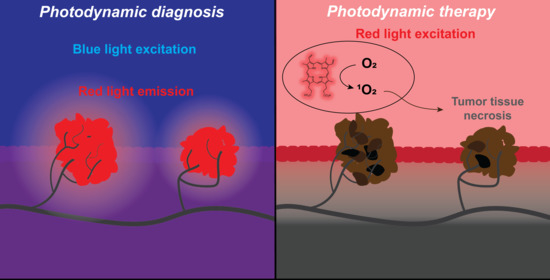
1.2. Photodynamic Diagnosis (PDD) and Photodynamic Therapy (PDT)
2. Photodynamic Diagnosis for Peritoneal Carcinomatosis
2.1. Clinical State-of-the-Art
2.1.1. Ovarian Cancer
2.1.2. Gastric Cancer
2.1.3. Colorectal Cancer
2.1.4. Pancreatic Cancer
2.1.5. Other Cancer Types
2.1.6. Predictive Diagnosis with PDD
2.2. PDD for Peritoneal Carcinomatosis: Challenges to Overcome
2.2.1. Tumor Heterogeneity
2.2.2. Increasing Specificity and Selectivity
2.2.3. Light Sources
2.3. Promising Experimental Studies on PDD for Peritoneal Carcinomatosis
2.3.1. Increasing Specificity and Selectivity
2.3.2. Light Sources
3. Photodynamic Therapy for Peritoneal Carcinomatosis
3.1. Clinical State-of-the-Art
3.1.1. Safety and Feasibility Studies
3.1.2. Ovarian Cancer
3.1.3. Gastric/Intestinal Cancer
3.1.4. Primary PCAR
3.1.5. Adverse Events
3.1.6. Ongoing Trials
3.2. PDT for Peritoneal Carcinomatosis: Challenges to Overcome
3.2.1. Tumor Heterogeneity
3.2.2. Selectivity and Efficacy of PDT
3.2.3. Integration into Clinical Practice
3.2.4. Excitation Sources
3.3. Promising Experimental Studies on PDT for Peritoneal Carcinomatosis
3.3.1. Selectivity and Efficacy of PDT
3.3.2. Integration into Clinical Practice
3.3.3. Light Sources
3.4. Radiotherapy-Activated PDT for PCAR
4. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Coccolini, F. Peritoneal carcinomatosis. WJG 2013, 19, 6979. [Google Scholar] [CrossRef] [PubMed]
- Desai, J.P.; Moustarah, F. Cancer, Peritoneal Metastasis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Vassos, N.; Piso, P. Metastatic Colorectal Cancer to the Peritoneum: Current Treatment Options. Curr. Treat. Options Oncol. 2018, 19, 49. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA: A Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, E. Ovarian Cancer Development and Metastasis. Am. J. Pathol. 2010, 177, 1053–1064. [Google Scholar] [CrossRef]
- Thomassen, I.; Lemmens, V.E.P.P.; Nienhuijs, S.W.; Luyer, M.D.; Klaver, Y.L.; de Hingh, I.H.J.T. Incidence, Prognosis, and Possible Treatment Strategies of Peritoneal Carcinomatosis of Pancreatic Origin: A Population-Based Study. Pancreas 2013, 42, 72–75. [Google Scholar] [CrossRef]
- Lambert, L.A. Looking up: Recent advances in understanding and treating peritoneal carcinomatosis: Recent Advances in Understanding and Treating Peritoneal Carcinomatosis. CA: A Cancer J. Clin. 2015, 65, 283–298. [Google Scholar] [CrossRef]
- Auer, R.C.; Sivajohanathan, D.; Biagi, J.; Conner, J.; Kennedy, E.; May, T. Indications for hyperthermic intraperitoneal chemotherapy with cytoreductive surgery: A systematic review. Eur. J. Cancer 2020, 127, 76–95. [Google Scholar] [CrossRef]
- Azaïs, H.; Estevez, J.P.; Foucher, P.; Kerbage, Y.; Mordon, S.; Collinet, P. Dealing with microscopic peritoneal metastases of epithelial ovarian cancer. A surgical challenge. Surg. Oncol. 2017, 26, 46–52. [Google Scholar] [CrossRef]
- Thomassen, I.; Verhoeven, R.H.A.; van Gestel, Y.R.B.M.; van de Wouw, A.J.; Lemmens, V.E.P.P.; de Hingh, I.H.J.T. Population-based incidence, treatment and survival of patients with peritoneal metastases of unknown origin. Eur. J. Cancer 2014, 50, 50–56. [Google Scholar] [CrossRef]
- Narasimhan, V.; Britto, M.; Pham, T.; Warrier, S.; Naik, A.; Lynch, A.C.; Michael, M.; Tie, J.; Ramsay, R.; Heriot, A. Evolution of Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Colorectal Peritoneal Metastases: 8-Year Single-Institutional Experience. Dis. Colon Rectum 2019, 62, 1195–1203. [Google Scholar] [CrossRef]
- Glockzin, G.; Zeman, F.; Croner, R.S.; Königsrainer, A.; Pelz, J.; Ströhlein, M.A.; Rau, B.; Arnold, D.; Koller, M.; Schlitt, H.J.; et al. Perioperative Systemic Chemotherapy, Cytoreductive Surgery, and Hyperthermic Intraperitoneal Chemotherapy in Patients with Colorectal Peritoneal Metastasis: Results of the Prospective Multicenter Phase 2 COMBATAC Trial. Clin. Colorectal Cancer 2018, 17, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Hompes, D.; D’Hoore, A.; Van Cutsem, E.; Fieuws, S.; Ceelen, W.; Peeters, M.; Van der Speeten, K.; Bertrand, C.; Legendre, H.; Kerger, J. The Treatment of Peritoneal Carcinomatosis of Colorectal Cancer with Complete Cytoreductive Surgery and Hyperthermic Intraperitoneal Peroperative Chemotherapy (HIPEC) with Oxaliplatin: A Belgian Multicentre Prospective Phase II Clinical Study. Ann. Surg. Oncol. 2012, 19, 2186–2194. [Google Scholar] [CrossRef] [PubMed]
- Quénet, F.; Élias, D.; Roca, L.; Goéré, D.; Ghouti, L.; Pocard, M.; Facy, O.; Arvieux, C.; Lorimier, G.; Pezet, D.; et al. A UNICANCER phase III trial of hyperthermic intra-peritoneal chemotherapy (HIPEC) for colorectal peritoneal carcinomatosis (PC): PRODIGE 7. J. Clin. Oncol. 2018, 36. [Google Scholar] [CrossRef]
- Abboud, K.; André, T.; Brunel, M.; Ducreux, M.; Eveno, C.; Glehen, O.; Goéré, D.; Gornet, J.-M.; Lefevre, J.H.; Mariani, P.; et al. Management of colorectal peritoneal metastases: Expert opinion. J. Visc. Surg. 2019, 156, 377–379. [Google Scholar] [CrossRef] [PubMed]
- Sinukumar, S.; Rajan, F.; Mehta, S.; Damodaran, D.; Zaveri, S.; Kammar, P.; Bhatt, A. A comparison of outcomes following total and selective peritonectomy performed at the time of interval cytoreductive surgery for advanced serous epithelial ovarian, fallopian tube and primary peritoneal cancer—A study by INDEPSO. Eur. J. Surg. Oncol. 2019. [Google Scholar] [CrossRef]
- Bhatt, A.; Sinukumar, S.; Mehta, S.; Damodaran, D.; Zaveri, S.; Kammar, P.; Mishra, S.; Parikh, L.; Ranade, R.; Penumadu, P.; et al. Patterns of pathological response to neoadjuvant chemotherapy and its clinical implications in patients undergoing interval cytoreductive surgery for advanced serous epithelial ovarian cancer—A study by the Indian Network for Development of Peritoneal Surface Oncology (INDEPSO). Eur. J. Surg. Oncol. 2019, 45, 666–671. [Google Scholar] [CrossRef]
- Rodriguez, L.; Batlle, A.; Di Venosa, G.; Battah, S.; Dobbin, P.; Macrobert, A.J.; Casas, A. Mechanisms of 5-aminolevulinic acid ester uptake in mammalian cells. Br. J. Pharm. 2006, 147, 825–833. [Google Scholar] [CrossRef] [Green Version]
- Wachowska, M.; Muchowicz, A.; Firczuk, M.; Gabrysiak, M.; Winiarska, M.; Wańczyk, M.; Bojarczuk, K.; Golab, J. Aminolevulinic Acid (ALA) as a Prodrug in Photodynamic Therapy of Cancer. Molecules 2011, 16, 4140–4164. [Google Scholar] [CrossRef] [Green Version]
- Celli, J.P.; Spring, B.Q.; Rizvi, I.; Evans, C.L.; Samkoe, K.S.; Verma, S.; Pogue, B.W.; Hasan, T. Imaging and photodynamic therapy: Mechanisms, monitoring, and optimization. Chem. Rev. 2010, 110, 2795–2838. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, A.E.; Gallagher, W.M.; Byrne, A.T. Porphyrin and nonporphyrin photosensitizers in oncology: Preclinical and clinical advances in photodynamic therapy. Photochem. Photobiol. 2009, 85, 1053–1074. [Google Scholar] [CrossRef]
- Landsman, M.L.; Kwant, G.; Mook, G.A.; Zijlstra, W.G. Light-absorbing properties, stability, and spectral stabilization of indocyanine green. J. Appl. Physiol. 1976, 40, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; Lindsey, J.S. Database of Absorption and Fluorescence Spectra of >300 Common Compounds for use in PhotochemCAD. Photochem. Photobiol. 2018, 94, 290–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aveline, B.; Hasan, T.; Redmond, R.W. Photophysical and photosensitizing properties of benzoporphyrin derivative monoacid ring A (BPD-MA). Photochem. Photobiol. 1994, 59, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Plaetzer, K.; Krammer, B.; Berlanda, J.; Berr, F.; Kiesslich, T. Photophysics and photochemistry of photodynamic therapy: Fundamental aspects. Lasers Med. Sci. 2009, 24, 259–268. [Google Scholar] [CrossRef]
- Broekgaarden, M.; Weijer, R.; van Gulik, T.M.; Hamblin, M.R.; Heger, M. Tumor cell survival pathways activated by photodynamic therapy: A molecular basis for pharmacological inhibition strategies. Cancer Metastasis Rev. 2015, 34, 643–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fingar, V.H. Vascular effects of photodynamic therapy. J. Clin. Laser Med. Surg. 1996, 14, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.P.; Mroz, P.; Hamblin, M.R. Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer 2006, 6, 535–545. [Google Scholar] [CrossRef] [Green Version]
- Kishi, K.; Fujiwara, Y.; Yano, M.; Inoue, M.; Miyashiro, I.; Motoori, M.; Shingai, T.; Gotoh, K.; Takahashi, H.; Noura, S.; et al. Staging laparoscopy using ALA-mediated photodynamic diagnosis improves the detection of peritoneal metastases in advanced gastric cancer. J. Surg. Oncol. 2012, 106, 294–298. [Google Scholar] [CrossRef]
- Murayama, Y.; Ichikawa, D.; Koizumi, N.; Komatsu, S.; Shiozaki, A.; Kuriu, Y.; Ikoma, H.; Kubota, T.; Nakanishi, M.; Harada, Y.; et al. Staging Fluorescence Laparoscopy for Gastric Cancer by Using 5-Aminolevulinic Acid. Anticancer Res. 2012, 32, 5421–5427. [Google Scholar]
- Satou, S.; Ishizawa, T.; Masuda, K.; Kaneko, J.; Aoki, T.; Sakamoto, Y.; Hasegawa, K.; Sugawara, Y.; Kokudo, N. Indocyanine green fluorescent imaging for detecting extrahepatic metastasis of hepatocellular carcinoma. J. Gastroenterol. 2013, 48, 1136–1143. [Google Scholar] [CrossRef]
- Kishi, K.; Fujiwara, Y.; Yano, M.; Motoori, M.; Sugimura, K.; Ohue, M.; Noura, S.; Marubashi, S.; Takahashi, H.; Sakon, M. Diagnostic Laparoscopy with 5-Aminolevulinic-Acid-Mediated Photodynamic Diagnosis Enhances the Detection of Peritoneal Micrometastases in Advanced Gastric Cancer. Oncology 2014, 87, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Murayama, Y.; Konishi, H.; Morimura, R.; Komatsu, S.; Shiozaki, A.; Kuriu, Y.; Ikoma, H.; Kubota, T.; Nakanishi, M.; et al. Fluorescent detection of peritoneal metastasis in human colorectal cancer using 5-aminolevulinic acid. Int. J. Oncol. 2014, 45, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Endo, Y.; Fujita, T.; Ishibashi, H.; Nishioka, T.; Canbay, E.; Li, Y.; Ogura, S.; Yonemura, Y. Cytoreductive Surgery Under Aminolevulinic Acid-Mediated Photodynamic Diagnosis Plus Hyperthermic Intraperitoneal Chemotherapy in Patients with Peritoneal Carcinomatosis from Ovarian Cancer and Primary Peritoneal Carcinoma: Results of a Phase I Trial. Ann. Surg. Oncol. 2014, 21, 4256–4262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yonemura, Y. Selection of Patients by Membrane Transporter Expressions for Aminolevulinic Acid (ALA)-Guided Photodynamic Detection of Peritoneal Metastases. Int. J. Sci. 2015, 4, 66–77. [Google Scholar] [CrossRef] [Green Version]
- Kishi, K.; Fujiwara, Y.; Yano, M.; Motoori, M.; Sugimura, K.; Takahashi, H.; Ohue, M.; Sakon, M. Usefulness of diagnostic laparoscopy with 5-aminolevulinic acid (ALA)-mediated photodynamic diagnosis for the detection of peritoneal micrometastasis in advanced gastric cancer after chemotherapy. Surg. Today 2016, 46, 1427–1434. [Google Scholar] [CrossRef]
- Yonemura, Y.; Canbay, E.; Ishibashi, H.; Nishino, E.; Endou, Y.; Sako, S.; Ogura, S.-I. 5-Aminolevulinic Acid Fluorescence in Detection of Peritoneal Metastases. Asian Pac. J. Cancer Prev. 2016, 17, 2271–2275. [Google Scholar] [CrossRef] [Green Version]
- Hillemanns, P.; Wimberger, P.; Reif, J.; Stepp, H.; Klapdor, R. Photodynamic diagnosis with 5-aminolevulinic acid for intraoperative detection of peritoneal metastases of ovarian cancer: A feasibility and dose finding study: PHOTODYNAMIC DIAGNOSIS WITH 5-AMINOLEVULINIC ACID. Lasers Surg. Med. 2017, 49, 169–176. [Google Scholar] [CrossRef]
- Ushimaru, Y.; Fujiwara, Y.; Kishi, K.; Sugimura, K.; Omori, T.; Moon, J.-H.; Yanagimoto, Y.; Ohue, M.; Yasui, M.; Takahashi, H.; et al. Prognostic Significance of Basing Treatment Strategy on the Results of Photodynamic Diagnosis in Advanced Gastric Cancer. Ann. Surg. Oncol. 2017, 24, 983–989. [Google Scholar] [CrossRef]
- Harada, K.; Murayama, Y.; Kubo, H.; Matsuo, H.; Morimura, R.; Ikoma, H.; Fujiwara, H.; Okamoto, K.; Tanaka, T.; Otsuji, E. Photodynamic diagnosis of peritoneal metastasis in human pancreatic cancer using 5-aminolevulinic acid during staging laparoscopy. Oncol. Lett. 2018. [Google Scholar] [CrossRef]
- Sugarbaker, P.H.; Alderman, R.; Edwards, G.; Marquardt, C.E.; Gushchin, V.; Esquivel, J.; Chang, D. Prospective morbidity and mortality assessment of cytoreductive surgery plus perioperative intraperitoneal chemotherapy to treat peritoneal dissemination of appendiceal mucinous malignancy. Ann. Surg. Oncol. 2006, 13, 635–644. [Google Scholar] [CrossRef]
- Dhir, M.; Ramalingam, L.; Shuai, Y.; Pakrafter, S.; Jones, H.L.; Hogg, M.E.; Zureikat, A.H.; Holtzman, M.P.; Ahrendt, S.A.; Bahary, N.; et al. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemoperfusion in Adolescent and Young Adults with Peritoneal Metastases. Ann. Surg. Oncol. 2017, 24, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Canbay, E.; Ishibashi, H.; Sako, S.; Kitai, T.; Nishino, E.; Hirano, M.; Mizumoto, A.; Endo, Y.; Ogura, S.; Yonemura, Y. Photodynamic detection and management of intraperitoneal spreading of primary peritoneal papillary serous carcinoma in a man: Report of a case. Surg. Today 2014, 44, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Azaïs, H.; Canlorbe, G.; Kerbage, Y.; Grabarz, A.; Collinet, P.; Mordon, S. Image-guided surgery in gynecologic oncology. Future Oncol. 2017, 13, 2321–2328. [Google Scholar] [CrossRef] [PubMed]
- Azaïs, H.; Betrouni, N.; Mordon, S.; Collinet, P. Targeted approaches and innovative illumination solutions: A new era for photodynamic therapy applications in gynecologic oncology? Photodiagnosis Photodyn. 2016, 13, 128–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azaïs, H.; Rebahi, C.; Baydoun, M.; Serouart, B.; Ziane, L.; Moralès, O.; Frochot, C.; Colombeau, L.; Thécua, E.; Collinet, P.; et al. A global approach for the development of photodynamic therapy of peritoneal metastases regardless of their origin. Photodiagnosis Photodyn. 2020, 101683. [Google Scholar] [CrossRef]
- Namikawa, T.; Fujisawa, K.; Munekage, E.; Iwabu, J.; Uemura, S.; Tsujii, S.; Maeda, H.; Kitagawa, H.; Fukuhara, H.; Inoue, K.; et al. Clinical application of photodynamic medicine technology using light-emitting fluorescence imaging based on a specialized luminous source. Med. Mol. Morphol. 2018, 51, 187–193. [Google Scholar] [CrossRef]
- Almerie, M.Q.; Gossedge, G.; Wright, K.E.; Jayne, D.G. Photodynamic diagnosis for detection of peritoneal carcinomatosis. J. Surg. Res. 2015, 195, 175–187. [Google Scholar] [CrossRef]
- Kushibiki, T.; Noji, T.; Ebihara, Y.; Hontani, K.; Ono, M.; Kuwabara, S.; Nakamura, T.; Tsuchikawa, T.; Okamura, K.; Ishizuka, M.; et al. 5-Aminolevulinic-acid-mediated Photodynamic Diagnosis Enhances the Detection of Peritoneal Metastases in Biliary Tract Cancer in Mice. In Vivo 2017, 31, 905–908. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.H.; Choi, M.-G.; Hasan, T. Application of photodynamic therapy in gastrointestinal disorders: An outdated or re-emerging technique? Korean J. Intern. Med. 2017, 32, 1–10. [Google Scholar] [CrossRef]
- Anand, S.; Honari, G.; Hasan, T.; Elson, P.; Maytin, E.V. Low-dose methotrexate enhances aminolevulinate-based photodynamic therapy in skin carcinoma cells in vitro and in vivo. Clin. Cancer Res. 2009, 15, 3333–3343. [Google Scholar] [CrossRef] [Green Version]
- Anand, S.; Wilson, C.; Hasan, T.; Maytin, E.V. Vitamin D3 enhances the apoptotic response of epithelial tumors to aminolevulinate-based photodynamic therapy. Cancer Res. 2011, 71, 6040–6050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anand, S.; Hasan, T.; Maytin, E.V. Mechanism of differentiation-enhanced photodynamic therapy for cancer: Upregulation of coproporphyrinogen oxidase by C/EBP transcription factors. Mol. Cancer 2013, 12, 1638–1650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anand, S.; Rollakanti, K.R.; Brankov, N.; Brash, D.E.; Hasan, T.; Maytin, E.V. Fluorouracil Enhances Photodynamic Therapy of Squamous Cell Carcinoma via a p53-Independent Mechanism that Increases Protoporphyrin IX levels and Tumor Cell Death. Mol. Cancer 2017, 16, 1092–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maytin, E.V.; Anand, S.; Riha, M.; Lohser, S.; Tellez, A.; Ishak, R.; Karpinski, L.; Sot, J.; Hu, B.; Denisyuk, A.; et al. 5-Fluorouracil Enhances Protoporphyrin IX Accumulation and Lesion Clearance during Photodynamic Therapy of Actinic Keratoses: A Mechanism-Based Clinical Trial. Clin. Cancer Res. 2018, 24, 3026–3035. [Google Scholar] [CrossRef] [Green Version]
- Berg, K.; Anholt, H.; Bech, O.; Moan, J. The influence of iron chelators on the accumulation of protoporphyrin IX in 5-aminolaevulinic acid-treated cells. Br. J. Cancer 1996, 74, 688–697. [Google Scholar] [CrossRef] [Green Version]
- Pourzand, C.; Reelfs, O.; Kvam, E.; Tyrrell, R.M. The iron regulatory protein can determine the effectiveness of 5-aminolevulinic acid in inducing protoporphyrin IX in human primary skin fibroblasts. J. Investig. Derm. 1999, 112, 419–425. [Google Scholar] [CrossRef] [Green Version]
- Alexander, V.M.; Sano, K.; Yu, Z.; Nakajima, T.; Choyke, P.L.; Ptaszek, M.; Kobayashi, H. Galactosyl human serum albumin-NMP1 conjugate: A near infrared (NIR)-activatable fluorescence imaging agent to detect peritoneal ovarian cancer metastases. Bioconjug. Chem. 2012, 23, 1671–1679. [Google Scholar] [CrossRef] [Green Version]
- van Dam, G.M.; Themelis, G.; Crane, L.M.A.; Harlaar, N.J.; Pleijhuis, R.G.; Kelder, W.; Sarantopoulos, A.; de Jong, J.S.; Arts, H.J.G.; van der Zee, A.G.J.; et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: First in-human results. Nat. Med. 2011, 17, 1315–1319. [Google Scholar] [CrossRef]
- Tummers, Q.R.J.G.; Hoogstins, C.E.S.; Gaarenstroom, K.N.; de Kroon, C.D.; van Poelgeest, M.I.E.; Vuyk, J.; Bosse, T.; Smit, V.T.H.B.M.; van de Velde, C.J.H.; Cohen, A.F.; et al. Intraoperative imaging of folate receptor alpha positive ovarian and breast cancer using the tumor specific agent EC17. Oncotarget 2016, 7, 32144–32155. [Google Scholar] [CrossRef] [Green Version]
- Hoogstins, C.E.S.; Tummers, Q.R.J.G.; Gaarenstroom, K.N.; de Kroon, C.D.; Trimbos, J.B.M.Z.; Bosse, T.; Smit, V.T.H.B.M.; Vuyk, J.; van de Velde, C.J.H.; Cohen, A.F.; et al. A Novel Tumor-Specific Agent for Intraoperative Near-Infrared Fluorescence Imaging: A Translational Study in Healthy Volunteers and Patients with Ovarian Cancer. Clin. Cancer Res. 2016, 22, 2929–2938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azaïs, H.; Schmitt, C.; Tardivel, M.; Kerdraon, O.; Stallivieri, A.; Frochot, C.; Betrouni, N.; Collinet, P.; Mordon, S. Assessment of the specificity of a new folate-targeted photosensitizer for peritoneal metastasis of epithelial ovarian cancer to enable intraperitoneal photodynamic therapy. A preclinical study. Photodiagnosis Photodyn. 2016, 13, 130–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gravier, J.; Schneider, R.; Frochot, C.; Bastogne, T.; Schmitt, F.; Didelon, J.; Guillemin, F.; Barberi-Heyob, M. Improvement of meta-tetra(hydroxyphenyl)chlorin-like photosensitizer selectivity with folate-based targeted delivery. synthesis and in vivo delivery studies. J. Med. Chem. 2008, 51, 3867–3877. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, P.; Lin, H.; Jiang, Z.; Guo, L.; Li, B. A novel chlorin-PEG-folate conjugate with higher water solubility, lower cytotoxicity, better tumor targeting and photodynamic activity. J. Photochem. Photobiol. B Biol. 2013, 127, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Moret, F.; Scheglmann, D.; Reddi, E. Folate-targeted PEGylated liposomes improve the selectivity of PDT with meta-tetra(hydroxyphenyl)chlorin (m-THPC). Photochem. Photobiol. Sci. 2013, 12, 823–834. [Google Scholar] [CrossRef]
- Tsujimoto, H.; Morimoto, Y.; Takahata, R.; Nomura, S.; Yoshida, K.; Horiguchi, H.; Hiraki, S.; Ono, S.; Miyazaki, H.; Saito, D.; et al. Photodynamic therapy using nanoparticle loaded with indocyanine green for experimental peritoneal dissemination of gastric cancer. Cancer Sci. 2014, 105, 1626–1630. [Google Scholar] [CrossRef]
- Folli, S.; Wagnières, G.; Pèlegrin, A.; Calmes, J.M.; Braichotte, D.; Buchegger, F.; Chalandon, Y.; Hardman, N.; Heusser, C.; Givel, J.C. Immunophotodiagnosis of colon carcinomas in patients injected with fluoresceinated chimeric antibodies against carcinoembryonic antigen. Proc. Natl. Acad. Sci. USA 1992, 89, 7973–7977. [Google Scholar] [CrossRef] [Green Version]
- Spring, B.Q.; Abu-Yousif, A.O.; Palanisami, A.; Rizvi, I.; Zheng, X.; Mai, Z.; Anbil, S.; Sears, R.B.; Mensah, L.B.; Goldschmidt, R.; et al. Selective treatment and monitoring of disseminated cancer micrometastases in vivo using dual-function, activatable immunoconjugates. Proc. Natl. Acad. Sci. USA 2014, 111, E933–E942. [Google Scholar] [CrossRef] [Green Version]
- Koizumi, N.; Harada, Y.; Minamikawa, T.; Tanaka, H.; Otsuji, E.; Takamatsu, T. Recent advances in photodynamic diagnosis of gastric cancer using 5-aminolevulinic acid. World J. Gastroenterol. 2016, 22, 1289–1296. [Google Scholar] [CrossRef]
- Harada, K.; Harada, Y.; Beika, M.; Koizumi, N.; Inoue, K.; Murayama, Y.; Kuriu, Y.; Nakanishi, M.; Minamikawa, T.; Yamaoka, Y.; et al. Detection of lymph node metastases in human colorectal cancer by using 5-aminolevulinic acid-induced protoporphyrin IX fluorescence with spectral unmixing. Int. J. Mol. Sci. 2013, 14, 23140–23152. [Google Scholar] [CrossRef] [Green Version]
- Mallidi, S.; Anbil, S.; Bulin, A.-L.; Obaid, G.; Ichikawa, M.; Hasan, T. Beyond the Barriers of Light Penetration: Strategies, Perspectives and Possibilities for Photodynamic Therapy. Theranostics 2016, 6, 2458–2487. [Google Scholar] [CrossRef] [Green Version]
- Sindelar, W.F. Technique of Photodynamic Therapy for Disseminated Intraperitoneal Malignant Neoplasms: Phase I Study. Arch. Surg. 1991, 126, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Delaney, T.F.; Sindelar, W.F.; Tochner, Z.; Smith, P.D.; Friauf, W.S.; Thomas, G.; Dachowski, L.; Cole, J.W.; Steinberg, S.M.; Glatstein, E. Phase I study of debulking surgery and photodynamic therapy for disseminated intraperitoneal tumors. Int. J. Radiat. Oncol. Biol. Phys. 1993, 25, 445–457. [Google Scholar] [CrossRef]
- Hahn, S.M. A Phase II Trial of Intraperitoneal Photodynamic Therapy for Patients with Peritoneal Carcinomatosis and Sarcomatosis. Clin. Cancer Res. 2006, 12, 2517–2525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canter, R.J.; Mick, R.; Kesmodel, S.B.; Raz, D.J.; Spitz, F.R.; Metz, J.M.; Glatstein, E.J.; Hahn, S.M.; Fraker, D.L. Intraperitoneal Photodynamic Therapy Causes a Capillary-Leak Syndrome. Ann. Surg. Oncol. 2003, 10, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Hendren, S.K.; Hahn, S.M.; Spitz, F.R.; Bauer, T.W.; Rubin, S.C.; Zhu, T.; Glatstein, E.; Fraker, D.L. Phase II Trial of Debulking Surgery and Photodynamic Therapy for Disseminated Intraperitoneal Tumors. Ann. Surg. Oncol. 2001, 8, 65–71. [Google Scholar] [CrossRef]
- Glehen, O.; Mohamed, F.; Gilly, F.N. Peritoneal carcinomatosis from digestive tract cancer: New management by cytoreductive surgery and intraperitoneal chemohyperthermia. Lancet Oncol. 2004, 5, 219–228. [Google Scholar] [CrossRef]
- Chen, J.; Liang, B.; Yuan, Y.; Liu, C.; Li, L.; Li, H.; Mu, F.; Zuo, J.; Xu, K. Comprehensive treatment of malignant mesothelioma patients after the failure of systemic chemotherapy. Cryobiology 2012, 65, 284–288. [Google Scholar] [CrossRef]
- Hahn, S.M.; Putt, M.E.; Metz, J.; Shin, D.B.; Rickter, E.; Menon, C.; Smith, D.; Glatstein, E.; Fraker, D.L.; Busch, T.M. Photofrin Uptake in the Tumor and Normal Tissues of Patients Receiving Intraperitoneal Photodynamic Therapy. Clin. Cancer Res. 2006, 12, 5464–5470. [Google Scholar] [CrossRef] [Green Version]
- Menon, C.; Kutney, S.N.; Lehr, S.C.; Hendren, S.K.; Busch, T.M.; Hahn, S.M.; Fraker, D.L. Vascularity and uptake of photosensitizer in small human tumor nodules: Implications for intraperitoneal photodynamic therapy. Clin. Cancer Res. 2001, 7, 3904–3911. [Google Scholar]
- Busch, T.M. Hypoxia and Photofrin Uptake in the Intraperitoneal Carcinomatosis and Sarcomatosis of Photodynamic Therapy Patients. Clin. Cancer Res. 2004, 10, 4630–4638. [Google Scholar] [CrossRef] [Green Version]
- Weijer, R.; Broekgaarden, M.; Kos, M.; van Vught, R.; Rauws, E.A.J.; Breukink, E.; van Gulik, T.M.; Storm, G.; Heger, M. Enhancing photodynamic therapy of refractory solid cancers: Combining second-generation photosensitizers with multi-targeted liposomal delivery. J. Photochem. Photobiol. C Photochem. Rev. 2015, 23, 103–131. [Google Scholar] [CrossRef]
- Maeda, H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv. Drug Deliv. Rev. 2015, 91, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.; Heukers, R.; Sornkom, J.; Kok, R.J.; van Bergen En Henegouwen, P.M.P. Targeting tumors with nanobodies for cancer imaging and therapy. J. Control. Release 2013, 172, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Broekgaarden, M.; van Vught, R.; Oliveira, S.; Roovers, R.C.; van Bergen en Henegouwen, P.M.P.; Pieters, R.J.; Van Gulik, T.M.; Breukink, E.; Heger, M. Site-specific conjugation of single domain antibodies to liposomes enhances photosensitizer uptake and photodynamic therapy efficacy. Nanoscale 2016, 8, 6490–6494. [Google Scholar] [CrossRef] [PubMed]
- Obaid, G.; Bano, S.; Mallidi, S.; Broekgaarden, M.; Kuriakose, J.; Silber, Z.; Bulin, A.-L.; Wang, Y.; Mai, Z.; Jin, W.; et al. Impacting pancreatic cancer therapy in heterotypic in vitro organoids and in vivo tumors with specificity-tuned, NIR-activable photoimmuno-nanoconjugates: Towards conquering desmoplasia? Nano Lett. 2019, 19, 7573–7587. [Google Scholar] [CrossRef]
- Obaid, G.; Broekgaarden, M.; Bulin, A.-L.; Huang, H.-C.; Kuriakose, J.; Liu, J.; Hasan, T. Photonanomedicine: A convergence of photodynamic therapy and nanotechnology. Nanoscale 2016, 8, 12471–12503. [Google Scholar] [CrossRef]
- Pinto, A.; Pocard, M. Photodynamic therapy and photothermal therapy for the treatment of peritoneal metastasis: A systematic review. Pleura Peritoneum 2018, 3. [Google Scholar] [CrossRef]
- Baydoun, M.; Moralès, O.; Frochot, C.; Ludovic, C.; Leroux, B.; Thecua, E.; Ziane, L.; Grabarz, A.; Kumar, A.; de Schutter, C.; et al. Photodynamic Therapy Using a New Folate Receptor-Targeted Photosensitizer on Peritoneal Ovarian Cancer Cells Induces the Release of Extracellular Vesicles with Immunoactivating Properties. J. Clin. Med. 2020, 9, 1185. [Google Scholar] [CrossRef]
- Almerie, M.Q.; Gossedge, G.; Wright, K.E.; Jayne, D.G. Treatment of peritoneal carcinomatosis with photodynamic therapy: Systematic review of current evidence. Photodiagnosis Photodyn. 2017, 20, 276–286. [Google Scholar] [CrossRef] [Green Version]
- Quilbe, A.; Moralès, O.; Baydoun, M.; Kumar, A.; Mustapha, R.; Murakami, T.; Leroux, B.; de Schutter, C.; Thecua, E.; Ziane, L.; et al. An Efficient Photodynamic Therapy Treatment for Human Pancreatic Adenocarcinoma. J. Clin. Med. 2020, 9, 192. [Google Scholar] [CrossRef] [Green Version]
- Michy, T.; Massias, T.; Bernard, C.; Vanwonterghem, L.; Henry, M.; Guidetti, M.; Royal, G.; Coll, J.-L.; Texier, I.; Josserand, V.; et al. Verteporfin-Loaded Lipid Nanoparticles Improve Ovarian Cancer Photodynamic Therapy In Vitro and In Vivo. Cancers 2019, 11, 1760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guyon, L.; Farine, M.-O.; Lesage, J.C.; Gevaert, A.-M.; Simonin, S.; Schmitt, C.; Collinet, P.; Mordon, S. Photodynamic therapy of ovarian cancer peritoneal metastasis with hexaminolevulinate: A toxicity study. Photodiagnosis Photodyn. 2014, 11, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Guyon, L.; Ascencio, M.; Collinet, P.; Mordon, S. Photodiagnosis and photodynamic therapy of peritoneal metastasis of ovarian cancer. Photodiagnosis Photodyn. 2012, 9, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, I.; Celli, J.P.; Evans, C.L.; Abu-Yousif, A.O.; Muzikansky, A.; Pogue, B.W.; Finkelstein, D.; Hasan, T. Synergistic enhancement of carboplatin efficacy with photodynamic therapy in a three-dimensional model for micrometastatic ovarian cancer. Cancer Res. 2010, 70, 9319–9328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debefve, E.; Pegaz, B.; Ballini, J.-P.; Konan, Y.N.; van den Bergh, H. Combination therapy using aspirin-enhanced photodynamic selective drug delivery. Vasc. Pharm. 2007, 46, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Broekgaarden, M.; Rizvi, I.; Bulin, A.-L.; Petrovic, L.; Goldschmidt, R.; Celli, J.P.; Hasan, T. Neoadjuvant photodynamic therapy augments immediate and prolonged oxaliplatin efficacy in metastatic pancreatic cancer organoids. Oncotarget 2018, 9, 13009–13022. [Google Scholar] [CrossRef]
- Xin, J.; Wang, S.; Zhang, L.; Xin, B.; He, Y.; Wang, J.; Wang, S.; Shen, L.; Zhang, Z.; Yao, C. Comparison of the synergistic anticancer activity of AlPcS4 photodynamic therapy in combination with different low-dose chemotherapeutic agents on gastric cancer cells. Oncol. Rep. 2018, 40, 165–178. [Google Scholar] [CrossRef]
- Ma, L.W.; Moan, J.; Berg, K.; Peng, Q.; Steen, H.B. Potentiation of photodynamic therapy by mitomycin C in cultured human colon adenocarcinoma cells. Radiat. Res. 1993, 134, 22–28. [Google Scholar] [CrossRef]
- Ma, L.W.; Moan, J.; Steen, H.B.; Iani, V. Anti-tumour activity of photodynamic therapy in combination with mitomycin C in nude mice with human colon adenocarcinoma. Br. J. Cancer 1995, 71, 950–956. [Google Scholar] [CrossRef]
- Guyon, L.; Lesage, J.C.; Betrouni, N.; Mordon, S. Development of a new illumination procedure for photodynamic therapy of the abdominal cavity. J. Biomed. Opt. 2012, 17, 038001. [Google Scholar] [CrossRef]
- Mordon, S.; Cochrane, C.; Tylcz, J.B.; Betrouni, N.; Mortier, L.; Koncar, V. Light emitting fabric technologies for photodynamic therapy. Photodiagnosis Photodyn. 2015, 12, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krueger, T.; Altermatt, H.J.; Mettler, D.; Scholl, B.; Magnusson, L.; Ris, H.-B. Experimental photodynamic therapy for malignant pleural mesothelioma with pegylated mTHPC. Lasers Surg. Med. 2003, 32, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Dujardin, C.; Auffray, E.; Bourret-Courchesne, E.; Dorenbos, P.; Lecoq, P.; Nikl, M.; Vasil’ev, A.N.; Yoshikawa, A.; Zhu, R.-Y. Needs, Trends, and Advances in Inorganic Scintillators. IEEE Trans. Nucl. Sci. 2018, 65, 1977–1997. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Tang, Y.; Elmenoufy, A.H.; Xu, H.; Cheng, Z.; Yang, X. Nanocomposite-Based Photodynamic Therapy Strategies for Deep Tumor Treatment. Small 2015, 11, 5860–5887. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Huang, P.; Chen, X. Overcoming the Achilles’ heel of photodynamic therapy. Chem. Soc. Rev. 2016, 45, 6488–6519. [Google Scholar] [CrossRef]
- Kamkaew, A.; Chen, F.; Zhan, Y.; Majewski, R.L.; Cai, W. Scintillating Nanoparticles as Energy Mediators for Enhanced Photodynamic Therapy. ACS Nano 2016, 10, 3918–3935. [Google Scholar] [CrossRef]
- Larue, L.; Ben Mihoub, A.; Youssef, Z.; Colombeau, L.; Acherar, S.; André, J.C.; Arnoux, P.; Baros, F.; Vermandel, M.; Frochot, C. Using X-rays in photodynamic therapy: An overview. Photochem. Photobiol. Sci. 2018, 17, 1612–1650. [Google Scholar] [CrossRef]
- Chen, X.; Song, J.; Chen, X.; Yang, H. X-ray-activated nanosystems for theranostic applications. Chem. Soc. Rev. 2019, 48, 3073–3101. [Google Scholar] [CrossRef]
- Sun, W.; Zhou, Z.; Pratx, G.; Chen, X.; Chen, H. Nanoscintillator-Mediated X-Ray Induced Photodynamic Therapy for Deep-Seated Tumors: From Concept to Biomedical Applications. Theranostics 2020, 10, 1296–1318. [Google Scholar] [CrossRef]
- Petit, T.; Velten, M.; d’Hombres, A.; Marchal, C.; Montbarbon, X.; Mornex, F.; Quetin, P.; Gérard, J.-P.; Romestaing, P.; Carrie, C. Long-term survival of 106 stage III ovarian cancer patients with minimal residual disease after second-look laparotomy and consolidation radiotherapy. Gynecol. Oncol. 2007, 104, 104–108. [Google Scholar] [CrossRef]
- Iorio, G.C.; Martini, S.; Arcadipane, F.; Ricardi, U.; Franco, P. The role of radiotherapy in epithelial ovarian cancer: A literature overview. Med. Oncol. 2019, 36, 64. [Google Scholar] [CrossRef] [PubMed]
- Goldhirsch, A.; Greiner, R.; Dreher, E.; Sessa, C.; Krauer, F.; Forni, M.; Jungi, F.W.; Brunner, K.W.; Veraguth, P.; Engeler, V. Treatment of advanced ovarian cancer with surgery, chemotherapy, and consolidation of response by whole-abdominal radiotherapy. Cancer 1988, 62, 40–47. [Google Scholar] [CrossRef]
- Pickel, H.; Lahousen, M.; Petru, E.; Stettner, H.; Hackl, A.; Kapp, K.; Winter, R. Consolidation radiotherapy after carboplatin-based chemotherapy in radically operated advanced ovarian cancer. Gynecol. Oncol. 1999, 72, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, N.; Lundell, M.; Nilsson, B.; Ragnarsson-Olding, B.; Sjövall, K. Is there place for radiotherapy in the treatment of advanced ovarian cancer? Radiother. Oncol. 1999, 53, 213–218. [Google Scholar] [CrossRef]
- Dinniwell, R.; Lock, M.; Pintilie, M.; Fyles, A.; Laframboise, S.; Depetrillo, D.; Levin, W.; Manchul, L.; Murphy, J.; Oza, A.; et al. Consolidative abdominopelvic radiotherapy after surgery and carboplatin/paclitaxel chemotherapy for epithelial ovarian cancer. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Sorbe, B. Consolidation treatment of advanced (FIGO stage III) ovarian carcinoma in complete surgical remission after induction chemotherapy: A randomized, controlled, clinical trial comparing whole abdominal radiotherapy, chemotherapy, and no further treatment. Int. J. Gynecol. Cancer 2003, 13, 278–286. [Google Scholar] [CrossRef]
- Hong, L.; Alektiar, K.; Chui, C.; LoSasso, T.; Hunt, M.; Spirou, S.; Yang, J.; Amols, H.; Ling, C.; Fuks, Z.; et al. IMRT of large fields: Whole-abdomen irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2002, 54, 278–289. [Google Scholar] [CrossRef]
- Arians, N.; Kieser, M.; Benner, L.; Rochet, N.; Katayama, S.; Sterzing, F.; Herfarth, K.; Schubert, K.; Schröder, L.; Leitzen, C.; et al. Adjuvant Intensity Modulated Whole-Abdominal Radiation Therapy for High-Risk Patients With Ovarian Cancer (International Federation of Gynecology and Obstetrics Stage III): First Results of a Prospective Phase 2 Study. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 912–920. [Google Scholar] [CrossRef]
- Arians, N.; Kieser, M.; Benner, L.; Rochet, N.; Schröder, L.; Katayama, S.; Herfarth, K.; Schubert, K.; Schneeweiss, A.; Sohn, C.; et al. Adjuvant intensity modulated whole-abdominal radiation therapy for high-risk patients with ovarian cancer FIGO stage III: Final results of a prospective phase 2 study. Radiat. Oncol. 2019, 14, 179. [Google Scholar] [CrossRef] [Green Version]
- Rochet, N.; Sterzing, F.; Jensen, A.D.; Dinkel, J.; Herfarth, K.K.; Schubert, K.; Eichbaum, M.H.; Schneeweiss, A.; Sohn, C.; Debus, J.; et al. Intensity-modulated whole abdominal radiotherapy after surgery and carboplatin/taxane chemotherapy for advanced ovarian cancer: Phase I study. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 1382–1389. [Google Scholar] [CrossRef]
- Rochet, N.; Kieser, M.; Sterzing, F.; Krause, S.; Lindel, K.; Harms, W.; Eichbaum, M.H.; Schneeweiss, A.; Sohn, C.; Debus, J. Phase II study evaluating consolidation whole abdominal intensity-modulated radiotherapy (IMRT) in patients with advanced ovarian cancer stage FIGO III-the. BMC Cancer 2011, 11, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reiss, K.A.; Herman, J.M.; Zahurak, M.; Brade, A.; Dawson, L.A.; Scardina, A.; Joffe, C.; Petito, E.; Hacker-Prietz, A.; Kinders, R.J.; et al. A Phase I study of veliparib (ABT-888) in combination with low-dose fractionated whole abdominal radiation therapy in patients with advanced solid malignancies and peritoneal carcinomatosis. Clin. Cancer Res. 2015, 21, 68–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broekgaarden, M.; Anbil, S.; Bulin, A.-L.; Obaid, G.; Mai, Z.; Baglo, Y.; Rizvi, I.; Hasan, T. Modulation of redox metabolism negates cancer-associated fibroblasts-induced treatment resistance in a heterotypic 3D culture platform of pancreatic cancer. Biomaterials 2019, 222, 119421. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K.; Yachida, S.; Sugimoto, M.; Oshima, M.; Nakagawa, T.; Akamoto, S.; Tabata, S.; Saitoh, K.; Kato, K.; Sato, S.; et al. Global metabolic reprogramming of colorectal cancer occurs at adenoma stage and is induced by MYC. Proc. Natl. Acad. Sci. USA 2017, 114, E7697–E7706. [Google Scholar] [CrossRef] [Green Version]
- McDonald, O.G.; Li, X.; Saunders, T.; Tryggvadottir, R.; Mentch, S.J.; Warmoes, M.O.; Word, A.E.; Carrer, A.; Salz, T.H.; Natsume, S.; et al. Epigenomic reprogramming during pancreatic cancer progression links anabolic glucose metabolism to distant metastasis. Nat. Genet. 2017, 49, 367–376. [Google Scholar] [CrossRef]
- Daemen, A.; Peterson, D.; Sahu, N.; McCord, R.; Du, X.; Liu, B.; Kowanetz, K.; Hong, R.; Moffat, J.; Gao, M.; et al. Metabolite profiling stratifies pancreatic ductal adenocarcinomas into subtypes with distinct sensitivities to metabolic inhibitors. Proc. Natl. Acad. Sci. USA 2015, 112, E4410–E4417. [Google Scholar] [CrossRef] [Green Version]
- Anbil, S.; Pigula, M.; Huang, H.-C.; Mallidi, S.; Broekgaarden, M.; Baglo, Y.; De Silva, P.; Simeone, D.M.; Mino-Kenudson, M.; Maytin, E.V.; et al. Vitamin D receptor activation and photodynamic priming enable durable low-dose chemotherapy. Mol. Cancer 2020. [Google Scholar] [CrossRef] [Green Version]




| Photosensitizer | Application | Peak Excitation Wavelengths | Molar Extinction Coefficient (M−1cm−1) | Peak Emission Wavelength | State of Development | Ref. |
|---|---|---|---|---|---|---|
| Aminolevulinic acid (PpIX) | PDD & PDT | 409 nm 630 nm | 1.2 × 105 (409 nm) 5.0 × 103 (630 nm) | 635 nm | Clinical trials | [21] |
| Indocyanine green | PDD | 780 nm | 2.6 × 105 | 835 nm | Clinical trials | [22] |
| Porfimer sodium | PDT | 630 nm | 1.2 × 103 | 635 nm | Clinical trials | [21] |
| Hypericin | PDD & PDT | 589 nm | 4.5 × 104 | 599 nm | Clinical trials | [21] |
| Pyropheophorbide A (Folate-conjugated) | PDD & PDT | 668 nm | 4.5 × 104 | 672 nm | Preclinical | [23] |
| Meso-tetrahydroxy-phenylchlorin (Folate-conjugated) | PDD & PDT | 652 nm | 2.9 × 104 | 655 nm | Preclinical | [21] |
| Benzoporphyrin derivative (anti-EGFR mAb-conjugated) | PDD & PDT | 692 nm | 3.3 × 104 | 695 nm | Preclinical | [24] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, S.; Bulin, A.-L.; Hurbin, A.; Elleaume, H.; Coll, J.-L.; Broekgaarden, M. Photodynamic Diagnosis and Therapy for Peritoneal Carcinomatosis: Emerging Perspectives. Cancers 2020, 12, 2491. https://doi.org/10.3390/cancers12092491
Xu S, Bulin A-L, Hurbin A, Elleaume H, Coll J-L, Broekgaarden M. Photodynamic Diagnosis and Therapy for Peritoneal Carcinomatosis: Emerging Perspectives. Cancers. 2020; 12(9):2491. https://doi.org/10.3390/cancers12092491
Chicago/Turabian StyleXu, Si, Anne-Laure Bulin, Amandine Hurbin, Hélène Elleaume, Jean-Luc Coll, and Mans Broekgaarden. 2020. "Photodynamic Diagnosis and Therapy for Peritoneal Carcinomatosis: Emerging Perspectives" Cancers 12, no. 9: 2491. https://doi.org/10.3390/cancers12092491