RNA-Sequencing of Tumor-Educated Platelets, a Novel Biomarker for Blood-Based Sarcoma Diagnostics
Abstract
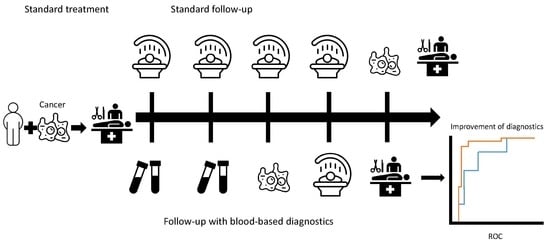
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Inclusion of Patients
4.2. Particle-Swarm Optimization-Enhanced ThromboSeq Analysis
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arifi, S.; Belbaraka, R.; Rahhali, R.; Ismaili, N. Treatment of Adult Soft Tissue Sarcomas: An Overview. Rare Cancers Ther. 2015, 3, 69–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burningham, Z.; Hashibe, M.; Spector, L.; Schiffman, J.D. The epidemiology of sarcoma. Clin. Sarcoma Res. 2012, 2, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fletcher, C.D.M.; Bridge, J.A.; Hogendoorn, P.; Mertens, F. WHO Classification of Tumours of Soft Tissue and Bone; International Agency for Research on Cancer: Lyon, France, 2013.
- Weitz, J.; Antonescu, C.R.; Brennan, M.F. Localized extremity soft tissue sarcoma: Improved knowledge with unchanged survival over time. J. Clin. Oncol. 2003, 21, 2719–2725. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, R.D.; Martin, R.G.; Romsdahl, M.M.; Barkley, H.T. Conservative surgery and postoperative radiotherapy in 300 adults with soft-tissue sarcomas. Cancer 1981, 47, 2391–2397. [Google Scholar] [CrossRef]
- Ballinger, M.L.; Pinese, M.; Thomas, D.M. Translating genomic risk into an early detection strategy for sarcoma. Genes Chromosom. Cancer 2019, 58, 130–136. [Google Scholar] [CrossRef]
- Primrose, J.N.; Perera, R.; Gray, A.; Rose, P.; Fuller, A.; Corkhill, A.; George, S.; MDFT. Effect of 3 to 5 years of scheduled CEA and CT follow-up to detect recurrence of colorectal cancer: The FACS randomized clinical trial. JAMA 2014, 311, 263–270. [Google Scholar] [CrossRef] [Green Version]
- Lilja, H.D.U.; Vickers, A.J. Prostate-specific antigen and prostate cancer: Prediction, detection and monitoring. Nat. Rev. Cancer 2008, 8, 268–278. [Google Scholar] [CrossRef]
- Best, M.G.; Sol, N.; Kooi, I.; Tannous, J.; Westerman, B.A.; Rustenburg, F.; Schellen, P.; Verschueren, H.; Post, E.; Koster, J.; et al. RNA-Seq of Tumor-Educated Platelets Enables Blood-Based Pan-Cancer, Multiclass, and Molecular Pathway Cancer Diagnostics. Cancer Cell 2015, 28, 666–676. [Google Scholar] [CrossRef] [Green Version]
- Lia, X.; Seebacher, N.A.; Hornicek, F.J.; Xiao, T.D.Z. Application of liquid biopsy in bone and soft tissue sarcomas: Present and Future. Cancer Lett. 2018, 439, 66–77. [Google Scholar] [CrossRef]
- Best, M.G.; Sol, N.; In ’t Veld, S.G.J.G.; Vancura, A.; Muller, M.; Niemeijer, A.-L.N.; Fejes, A.V.; Tjon Kon Fat, L.-A.; Huis In ’t Veld, A.E.; Leurs, C.; et al. Swarm Intelligence-Enhanced Detection of Non-Small-Cell Lung Cancer Using Tumor-Educated Platelets. Cancer Cell 2017, 32, 238–252.e9. [Google Scholar] [CrossRef]
- In ’t Veld, S.G.J.G.; Wurdinger, T. Tumor-educated platelets. Blood Spotlight 2019, 133, 2359–2364. [Google Scholar] [CrossRef] [PubMed]
- Wurdinger, T.; In ’t Veld, S.G.J.G.; Best, M.G. Platelet RNA as Pan-Tumor Biomarker for Cancer Detection. Cancer Res. 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McAllister, S.S.W.R. The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nat. Cell Biol. 2014, 16, 717–727. [Google Scholar] [CrossRef]
- Saito, M.; Ichikawa, J.; Ando, T.; Schoenecker, J.G.; Ohba, T.; Koyama, K.; Suzuki-Inoue, K.H.H. Platelet-Derived TGF-β Induces Tissue Factor Expression via the Smad3 Pathway in Osteosarcoma Cells. J. Bone Min. Res. 2018, 33, 2048–2058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, G.; Yamazaki, H.; Aibe, N.; Masui, K.; Sasaki, N.; Shimizu, D.; Kimoto, T.; Asai, J.; Wada, M.; Komori, S.; et al. Clinical Usefulness of the Platelet-to Lymphocyte Ratio in Patients with Angiosarcoma of the Face and Scalp. Int. J. Mol. Sci. 2017, 18, 2402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, W.K.; Liu, Z.L.; Shen, D.; Lin, Q.F.; Su, J.M.W. Prognostic performance of pre-treatment NLR and PLR in patients suffering from osteosarcoma. World J. Surg. Oncol. 2016, 14, 127. [Google Scholar] [CrossRef] [Green Version]
- Wysoczynski, M.; Liu, R.; Kucia, M.; Drukala, J.R.M. Thrombin regulates the metastatic potential of human rhabdomyosarcoma cells: Distinct role of PAR1 and PAR3 signaling. Mol. Cancer Res. 2010, 8, 677–690. [Google Scholar] [CrossRef] [Green Version]
- Skog, J.; Würdinger, T.; van Rijn, S.; Meijer, D.H.; Gainche, L.; Curry, W.T.; Carter, B.S.; Krichevsky, A.M.; Breakefield, X.O. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008, 10, 1470–1476. [Google Scholar] [CrossRef]
- Best, M.G.; In ’t Veld, S.G.J.G.; Sol, N.; Wurdinger, T. RNA sequencing and swarm intelligence–enhanced classification algorithm development for blood-based disease diagnostics using spliced blood platelet RNA. Nat. Protoc. 2019, 14, 1206–1234. [Google Scholar] [CrossRef]
- Nilsson, R.J.A.; Balaj, L.; Hulleman, E.; van Rijn, S.; Pegtel, D.M.; Walraven, M.; Widmark, A.; Gerritsen, W.R.; Verheul, H.M.; Vandertop, W.P.; et al. Blood platelets contain tumor-derived RNA biomarkers. Blood 2011, 118, 3680–3683. [Google Scholar] [CrossRef]
- Hofman, P.P.H. Pathologists and liquid biopsies: To be or not to be? Virchows Arch. 2016, 469, 601–609. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Training | Evaluation | Validation | |||
|---|---|---|---|---|---|---|
| Sarcoma cohort N | 21 | 19 | 17 | |||
| Median age (IQR) in years | 56 (19) | 60 (18) | 60 (19) | |||
| F/M % | 33/67 | 74/26 | 35/65 | |||
| Localized N (%) | 5 (24%) | 7 (37%) | 5 (29%) | |||
| Metastasized N (%) | 16 (76%) | 12 (63%) | 12 (71%) | |||
| Controls cohort N | 34 | 33 | 36 | |||
| Median age (IQR) in years | 61 (20.5) | 59 (18) | 54 (26.5) | |||
| F/M % | 55/45 | 85/15 | 58/42 | |||
| Former sarcoma /healthy donors N | 11 | 23 | 10 | 23 | 12 | 24 |
| Median age (IQR) in years | 72 (13.5) | 57 (23) | 59.5 (17) | 59 (17) | 64 (11.5) | 48 (22.5) |
| F/M % | 55/45 | 100/0 | 50/50 | 100/0 | 58/42 | 100/0 |
| Histologic Subtype | Training | Evaluation | Validation |
|---|---|---|---|
| Dedifferentiated liposarcoma | 3 | 1 | 2 |
| Myxoid liposarcoma | 3 | 2 | 1 |
| Pleomorphic liposarcoma | 1 | 1 | 0 |
| Leiomyosarcoma | 3 | 3 | 5 |
| Gastrointestinal Stromal Tumor | 5 | 5 | 6 |
| Myxofibrosarcoma | 1 | 2 | 0 |
| Undifferentiated pleomorphic sarcoma | 1 | 1 | 1 |
| Others | 1 | 1 | 0 |
| Angiosarcoma | 1 | 0 | 1 |
| Ewing sarcoma | 0 | 0 | 1 |
| Extraskeletal chondrosarcoma | 1 | 1 | 0 |
| Malignant peripheral nerve sheath tumor | |||
| Synovial sarcoma | 1 | 2 | 0 |
| Total | 21 | 19 | 17 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heinhuis, K.M.; In ’t Veld, S.G.J.G.; Dwarshuis, G.; van den Broek, D.; Sol, N.; Best, M.G.; van Coevorden, F.; Haas, R.L.; Beijnen, J.H.; van Houdt, W.J.; et al. RNA-Sequencing of Tumor-Educated Platelets, a Novel Biomarker for Blood-Based Sarcoma Diagnostics. Cancers 2020, 12, 1372. https://doi.org/10.3390/cancers12061372
Heinhuis KM, In ’t Veld SGJG, Dwarshuis G, van den Broek D, Sol N, Best MG, van Coevorden F, Haas RL, Beijnen JH, van Houdt WJ, et al. RNA-Sequencing of Tumor-Educated Platelets, a Novel Biomarker for Blood-Based Sarcoma Diagnostics. Cancers. 2020; 12(6):1372. https://doi.org/10.3390/cancers12061372
Chicago/Turabian StyleHeinhuis, Kimberley M., Sjors G. J. G. In ’t Veld, Govert Dwarshuis, Daan van den Broek, Nik Sol, Myron G. Best, Frits van Coevorden, Rick L. Haas, Jos H. Beijnen, Winan J. van Houdt, and et al. 2020. "RNA-Sequencing of Tumor-Educated Platelets, a Novel Biomarker for Blood-Based Sarcoma Diagnostics" Cancers 12, no. 6: 1372. https://doi.org/10.3390/cancers12061372