Transcranial Direct Current Stimulation of Primary Motor Cortex over Multiple Days Improves Motor Learning of a Complex Overhand Throwing Task
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
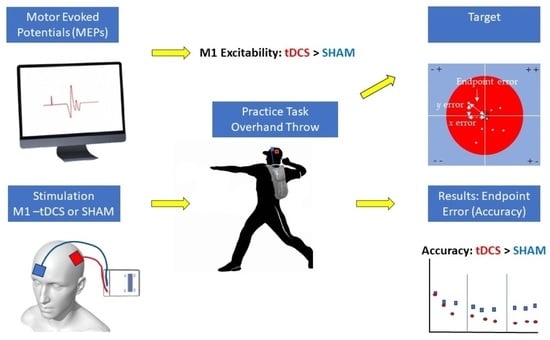
2.2. Experimental Design
2.3. Experimental Procedures
2.3.1. Pre-Test Block
2.3.2. Transcranial Magnetic Stimulation Quantification of tDCS Effects on Primary Motor Cortex Excitability
2.3.3. Practice Blocks
2.3.4. Post-Test Blocks
2.4. tDCS
2.5. Overhand Throwing Task
2.6. Data Analysis
2.7. Statistical Analysis
3. Results
3.1. Endpoint Error
3.2. MEP Amplitude
3.3. Associations between Increases in MEPs and Increases in Endpoint Accuracy
3.4. Control Measures
4. Discussion
4.1. Influence of M1-tDCS on Motor Learning
4.2. The Influence of tDCS on Primary Motor Cortex Excitability Measured by Transcranial Magnetic Stimulation
4.3. Clinical Implications
4.4. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buch, E.R.; Santarnecchi, E.; Antal, A.; Born, J.; Celnik, P.A.; Classen, J.; Gerloff, C.; Hallett, M.; Hummel, F.C.; Nitsche, M.A.; et al. Effects of tDCS on motor learning and memory formation: A consensus and critical position paper. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2017, 128, 589–603. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, A.N.; Porto, A.B.; Marcori, A.J.; Lage, G.M.; Altimari, L.R.; Alves Okazaki, V.H. Motor learning and tDCS: A systematic review on the dependency of the stimulation effect on motor task characteristics or tDCS assembly specifications. Neuropsychologia 2023, 179, 108463. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Liang, Z.; Wei, Z.; Liu, Y.; Wang, X. Effects of transcranial direct current stimulation on motor skills learning in healthy adults through the activation of different brain regions: A systematic review. Front. Hum. Neurosci. 2022, 16, 1021375. [Google Scholar] [CrossRef]
- Wang, B.; Xiao, S.; Yu, C.; Zhou, J.; Fu, W. Effects of Transcranial Direct Current Stimulation Combined With Physical Training on the Excitability of the Motor Cortex, Physical Performance, and Motor Learning: A Systematic Review. Front. Neurosci. 2021, 15, 648354. [Google Scholar] [CrossRef] [PubMed]
- Halakoo, S.; Ehsani, F.; Hosnian, M.; Zoghi, M.; Jaberzadeh, S. The comparative effects of unilateral and bilateral transcranial direct current stimulation on motor learning and motor performance: A systematic review of literature and meta-analysis. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2020, 72, 8–14. [Google Scholar] [CrossRef]
- Pascual-Leone, A.; Cammarota, A.; Wassermann, E.M.; Brasil-Neto, J.P.; Cohen, L.G.; Hallett, M. Modulation of motor cortical outputs to the reading hand of braille readers. Ann. Neurol. 1993, 34, 33–37. [Google Scholar] [CrossRef]
- Pascual-Leone, A.; Nguyet, D.; Cohen, L.G.; Brasil-Neto, J.P.; Cammarota, A.; Hallett, M. Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. J. Neurophysiol. 1995, 74, 1037–1045. [Google Scholar] [CrossRef]
- Pascual-Leone, A.; Wassermann, E.M.; Sadato, N.; Hallett, M. The role of reading activity on the modulation of motor cortical outputs to the reading hand in Braille readers. Ann. Neurol. 1995, 38, 910–915. [Google Scholar] [CrossRef]
- Broeder, S.; Nackaerts, E.; Heremans, E.; Vervoort, G.; Meesen, R.; Verheyden, G.; Nieuwboer, A. Transcranial direct current stimulation in Parkinson’s disease: Neurophysiological mechanisms and behavioral effects. Neurosci. Biobehav. Rev. 2015, 57, 105–117. [Google Scholar] [CrossRef]
- Stagg, C.J.; Nitsche, M.A. Physiological basis of transcranial direct current stimulation. Neurosci. A Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2011, 17, 37–53. [Google Scholar] [CrossRef]
- Bliem, B.; Muller-Dahlhaus, J.F.; Dinse, H.R.; Ziemann, U. Homeostatic metaplasticity in the human somatosensory cortex. J. Cogn. Neurosci. 2008, 20, 1517–1528. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.; Fischer, J.T.; Prichard, G.; Weiller, C.; Cohen, L.G.; Fritsch, B. Time- but Not Sleep-Dependent Consolidation of tDCS-Enhanced Visuomotor Skills. Cereb. Cortex 2015, 25, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.; Schambra, H.M.; Cohen, L.G.; Buch, E.R.; Fritsch, B.; Zarahn, E.; Celnik, P.A.; Krakauer, J.W. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc. Natl. Acad. Sci. USA 2009, 106, 1590–1595. [Google Scholar] [CrossRef]
- van Dun, K.; Bodranghien, F.; Manto, M.; Marien, P. Targeting the Cerebellum by Noninvasive Neurostimulation: A Review. Cerebellum 2017, 16, 695–741. [Google Scholar] [CrossRef]
- Meek, A.W.; Greenwell, D.; Poston, B.; Riley, Z.A. Anodal tDCS accelerates on-line learning of dart throwing. Neurosci. Lett. 2021, 764, 136211. [Google Scholar] [CrossRef]
- Wilson, M.A.; Greenwell, D.; Meek, A.W.; Poston, B.; Riley, Z.A. Neuroenhancement of a dexterous motor task with anodal tDCS. Brain Res. 2022, 1790, 147993. [Google Scholar] [CrossRef]
- Cordo, P.J.; Gurfinkel, V.S. Motor coordination can be fully understood only by studying complex movements. Prog. Brain Res. 2004, 143, 29–38. [Google Scholar] [CrossRef]
- Wulf, G.; Shea, C.H. Principles derived from the study of simple skills do not generalize to complex skill learning. Psychon. Bull. Rev. 2002, 9, 185–211. [Google Scholar] [CrossRef] [PubMed]
- Lemon, R.N. Neural control of dexterity: What has been achieved? Exp. Brain Res. 1999, 128, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Fregni, F.; Boggio, P.S.; Santos, M.C.; Lima, M.; Vieira, A.L.; Rigonatti, S.P.; Silva, M.T.; Barbosa, E.R.; Nitsche, M.A.; Pascual-Leone, A. Noninvasive cortical stimulation with transcranial direct current stimulation in Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2006, 21, 1693–1702. [Google Scholar] [CrossRef]
- Hummel, F.; Celnik, P.; Giraux, P.; Floel, A.; Wu, W.H.; Gerloff, C.; Cohen, L.G. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain A J. Neurol. 2005, 128, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Groppa, S.; Oliviero, A.; Eisen, A.; Quartarone, A.; Cohen, L.G.; Mall, V.; Kaelin-Lang, A.; Mima, T.; Rossi, S.; Thickbroom, G.W.; et al. A practical guide to diagnostic transcranial magnetic stimulation: Report of an IFCN committee. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2012, 123, 858–882. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.K.; de Albuquerque, L.L.; Pantovic, M.; Fischer, K.M.; Guadagnoli, M.A.; Riley, Z.A.; Poston, B. Cerebellar Transcranial Direct Current Stimulation Enhances Motor Learning in a Complex Overhand Throwing Task. Cerebellum 2019, 18, 813–816. [Google Scholar] [CrossRef] [PubMed]
- Poston, B.; Kukke, S.N.; Paine, R.W.; Francis, S.; Hallett, M. Cortical silent period duration and its implications for surround inhibition of a hand muscle. Eur. J. Neurosci. 2012, 36, 2964–2971. [Google Scholar] [CrossRef] [PubMed]
- Fricke, K.; Seeber, A.A.; Thirugnanasambandam, N.; Paulus, W.; Nitsche, M.A.; Rothwell, J.C. Time course of the induction of homeostatic plasticity generated by repeated transcranial direct current stimulation of the human motor cortex. J. Neurophysiol. 2011, 105, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Monte-Silva, K.; Kuo, M.F.; Hessenthaler, S.; Fresnoza, S.; Liebetanz, D.; Paulus, W.; Nitsche, M.A. Induction of late LTP-like plasticity in the human motor cortex by repeated non-invasive brain stimulation. Brain Stimul. 2013, 6, 424–432. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000, 527 Pt. 3, 633–639. [Google Scholar] [CrossRef]
- Thirugnanasambandam, N.; Sparing, R.; Dafotakis, M.; Meister, I.G.; Paulus, W.; Nitsche, M.A.; Fink, G.R. Isometric contraction interferes with transcranial direct current stimulation (tDCS) induced plasticity: Evidence of state-dependent neuromodulation in human motor cortex. Restor. Neurol. Neurosci. 2011, 29, 311–320. [Google Scholar] [CrossRef]
- Horvath, J.C.; Carter, O.; Forte, J.D. Transcranial direct current stimulation: Five important issues we aren’t discussing (but probably should be). Front. Syst. Neurosci. 2014, 8, 2. [Google Scholar] [CrossRef]
- Quartarone, A.; Morgante, F.; Bagnato, S.; Rizzo, V.; Sant’Angelo, A.; Aiello, E.; Reggio, E.; Battaglia, F.; Messina, C.; Girlanda, P. Long lasting effects of transcranial direct current stimulation on motor imagery. Neuroreport 2004, 15, 1287–1291. [Google Scholar] [CrossRef] [PubMed]
- Antal, A.; Terney, D.; Poreisz, C.; Paulus, W. Towards unravelling task-related modulations of neuroplastic changes induced in the human motor cortex. Eur. J. Neurosci. 2007, 26, 2687–2691. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Alonso, V.; Cheeran, B.; Fernandez-del-Olmo, M. Relationship Between Non-invasive Brain Stimulation-induced Plasticity and Capacity for Motor Learning. Brain Stimul. 2015, 8, 1209–1219. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Cohen, L.G.; Wassermann, E.M.; Priori, A.; Lang, N.; Antal, A.; Paulus, W.; Hummel, F.; Boggio, P.S.; Fregni, F.; et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008, 1, 206–223. [Google Scholar] [CrossRef] [PubMed]
- Urbin, M.A. Sensorimotor control in overarm throwing. Mot. Control 2012, 16, 560–578. [Google Scholar] [CrossRef] [PubMed]
- Fleisig, G.S.; Barrentine, S.W.; Escamilla, R.F.; Andrews, J.R. Biomechanics of overhand throwing with implications for injuries. Sports Med. 1996, 21, 421–437. [Google Scholar] [CrossRef]
- Timmann, D.; Lee, P.; Watts, S.; Hore, J. Kinematics of arm joint rotations in cerebellar and unskilled subjects associated with the inability to throw fast. Cerebellum 2008, 7, 366–378. [Google Scholar] [CrossRef]
- Hirashima, M.; Kudo, K.; Ohtsuki, T. Utilization and compensation of interaction torques during ball-throwing movements. J. Neurophysiol. 2003, 89, 1784–1796. [Google Scholar] [CrossRef]
- Hirashima, M.; Kudo, K.; Watarai, K.; Ohtsuki, T. Control of 3D limb dynamics in unconstrained overarm throws of different speeds performed by skilled baseball players. J. Neurophysiol. 2007, 97, 680–691. [Google Scholar] [CrossRef]
- Hirashima, M.; Ohtsuki, T. Exploring the mechanism of skilled overarm throwing. Exerc. Sport. Sci. Rev. 2008, 36, 205–211. [Google Scholar] [CrossRef]
- Debicki, D.B.; Gribble, P.L.; Watts, S.; Hore, J. Kinematics of wrist joint flexion in overarm throws made by skilled subjects. Exp. Brain Res. 2004, 154, 382–394. [Google Scholar] [CrossRef]
- Hore, J.; Timmann, D.; Watts, S. Disorders in timing and force of finger opening in overarm throws made by cerebellar subjects. Ann. N. Y. Acad. Sci. 2002, 978, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hore, J.; Watts, S. Skilled throwers use physics to time ball release to the nearest millisecond. J. Neurophysiol. 2011, 106, 2024–2033. [Google Scholar] [CrossRef] [PubMed]
- Flament, D.; Hore, J. Movement and electromyographic disorders associated with cerebellar dysmetria. J. Neurophysiol. 1986, 55, 1221–1233. [Google Scholar] [CrossRef]
- Timmann, D.; Citron, R.; Watts, S.; Hore, J. Increased variability in finger position occurs throughout overarm throws made by cerebellar and unskilled subjects. J. Neurophysiol. 2001, 86, 2690–2702. [Google Scholar] [CrossRef] [PubMed]
- Timmann, D.; Watts, S.; Hore, J. Failure of cerebellar patients to time finger opening precisely causes ball high-low inaccuracy in overarm throws. J. Neurophysiol. 1999, 82, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, L.L.; Fischer, K.M.; Pauls, A.L.; Pantovic, M.; Guadagnoli, M.A.; Riley, Z.A.; Poston, B. An acute application of transcranial random noise stimulation does not enhance motor skill acquisition or retention in a golf putting task. Hum. Mov. Sci. 2019, 66, 241–248. [Google Scholar] [CrossRef]
- Lima de Albuquerque, L.; Pantovic, M.; Clingo, M.; Fischer, K.; Jalene, S.; Landers, M.; Mari, Z.; Poston, B. An Acute Application of Cerebellar Transcranial Direct Current Stimulation Does Not Improve Motor Performance in Parkinson’s Disease. Brain Sci. 2020, 10, 735. [Google Scholar] [CrossRef]
- de Albuquerque, L.L.; Pantovic, M.; Clingo, M.G.; Fischer, K.M.; Jalene, S.; Landers, M.R.; Mari, Z.; Poston, B. Long-Term Application of Cerebellar Transcranial Direct Current Stimulation Does Not Improve Motor Learning in Parkinson’s Disease. Cerebellum 2022, 21, 333–349. [Google Scholar] [CrossRef]
- Poston, B.; Van Gemmert, A.W.; Sharma, S.; Chakrabarti, S.; Zavaremi, S.H.; Stelmach, G. Movement trajectory smoothness is not associated with the endpoint accuracy of rapid multi-joint arm movements in young and older adults. Acta Psychol. 2013, 143, 157–167. [Google Scholar] [CrossRef]
- Cantarero, G.; Spampinato, D.; Reis, J.; Ajagbe, L.; Thompson, T.; Kulkarni, K.; Celnik, P. Cerebellar direct current stimulation enhances on-line motor skill acquisition through an effect on accuracy. J. Neurosci. Off. J. Soc. Neurosci. 2015, 35, 3285–3290. [Google Scholar] [CrossRef] [PubMed]
- Bologna, M.; Rocchi, L.; Paparella, G.; Nardella, A.; Li Voti, P.; Conte, A.; Kojovic, M.; Rothwell, J.C.; Berardelli, A. Reversal of Practice-related Effects on Corticospinal Excitability has no Immediate Effect on Behavioral Outcome. Brain Stimul. 2015, 8, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, D.; Celnik, P. Temporal dynamics of cerebellar and motor cortex physiological processes during motor skill learning. Sci. Rep. 2017, 7, 40715. [Google Scholar] [CrossRef]
- Spampinato, D.; Celnik, P. Multiple Motor Learning Processes in Humans: Defining Their Neurophysiological Bases. Neurosci. A Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2021, 27, 246–267. [Google Scholar] [CrossRef]
- Krakauer, J.W.; Mazzoni, P. Human sensorimotor learning: Adaptation, skill, and beyond. Curr. Opin. Neurobiol. 2011, 21, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Labruna, L.; Jamil, A.; Fresnoza, S.; Batsikadze, G.; Kuo, M.F.; Vanderschelden, B.; Ivry, R.B.; Nitsche, M.A. Efficacy of Anodal Transcranial Direct Current Stimulation is Related to Sensitivity to Transcranial Magnetic Stimulation. Brain Stimul. 2016, 9, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Bestmann, S.; Krakauer, J.W. The uses and interpretations of the motor-evoked potential for understanding behaviour. Exp. Brain Res. 2015, 233, 679–689. [Google Scholar] [CrossRef]
- Horvath, J.C.; Forte, J.D.; Carter, O. Evidence that transcranial direct current stimulation (tDCS) generates little-to-no reliable neurophysiologic effect beyond MEP amplitude modulation in healthy human subjects: A systematic review. Neuropsychologia 2015, 66, 213–236. [Google Scholar] [CrossRef]
- Dissanayaka, T.; Zoghi, M.; Farrell, M.; Egan, G.F.; Jaberzadeh, S. Does transcranial electrical stimulation enhance corticospinal excitability of the motor cortex in healthy individuals? A systematic review and meta-analysis. Eur. J. Neurosci. 2017, 46, 1968–1990. [Google Scholar] [CrossRef]
- Wiethoff, S.; Hamada, M.; Rothwell, J.C. Variability in response to transcranial direct current stimulation of the motor cortex. Brain Stimul. 2014, 7, 468–475. [Google Scholar] [CrossRef]
- Lidstone, D.E.; Miah, F.Z.; Poston, B.; Beasley, J.F.; Dufek, J.S. Manual dexterity in children with autism spectrum disorder: A cross-syndrome approach. Res. Autism Spect. Dis. 2020, 73, 1–8. [Google Scholar] [CrossRef]
- Lidstone, D.E.; Miah, F.Z.; Poston, B.; Beasley, J.F.; Mostofsky, S.H.; Dufek, J.S. Children with Autism Spectrum Disorder Show Impairments During Dynamic Versus Static Grip-force Tracking. Autism Res. 2020, 13, 2177–2189. [Google Scholar] [CrossRef] [PubMed]
- Di Benedetto, S.; Battaglino, A.; Abuin-Porras, V.; Sanchez-Romero, E.A.; Cantero-Tellez, R.; Valdes, K.; Villafane, J.H. Evaluation of the factors that impact upper limb coordination in children with cerebral palsy: A narrative review. Retos-Nuev. Tend. Educ. 2023, 48, 470–480. [Google Scholar] [CrossRef]
- Moura, R.C.F.; Santos, C.; Collange Grecco, L.; Albertini, G.; Cimolin, V.; Galli, M.; Oliveira, C. Effects of a single session of transcranial direct current stimulation on upper limb movements in children with cerebral palsy: A randomized, sham-controlled study. Dev. Neurorehabilit. 2017, 20, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Metelski, N.; Gu, Y.; Quinn, L.; Friel, K.M.; Gordon, A.M. Safety and efficacy of non-invasive brain stimulation for the upper extremities in children with cerebral palsy: A systematic review. Dev. Med. Child. Neurol. 2023; Online ahead of print. [Google Scholar] [CrossRef]
- Luckhardt, C.; Boxhoorn, S.; Schutz, M.; Fann, N.; Freitag, C.M. Brain stimulation by tDCS as treatment option in Autism Spectrum Disorder-A systematic literature review. Prog. Brain Res. 2021, 264, 233–257. [Google Scholar] [CrossRef]
- Simpson, M.W.; Mak, M. The effect of transcranial direct current stimulation on upper limb motor performance in Parkinson’s disease: A systematic review. J. Neurol. 2020, 267, 3479–3488. [Google Scholar] [CrossRef]
- Chen, G.; Wu, M.; Chen, J.; Cai, G.; Liu, Q.; Zhao, Y.; Huang, Z.; Lan, Y. Non-invasive brain stimulation effectively improves post-stroke sensory impairment: A systematic review and meta-analysis. J. Neural. Transm. 2023, 130, 1219–1230. [Google Scholar] [CrossRef]
- Schlaug, G.; Renga, V.; Nair, D. Transcranial direct current stimulation in stroke recovery. Arch. Neurol. 2008, 65, 1571–1576. [Google Scholar] [CrossRef]
- Hamilton, K.; Smith, K.; Winn, K.; Oliver, B.; Newland, P.; Hendricks-Ferguson, V. Quantifying Fatigue Using Electrophysiological Techniques and Non-invasive Brain Stimulation in People With Multiple Sclerosis- A Review and Discussion. Biol. Res. Nurs. 2023; Online ahead of print. [Google Scholar] [CrossRef]
- Proessl, F.; Poston, B.; Rudroff, T. Does a single application of anodal tDCS improve knee extensor fatigability in people with multiple sclerosis? Brain Stimul. 2018, 11, 1388–1390. [Google Scholar] [CrossRef]
- Consideration of Sample Size in Neuroscience Studies. J. Neurosci. Off. J. Soc. Neurosci. 2020, 40, 4076–4077. [CrossRef]
- Szucs, D.; Ioannidis, J.P. Sample size evolution in neuroimaging research: An evaluation of highly-cited studies (1990–2012) and of latest practices (2017–2018) in high-impact journals. NeuroImage 2020, 221, 117164. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, M.; Zoghi, M.; Jaberzadeh, S. Biological and anatomical factors influencing interindividual variability to noninvasive brain stimulation of the primary motor cortex: A systematic review and meta-analysis. Rev. Neurosci. 2018, 29, 199–222. [Google Scholar] [CrossRef] [PubMed]
- Li, L.M.; Uehara, K.; Hanakawa, T. The contribution of interindividual factors to variability of response in transcranial direct current stimulation studies. Front. Cell Neurosci. 2015, 9, 181. [Google Scholar] [CrossRef] [PubMed]
- Miterko, L.N.; Baker, K.B.; Beckinghausen, J.; Bradnam, L.V.; Cheng, M.Y.; Cooperrider, J.; DeLong, M.R.; Gornati, S.V.; Hallett, M.; Heck, D.H.; et al. Consensus Paper: Experimental Neurostimulation of the Cerebellum. Cerebellum 2019, 18, 1064–1097. [Google Scholar] [CrossRef]
- Herzog, R.; Bolte, C.; Radecke, J.O.; von Moller, K.; Lencer, R.; Tzvi, E.; Munchau, A.; Baumer, T.; Weissbach, A. Neuronavigated Cerebellar 50 Hz tACS: Attenuation of Stimulation Effects by Motor Sequence Learning. Biomedicines 2023, 11, 2218. [Google Scholar] [CrossRef]
- Hsu, G.; Shereen, A.D.; Cohen, L.G.; Parra, L.C. Robust enhancement of motor sequence learning with 4 mA transcranial electric stimulation. Brain Stimul. 2023, 16, 56–67. [Google Scholar] [CrossRef]
- Naros, G.; Geyer, M.; Koch, S.; Mayr, L.; Ellinger, T.; Grimm, F.; Gharabaghi, A. Enhanced motor learning with bilateral transcranial direct current stimulation: Impact of polarity or current flow direction? Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2016, 127, 2119–2126. [Google Scholar] [CrossRef]
- Workman, C.D.; Fietsam, A.C.; Uc, E.Y.; Rudroff, T. Cerebellar Transcranial Direct Current Stimulation in People with Parkinson’s Disease: A Pilot Study. Brain Sci. 2020, 10, 96. [Google Scholar] [CrossRef]
- Priori, A.; Hallett, M.; Rothwell, J.C. Repetitive transcranial magnetic stimulation or transcranial direct current stimulation? Brain Stimul. 2009, 2, 241–245. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pantovic, M.; Albuquerque, L.L.d.; Mastrantonio, S.; Pomerantz, A.S.; Wilkins, E.W.; Riley, Z.A.; Guadagnoli, M.A.; Poston, B. Transcranial Direct Current Stimulation of Primary Motor Cortex over Multiple Days Improves Motor Learning of a Complex Overhand Throwing Task. Brain Sci. 2023, 13, 1441. https://doi.org/10.3390/brainsci13101441
Pantovic M, Albuquerque LLd, Mastrantonio S, Pomerantz AS, Wilkins EW, Riley ZA, Guadagnoli MA, Poston B. Transcranial Direct Current Stimulation of Primary Motor Cortex over Multiple Days Improves Motor Learning of a Complex Overhand Throwing Task. Brain Sciences. 2023; 13(10):1441. https://doi.org/10.3390/brainsci13101441
Chicago/Turabian StylePantovic, Milan, Lidio Lima de Albuquerque, Sierra Mastrantonio, Austin S. Pomerantz, Erik W. Wilkins, Zachary A. Riley, Mark A. Guadagnoli, and Brach Poston. 2023. "Transcranial Direct Current Stimulation of Primary Motor Cortex over Multiple Days Improves Motor Learning of a Complex Overhand Throwing Task" Brain Sciences 13, no. 10: 1441. https://doi.org/10.3390/brainsci13101441