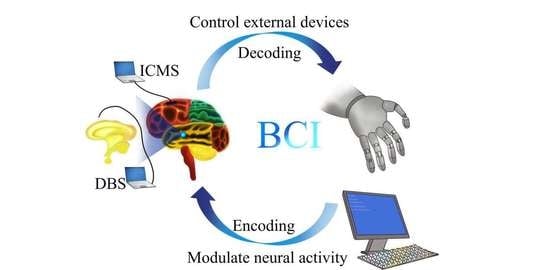
Modulating Brain Activity with Invasive Brain–Computer Interface: A Narrative Review
Abstract
:1. Introduction
2. The Framework of Neural Decoding and Encoding
3. Application of Decoding for Controlling External Devices
4. Modulating Cortical Activity by ICMS
4.1. ICMS for Restoration of Tactile Feedback
4.2. ICMS for Restoration of Visual Sense
5. Modulating Brain Activity by DBS
5.1. DBS for Neurological Disorders
5.2. DBS for Neuropsychiatric Disorders
6. Technological Challenges and Future Directions
7. Ethical Issues of Invasive BCI
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wolpaw, J.R.; Birbaumer, N.; Heetderks, W.J.; McFarland, D.J.; Peckham, P.H.; Schalk, G.; Donchin, E.; Quatrano, L.A.; Robinson, C.J.; Vaughan, T.M. Brain-computer interface technology: A review of the first international meeting. IEEE Trans. Rehabil. Eng. 2000, 8, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Lebedev, M.A.; Nicolelis, M.A. Brain-Machine Interfaces: From Basic Science to Neuroprostheses and Neurorehabilitation. Physiol. Rev. 2017, 97, 767–837. [Google Scholar] [CrossRef] [PubMed]
- Moran, D. Evolution of brain-computer interface: Action potentials, local field potentials and electrocorticograms. Curr. Opin. Neurobiol. 2010, 20, 741–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, S.; Mamun, K.A.; Ahmed, K.; Mostafa, R.; Naik, G.R.; Darvishi, S.; Khandoker, A.H.; Baumert, M. Progress in Brain Computer Interface: Challenges and Opportunities. Front. Syst. Neurosci. 2021, 15, 578875. [Google Scholar] [CrossRef] [PubMed]
- Hubel, D.H. Tungsten Microelectrode for Recording from Single Units. Science 1957, 125, 549–550. [Google Scholar] [CrossRef] [PubMed]
- Bekhtereva, N.P.; Grachev, K.V.; Orlova, A.N.; Iatsuksl. Utilization of multiple electrodes implanted in the subcortical structure of the human brain for the treatment of hyperkinesis. Zh. Nevropatol. Psikhiatr. Im. S. S. Korsakova 1963, 63, 3–8. [Google Scholar]
- Fetz, E.E. Operant conditioning of cortical unit activity. Science 1969, 163, 955–958. [Google Scholar] [CrossRef] [Green Version]
- Asanuma, H.; Arnold, A.; Zarzecki, P. Further study on the excitation of pyramidal tract cells by intracortical microstimulation. Exp. Brain Res. 1976, 26, 443–461. [Google Scholar] [CrossRef]
- Dobelle, W.H.; Mladejovsky, M.G.; Evans, J.R.; Roberts, T.S.; Girvin, J.P. “Braille” reading by a blind volunteer by visual cortex stimulation. Nature 1976, 259, 111–112. [Google Scholar] [CrossRef]
- Georgopoulos, A.P.; Schwartz, A.B.; Kettner, R.E. Neuronal population coding of movement direction. Science 1986, 233, 1416–1419. [Google Scholar] [CrossRef] [Green Version]
- Brice, J.; McLellan, L. Suppression of intention tremor by contingent deep-brain stimulation. Lancet 1980, 1, 1221–1222. [Google Scholar] [CrossRef]
- Benabid, A.L.; Pollak, P.; Gao, D.; Hoffmann, D.; Limousin, P.; Gay, E.; Payen, I.; Benazzouz, A. Chronic electrical stimulation of the ventralis intermedius nucleus of the thalamus as a treatment of movement disorders. J. Neurosurg. 1996, 84, 203–214. [Google Scholar] [CrossRef]
- Benabid, A.L.; Benazzouz, A.; Hoffmann, D.; Limousin, P.; Krack, P.; Pollak, P. Long-term electrical inhibition of deep brain targets in movement disorders. Mov. Disord. 1998, 13 (Suppl. 3), 119–125. [Google Scholar] [CrossRef]
- Boraud, T.; Bezard, E.; Bioulac, B.; Gross, C. High frequency stimulation of the internal Globus Pallidus (GPi) simultaneously improves parkinsonian symptoms and reduces the firing frequency of GPi neurons in the MPTP-treated monkey. Neurosci. Lett. 1996, 215, 17–20. [Google Scholar] [CrossRef]
- Velliste, M.; Perel, S.; Spalding, M.C.; Whitford, A.S.; Schwartz, A.B. Cortical control of a prosthetic arm for self-feeding. Nature 2008, 453, 1098–1101. [Google Scholar] [CrossRef] [Green Version]
- Hochberg, L.R.; Bacher, D.; Jarosiewicz, B.; Masse, N.Y.; Simeral, J.D.; Vogel, J.; Haddadin, S.; Liu, J.; Cash, S.S.; van der Smagt, P.; et al. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature 2012, 485, 372–375. [Google Scholar] [CrossRef] [Green Version]
- Capogrosso, M.; Milekovic, T.; Borton, D.; Wagner, F.; Moraud, E.M.; Mignardot, J.B.; Buse, N.; Gandar, J.; Barraud, Q.; Xing, D.; et al. A brain-spine interface alleviating gait deficits after spinal cord injury in primates. Nature 2016, 539, 284–288. [Google Scholar] [CrossRef] [Green Version]
- Musk, E.; Neuralink. An Integrated Brain-Machine Interface Platform with Thousands of Channels. J. Med. Internet Res. 2019, 21, e16194. [Google Scholar] [CrossRef]
- Scangos, K.W.; Khambhati, A.N.; Daly, P.M.; Makhoul, G.S.; Sugrue, L.P.; Zamanian, H.; Liu, T.X.; Rao, V.R.; Sellers, K.K.; Dawes, H.E.; et al. Closed-loop neuromodulation in an individual with treatment-resistant depression. Nat. Med. 2021, 27, 1696–1700. [Google Scholar] [CrossRef]
- Bacher, D.; Jarosiewicz, B.; Masse, N.Y.; Stavisky, S.D.; Simeral, J.D.; Newell, K.; Oakley, E.M.; Cash, S.S.; Friehs, G.; Hochberg, L.R. Neural Point-and-Click Communication by a Person with Incomplete Locked-In Syndrome. Neurorehabil. Neural Repair 2015, 29, 462–471. [Google Scholar] [CrossRef] [Green Version]
- Oxley, T.J.; Yoo, P.E.; Rind, G.S.; Ronayne, S.M.; Lee, C.M.S.; Bird, C.; Hampshire, V.; Sharma, R.P.; Morokoff, A.; Williams, D.L.; et al. Motor neuroprosthesis implanted with neurointerventional surgery improves capacity for activities of daily living tasks in severe paralysis: First in-human experience. J. Neurointerv. Surg. 2021, 13, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Brandman, D.M.; Hosman, T.; Saab, J.; Burkhart, M.C.; Shanahan, B.E.; Ciancibello, J.G.; Sarma, A.A.; Milstein, D.J.; Vargas-Irwin, C.E.; Franco, B.; et al. Rapid calibration of an intracortical brain-computer interface for people with tetraplegia. J. Neural Eng. 2018, 15, 026007. [Google Scholar] [CrossRef] [PubMed]
- Zabcikova, M.; Koudelkova, Z.; Jasek, R.; Lorenzo Navarro, J.J. Recent advances and current trends in brain-computer interface research and their applications. Int. J. Dev. Neurosci. 2021, 82, 107–123. [Google Scholar] [CrossRef] [PubMed]
- Vidal, G.W.; Rynes, M.L.; Kelliher, Z.; Goodwin, S.J. Review of Brain-Machine Interfaces Used in Neural Prosthetics with New Perspective on Somatosensory Feedback through Method of Signal Breakdown. Scientifica 2016, 2016, 8956432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camacho-Conde, J.A.; Gonzalez-Bermudez, M.D.R.; Carretero-Rey, M.; Khan, Z.U. Brain stimulation: A therapeutic approach for the treatment of neurological disorders. CNS Neurosci. Ther. 2022, 28, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Osborn, L.E.; Christie, B.P.; McMullen, D.P.; Nickl, R.W.; Thompson, M.C.; Pawar, A.S.; Thomas, T.M.; Alejandro Anaya, M.; Crone, N.E.; Wester, B.A.; et al. Intracortical microstimulation of somatosensory cortex enables object identification through perceived sensations. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC) Mexico, 1–5 November 2021; pp. 6259–6262. [Google Scholar] [CrossRef]
- Hughes, C.L.; Flesher, S.N.; Weiss, J.M.; Downey, J.E.; Boninger, M.; Collinger, J.L.; Gaunt, R.A. Neural stimulation and recording performance in human sensorimotor cortex over 1500 days. J. Neural Eng. 2021, 18, 045012. [Google Scholar] [CrossRef]
- Bashford, L.; Rosenthal, I.; Kellis, S.; Pejsa, K.; Kramer, D.; Lee, B.; Liu, C.; Andersen, R.A. The Neurophysiological Representation of Imagined Somatosensory Percepts in Human Cortex. J. Neurosci. 2021, 41, 2177–2185. [Google Scholar] [CrossRef]
- Bucur, M.; Papagno, C. Deep Brain Stimulation in Parkinson Disease: A Meta-analysis of the Long-term Neuropsychological Outcomes. Neuropsychol. Rev. 2022, 103, 956–967. [Google Scholar] [CrossRef]
- Lisoni, J.; Barlati, S.; Deste, G.; Ceraso, A.; Nibbio, G.; Baldacci, G.; Vita, A. Efficacy and tolerability of Brain Stimulation interventions in Borderline Personality Disorder: State of the art and future perspectives—A systematic review. Prog. Neuropsychopharmacol. Biol. Psychiatry 2022, 116, 110537. [Google Scholar] [CrossRef]
- Chandra, V.; Hilliard, J.D.; Foote, K.D. Deep brain stimulation for the treatment of tremor. J. Neurol. Sci. 2022, 435, 120190. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, Z.; Zheng, H.; Li, T.; Chen, K.; Wang, X.; Liu, C.; Xu, L.; Wu, X.; Lin, D.; et al. The combination of brain-computer interfaces and artificial intelligence: Applications and challenges. Ann. Transl. Med. 2020, 8, 712. [Google Scholar] [CrossRef]
- Hughes, C.; Herrera, A.; Gaunt, R.; Collinger, J. Bidirectional brain-computer interfaces. Handb. Clin. Neurol. 2020, 168, 163–181. [Google Scholar] [CrossRef]
- Adrian, E.D. The impulses produced by sensory nerve endings: Part I. J. Physiol. 1926, 61, 49–72. [Google Scholar] [CrossRef]
- Long, K.H.; Lieber, J.D.; Bensmaia, S.J. Texture is encoded in precise temporal spiking patterns in primate somatosensory cortex. Nat. Commun. 2022, 13, 1311. [Google Scholar] [CrossRef]
- Xu, Q.; Shen, J.; Ran, X.; Tang, H.; Pan, G.; Liu, J.K. Robust Transcoding Sensory Information with Neural Spikes. IEEE Trans. Neural Netw. Learn. Syst. 2021, 33, 1935–1946. [Google Scholar] [CrossRef]
- Kennedy, P.R.; Bakay, R.A. Restoration of neural output from a paralyzed patient by a direct brain connection. Neuroreport 1998, 9, 1707–1711. [Google Scholar] [CrossRef]
- Kettner, R.E.; Schwartz, A.B.; Georgopoulos, A.P. Primate motor cortex and free arm movements to visual targets in three-dimensional space. III. Positional gradients and population coding of movement direction from various movement origins. J. Neurosci. 1988, 8, 2938–2947. [Google Scholar] [CrossRef]
- Salinas, E.; Abbott, L.F. Vector reconstruction from firing rates. J. Comput. Neurosci. 1994, 1, 89–107. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.H.; Chen, Y.Y.; Lin, S.H.; Liao, L.D.; Lu, H.H.; Wang, C.F.; Chen, P.C.; Lo, Y.C.; Phan, T.D.; Chao, H.Y.; et al. A Sliced Inverse Regression (SIR) Decoding the Forelimb Movement from Neuronal Spikes in the Rat Motor Cortex. Front. Neurosci. 2016, 10, 556. [Google Scholar] [CrossRef] [Green Version]
- Yu, B.M.; Kemere, C.; Santhanam, G.; Afshar, A.; Ryu, S.I.; Meng, T.H.; Sahani, M.; Shenoy, K.V. Mixture of trajectory models for neural decoding of goal-directed movements. J. Neurophysiol. 2007, 97, 3763–3780. [Google Scholar] [CrossRef]
- Shenoy, K.V.; Carmena, J.M. Combining decoder design and neural adaptation in brain-machine interfaces. Neuron 2014, 84, 665–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapin, J.K.; Moxon, K.A.; Markowitz, R.S.; Nicolelis, M.A. Real-time control of a robot arm using simultaneously recorded neurons in the motor cortex. Nat. Neurosci. 1999, 2, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Serruya, M.D.; Hatsopoulos, N.G.; Paninski, L.; Fellows, M.R.; Donoghue, J.P. Instant neural control of a movement signal. Nature 2002, 416, 141–142. [Google Scholar] [CrossRef] [PubMed]
- Donoghue, J.P.; Nurmikko, A.; Black, M.; Hochberg, L.R. Assistive technology and robotic control using motor cortex ensemble-based neural interface systems in humans with tetraplegia. J. Physiol. 2007, 579, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Simeral, J.D.; Kim, S.P.; Black, M.J.; Donoghue, J.P.; Hochberg, L.R. Neural control of cursor trajectory and click by a human with tetraplegia 1000 days after implant of an intracortical microelectrode array. J. Neural Eng. 2011, 8, 025027. [Google Scholar] [CrossRef] [Green Version]
- Handelman, D.A.; Osborn, L.E.; Thomas, T.M.; Badger, A.R.; Thompson, M.; Nickl, R.W.; Anaya, M.A.; Wormley, J.M.; Cantarero, G.L.; McMullen, D.; et al. Shared Control of Bimanual Robotic Limbs with a Brain-Machine Interface for Self-Feeding. Front. Neurorobot. 2022, 16, 918001. [Google Scholar] [CrossRef]
- O’Doherty, J.; Lebedev, M.; Hanson, T.; Fitzsimmons, N.; Nicolelis, M. A brain-machine interface instructed by direct intracortical microstimulation. Front. Integr. Neurosci. 2009, 3, 20. [Google Scholar] [CrossRef] [Green Version]
- Anumanchipalli, G.K.; Chartier, J.; Chang, E.F. Speech synthesis from neural decoding of spoken sentences. Nature 2019, 568, 493–498. [Google Scholar] [CrossRef]
- Willett, F.R.; Avansino, D.T.; Hochberg, L.R.; Henderson, J.M.; Shenoy, K.V. High-performance brain-to-text communication via handwriting. Nature 2021, 593, 249–254. [Google Scholar] [CrossRef]
- Dekleva, B.M.; Weiss, J.M.; Boninger, M.L.; Collinger, J.L. Generalizable cursor click decoding using grasp-related neural transients. J. Neural Eng. 2021, 18, 0460e9. [Google Scholar] [CrossRef]
- Yoo, J.; Shoaran, M. Neural interface systems with on-device computing: Machine learning and neuromorphic architectures. Curr. Opin. Biotechnol. 2021, 72, 95–101. [Google Scholar] [CrossRef]
- Cao, C.; Liu, F.; Tan, H.; Song, D.; Shu, W.; Li, W.; Zhou, Y.; Bo, X.; Xie, Z. Deep Learning and Its Applications in Biomedicine. Genom. Proteom. Bioinform. 2018, 16, 17–32. [Google Scholar] [CrossRef]
- Ghanbari, A.; Lee, C.M.; Read, H.L.; Stevenson, I.H. Modeling stimulus-dependent variability improves decoding of population neural responses. J. Neural Eng. 2019, 16, 066018. [Google Scholar] [CrossRef]
- Hosokawa, T.; Xu, M.; Katori, Y.; Yamada, M.; Aihara, K.; Tsutsui, K.I. Monkey Prefrontal Single-Unit Activity Reflecting Category-Based Logical Thinking Process and Its Neural Network Model. J. Neurosci. 2022, 42, 6380–6391. [Google Scholar] [CrossRef]
- Hagen, E.; Magnusson, S.H.; Ness, T.V.; Halnes, G.; Babu, P.N.; Linssen, C.; Morrison, A.; Einevoll, G.T. Brain signal predictions from multi-scale networks using a linearized framework. PLoS Comput. Biol. 2022, 18, e1010353. [Google Scholar] [CrossRef]
- Yamazaki, K.; Vo-Ho, V.K.; Bulsara, D.; Le, N. Spiking Neural Networks and Their Applications: A Review. Brain Sci. 2022, 12, 863. [Google Scholar] [CrossRef]
- Heydari Beni, N.; Foodeh, R.; Shalchyan, V.; Daliri, M.R. Force decoding using local field potentials in primary motor cortex: PLS or Kalman filter regression? Australas. Phys. Eng. Sci. Med. 2020, 43, 175–186. [Google Scholar] [CrossRef]
- Asgharpour, M.; Foodeh, R.; Daliri, M.R. Regularized Kalman filter for brain-computer interfaces using local field potential signals. J. Neurosci. Methods 2021, 350, 109022. [Google Scholar] [CrossRef]
- Liu, D.; Xu, X.; Li, D.; Li, J.; Yu, X.; Ling, Z.; Hong, B. Intracranial brain-computer interface spelling using localized visual motion response. Neuroimage 2022, 258, 119363. [Google Scholar] [CrossRef]
- Kashefi, M.; Daliri, M.R. A stack LSTM structure for decoding continuous force from local field potential signal of primary motor cortex (M1). BMC Bioinform. 2021, 22, 26. [Google Scholar] [CrossRef]
- Penfield, W.; Boldrey, E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain 1937, 60, 389–443. [Google Scholar] [CrossRef]
- Murphy, J.T.; Kwan, H.C.; Wong, Y.C. Differential effects of reciprocal wrist torques on responses of somatotopically identified neurons of precentral cortex in awake primates. Brain Res. 1979, 172, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Sessle, B.J.; Wiesendanger, M. Structural and functional definition of the motor cortex in the monkey (Macaca fascicularis). J. Physiol. 1982, 323, 245–265. [Google Scholar] [CrossRef] [PubMed]
- Waters, R.S.; Samulack, D.D.; Dykes, R.W.; McKinley, P.A. Topographic organization of baboon primary motor cortex: Face, hand, forelimb, and shoulder representation. Somatosens. Mot. Res. 1990, 7, 485–514. [Google Scholar] [CrossRef] [PubMed]
- Salzman, C.D.; Murasugi, C.M.; Britten, K.H.; Newsome, W.T. Microstimulation in visual area MT: Effects on direction discrimination performance. J. Neurosci. 1992, 12, 2331–2355. [Google Scholar] [CrossRef] [Green Version]
- Fujii, N.; Mushiake, H.; Tanji, J. Intracortical microstimulation of bilateral frontal eye field. J. Neurophysiol. 1998, 79, 2240–2244. [Google Scholar] [CrossRef] [Green Version]
- Romo, R.; Hernandez, A.; Zainos, A.; Brody, C.D.; Lemus, L. Sensing without touching: Psychophysical performance based on cortical microstimulation. Neuron 2000, 26, 273–278. [Google Scholar] [CrossRef] [Green Version]
- Flesher, S.N.; Collinger, J.L.; Foldes, S.T.; Weiss, J.M.; Downey, J.E.; Tyler-Kabara, E.C.; Bensmaia, S.J.; Schwartz, A.B.; Boninger, M.L.; Gaunt, R.A. Intracortical microstimulation of human somatosensory cortex. Sci. Transl. Med. 2016, 8, 361ra141. [Google Scholar] [CrossRef]
- Rajan, A.T.; Boback, J.L.; Dammann, J.F.; Tenore, F.V.; Wester, B.A.; Otto, K.J.; Gaunt, R.A.; Bensmaia, S.J. The effects of chronic intracortical microstimulation on neural tissue and fine motor behavior. J. Neural Eng. 2015, 12, 066018. [Google Scholar] [CrossRef]
- O’Doherty, J.E.; Lebedev, M.A.; Ifft, P.J.; Zhuang, K.Z.; Shokur, S.; Bleuler, H.; Nicolelis, M.A. Active tactile exploration using a brain-machine-brain interface. Nature 2011, 479, 228–231. [Google Scholar] [CrossRef]
- Klaes, C.; Shi, Y.; Kellis, S.; Minxha, J.; Revechkis, B.; Andersen, R.A. A cognitive neuroprosthetic that uses cortical stimulation for somatosensory feedback. J. Neural Eng. 2014, 11, 056024. [Google Scholar] [CrossRef]
- Flesher, S.N.; Downey, J.E.; Weiss, J.M.; Hughes, C.L.; Herrera, A.J.; Tyler-Kabara, E.C.; Boninger, M.L.; Collinger, J.L.; Gaunt, R.A. A brain-computer interface that evokes tactile sensations improves robotic arm control. Science 2021, 372, 831–836. [Google Scholar] [CrossRef]
- Raspopovic, S.; Capogrosso, M.; Petrini, F.M.; Bonizzato, M.; Rigosa, J.; Di Pino, G.; Carpaneto, J.; Controzzi, M.; Boretius, T.; Fernandez, E.; et al. Restoring natural sensory feedback in real-time bidirectional hand prostheses. Sci. Transl. Med. 2014, 6, 222ra219. [Google Scholar] [CrossRef]
- D’Anna, E.; Valle, G.; Mazzoni, A.; Strauss, I.; Iberite, F.; Patton, J.; Petrini, F.M.; Raspopovic, S.; Granata, G.; Di Iorio, R.; et al. A closed-loop hand prosthesis with simultaneous intraneural tactile and position feedback. Sci. Robot. 2019, 4, eaau8892. [Google Scholar] [CrossRef]
- Zollo, L.; Di Pino, G.; Ciancio, A.L.; Ranieri, F.; Cordella, F.; Gentile, C.; Noce, E.; Romeo, R.A.; Bellingegni, A.D.; Vadala, G.; et al. Restoring Tactile sensations via neural interfaces for real-time force-and-slippage closed-loop control of bionic hands. Sci. Robot. 2019, 4, eaau9924. [Google Scholar] [CrossRef]
- Marasco, P.D.; Hebert, J.S.; Sensinger, J.W.; Beckler, D.T.; Thumser, Z.C.; Shehata, A.W.; Williams, H.E.; Wilson, K.R. Neurorobotic fusion of prosthetic touch, kinesthesia, and movement in bionic upper limbs promotes intrinsic brain behaviors. Sci. Robot. 2021, 6, eabf3368. [Google Scholar] [CrossRef]
- Schofield, J.S.; Shell, C.E.; Beckler, D.T.; Thumser, Z.C.; Marasco, P.D. Long-Term Home-Use of Sensory-Motor-Integrated Bidirectional Bionic Prosthetic Arms Promotes Functional, Perceptual, and Cognitive Changes. Front. Neurosci. 2020, 14, 120. [Google Scholar] [CrossRef] [Green Version]
- Brindley, G.S.; Lewin, W.S. The sensations produced by electrical stimulation of the visual cortex. J. Physiol. 1968, 196, 479–493. [Google Scholar] [CrossRef]
- Dobelle, W.H.; Mladejovsky, M.G. Phosphenes produced by electrical stimulation of human occipital cortex, and their application to the development of a prosthesis for the blind. J. Physiol. 1974, 243, 553–576. [Google Scholar] [CrossRef]
- Schmidt, E.M.; Bak, M.J.; Hambrecht, F.T.; Kufta, C.V.; O’Rourke, D.K.; Vallabhanath, P. Feasibility of a visual prosthesis for the blind based on intracortical microstimulation of the visual cortex. Brain 1996, 119 Pt 2, 507–522. [Google Scholar] [CrossRef]
- Tehovnik, E.J.; Slocum, W.M. Phosphene induction by microstimulation of macaque V1. Brain Res. Rev. 2007, 53, 337–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tehovnik, E.J.; Slocum, W.M.; Smirnakis, S.M.; Tolias, A.S. Microstimulation of visual cortex to restore vision. Prog. Brain Res. 2009, 175, 347–375. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, F.; Fernandez, E.; Roelfsema, P.R. Shape perception via a high-channel-count neuroprosthesis in monkey visual cortex. Science 2020, 370, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, E.; Alfaro, A.; Soto-Sanchez, C.; Gonzalez-Lopez, P.; Lozano, A.M.; Pena, S.; Grima, M.D.; Rodil, A.; Gomez, B.; Chen, X.; et al. Visual percepts evoked with an intracortical 96-channel microelectrode array inserted in human occipital cortex. J. Clin. Investig. 2021, 131, e151331. [Google Scholar] [CrossRef] [PubMed]
- Hanson, T.; Fitzsimmons, N.; O’Doherty, J.E. Technology for Multielectrode MicroStimulation of Brain Tissue. In Methods for Neural Ensemble Recordings, 2nd ed.; Nicolelis, M.A.L., Ed.; Frontiers in Neuroscience; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Dougherty, D.D. Deep Brain Stimulation: Clinical Applications. Psychiatr. Clin. N. Am. 2018, 41, 385–394. [Google Scholar] [CrossRef]
- Benabid, A.L.; Pollak, P.; Gervason, C.; Hoffmann, D.; Gao, D.M.; Hommel, M.; Perret, J.E.; de Rougemont, J. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet 1991, 337, 403–406. [Google Scholar] [CrossRef]
- Rajan, R.; Garg, K.; Saini, A.; Radhakrishnan, D.M.; Carecchio, M.; Bk, B.; Singh, M.; Srivastava, A.K. GPi-DBS for KMT2B-Associated Dystonia: Systematic Review and Meta-Analysis. Mov. Disord. Clin. Pract. 2022, 9, 31–37. [Google Scholar] [CrossRef]
- Gonzalez, V.; Cif, L.; Biolsi, B.; Garcia-Ptacek, S.; Seychelles, A.; Sanrey, E.; Descours, I.; Coubes, C.; de Moura, A.M.; Corlobe, A.; et al. Deep brain stimulation for Huntington’s disease: Long-term results of a prospective open-label study. J. Neurosurg. 2014, 121, 114–122. [Google Scholar] [CrossRef] [Green Version]
- Smeets, A.; Duits, A.A.; Plantinga, B.R.; Leentjens, A.F.G.; Oosterloo, M.; Visser-Vandewalle, V.; Temel, Y.; Ackermans, L. Deep Brain Stimulation of the internal globus pallidus in refractory Tourette Syndrome. Clin. Neurol. Neurosurg. 2016, 142, 54–59. [Google Scholar] [CrossRef]
- Benazzouz, A.; Gao, D.M.; Ni, Z.G.; Piallat, B.; Bouali-Benazzouz, R.; Benabid, A.L. Effect of high-frequency stimulation of the subthalamic nucleus on the neuronal activities of the substantia nigra pars reticulata and ventrolateral nucleus of the thalamus in the rat. Neuroscience 2000, 99, 289–295. [Google Scholar] [CrossRef]
- Anderson, M.E.; Postupna, N.; Ruffo, M. Effects of high-frequency stimulation in the internal globus pallidus on the activity of thalamic neurons in the awake monkey. J. Neurophysiol. 2003, 89, 1150–1160. [Google Scholar] [CrossRef] [Green Version]
- Fariba, K.A.; Gupta, V. Deep Brain Stimulation; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Shetty, N. Essential Tremor-Do We Have Better Therapeutics? A Review of Recent Advances and Future Directions. Curr. Neurol. Neurosci. Rep. 2022, 22, 197–208. [Google Scholar] [CrossRef]
- Velasco, F.; Saucedo-Alvarado, P.E.; Vazquez-Barron, D.; Trejo, D.; Velasco, A.L. Deep Brain Stimulation for Refractory Temporal Lobe Epilepsy. Current Status and Future Trends. Front. Neurol. 2022, 13, 796846. [Google Scholar] [CrossRef]
- Touma, L.; Dansereau, B.; Chan, A.Y.; Jette, N.; Kwon, C.S.; Braun, K.P.J.; Friedman, D.; Jehi, L.; Rolston, J.D.; Vadera, S.; et al. Neurostimulation in People with Drug-Resistant Epilepsy: Systematic Review and Meta-Analysis from the ILAE Surgical Therapies Commission. Epilepsia 2022, 63, 1314–1329. [Google Scholar] [CrossRef]
- Bewernick, B.H.; Kayser, S.; Sturm, V.; Schlaepfer, T.E. Long-term effects of nucleus accumbens deep brain stimulation in treatment-resistant depression: Evidence for sustained efficacy. Neuropsychopharmacology 2012, 37, 1975–1985. [Google Scholar] [CrossRef] [Green Version]
- Denys, D.; Mantione, M.; Figee, M.; van den Munckhof, P.; Koerselman, F.; Westenberg, H.; Bosch, A.; Schuurman, R. Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive-compulsive disorder. Arch. Gen. Psychiatry 2010, 67, 1061–1068. [Google Scholar] [CrossRef]
- Wu, H.; Van Dyck-Lippens, P.J.; Santegoeds, R.; van Kuyck, K.; Gabriels, L.; Lin, G.; Pan, G.; Li, Y.; Li, D.; Zhan, S.; et al. Deep-brain stimulation for anorexia nervosa. World Neurosurg. 2013, 80, S29e21-10. [Google Scholar] [CrossRef]
- Corripio, I.; Roldan, A.; Sarro, S.; McKenna, P.J.; Alonso-Solis, A.; Rabella, M.; Diaz, A.; Puigdemont, D.; Perez-Sola, V.; Alvarez, E.; et al. Deep brain stimulation in treatment resistant schizophrenia: A pilot randomized cross-over clinical trial. eBioMedicine 2020, 51, 102568. [Google Scholar] [CrossRef] [Green Version]
- Huys, D.; Kohl, S.; Baldermann, J.C.; Timmermann, L.; Sturm, V.; Visser-Vandewalle, V.; Kuhn, J. Open-label trial of anterior limb of internal capsule-nucleus accumbens deep brain stimulation for obsessive-compulsive disorder: Insights gained. J. Neurol. Neurosurg. Psychiatry 2019, 90, 805–812. [Google Scholar] [CrossRef]
- Welter, M.L.; Alves Dos Santos, J.F.; Clair, A.H.; Lau, B.; Diallo, H.M.; Fernandez-Vidal, S.; Belaid, H.; Pelissolo, A.; Domenech, P.; Karachi, C.; et al. Deep Brain Stimulation of the Subthalamic, Accumbens, or Caudate Nuclei for Patients with Severe Obsessive-Compulsive Disorder: A Randomized Crossover Controlled Study. Biol. Psychiatry 2021, 90, e45–e47. [Google Scholar] [CrossRef]
- Langevin, J.P.; Chen, J.W.; Koek, R.J.; Sultzer, D.L.; Mandelkern, M.A.; Schwartz, H.N.; Krahl, S.E. Deep Brain Stimulation of the Basolateral Amygdala: Targeting Technique and Electrodiagnostic Findings. Brain Sci. 2016, 6, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Rooij, S.J.H.; Sippel, L.M.; McDonald, W.M.; Holtzheimer, P.E. Defining focal brain stimulation targets for PTSD using neuroimaging. Depress. Anxiety 2021, 38, 768–785. [Google Scholar] [CrossRef] [PubMed]
- Meeres, J.; Hariz, M. Deep Brain Stimulation for Post-Traumatic Stress Disorder: A Review of the Experimental and Clinical Literature. Stereotact. Funct. Neurosurg. 2022, 100, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Castillo, P.R.; Middlebrooks, E.H.; Grewal, S.S.; Okromelidze, L.; Meschia, J.F.; Quinones-Hinojosa, A.; Uitti, R.J.; Wharen, R.E., Jr. Globus Pallidus Externus Deep Brain Stimulation Treats Insomnia in a Patient with Parkinson Disease. Mayo Clin. Proc. 2020, 95, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Marceglia, S.; Guidetti, M.; Harmsen, I.E.; Loh, A.; Meoni, S.; Foffani, G.; Lozano, A.M.; Volkmann, J.; Moro, E.; Priori, A. Deep brain stimulation: Is it time to change gears by closing the loop? J. Neural Eng. 2021, 18, 046002. [Google Scholar] [CrossRef]
- Sigrist, C.; Vockel, J.; MacMaster, F.P.; Farzan, F.; Croarkin, P.E.; Galletly, C.; Kaess, M.; Bender, S.; Koenig, J. Transcranial magnetic stimulation in the treatment of adolescent depression: A systematic review and meta-analysis of aggregated and individual-patient data from uncontrolled studies. Eur. Child Adolesc. Psychiatry 2022, 31, 1501–1525. [Google Scholar] [CrossRef]
- Mosilhy, E.A.; Alshial, E.E.; Eltaras, M.M.; Rahman, M.M.A.; Helmy, H.I.; Elazoul, A.H.; Hamdy, O.; Mohammed, H.S. Non-invasive transcranial brain modulation for neurological disorders treatment: A narrative review. Life Sci. 2022, 307, 120869. [Google Scholar] [CrossRef]
- Zhang, T.; Pan, N.; Wang, Y.; Liu, C.; Hu, S. Transcranial Focused Ultrasound Neuromodulation: A Review of the Excitatory and Inhibitory Effects on Brain Activity in Human and Animals. Front. Hum. Neurosci. 2021, 15, 749162. [Google Scholar] [CrossRef]
- Simko, P.; Kent, J.A.; Rektorova, I. Is non-invasive brain stimulation effective for cognitive enhancement in Alzheimer’s disease? An updated meta-analysis. Clin. Neurophysiol. 2022, 144, 23–40. [Google Scholar] [CrossRef]
- Kumar, S.; Khammash, M. Platforms for Optogenetic Stimulation and Feedback Control. Front. Bioeng. Biotechnol. 2022, 10, 918917. [Google Scholar] [CrossRef]
- Lan, T.H.; He, L.; Huang, Y.; Zhou, Y. Optogenetics for transcriptional programming and genetic engineering. Trends Genet. 2022, 38, 1253–1270. [Google Scholar] [CrossRef]
- Mian, S.Y.; Honey, J.R.; Carnicer-Lombarte, A.; Barone, D.G. Large Animal Studies to Reduce the Foreign Body Reaction in Brain-Computer Interfaces: A Systematic Review. Biosensors 2021, 11, 275. [Google Scholar] [CrossRef]
- Neudorfer, C.; Bhatia, K.; Boutet, A.; Germann, J.; Elias, G.J.; Loh, A.; Paff, M.; Krings, T.; Lozano, A.M. Endovascular deep brain stimulation: Investigating the relationship between vascular structures and deep brain stimulation targets. Brain Stimul. 2020, 13, 1668–1677. [Google Scholar] [CrossRef]
- Cabral, A.M.; Pereira, A.A.; Vieira, M.F.; Pessoa, B.L.; de Oliveira Andrade, A. Prevalence of distinct types of hardware failures related to deep brain stimulation. Neurosurg. Rev. 2021, 45, 1123–1134. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, D. On the Vulnerability of CNN Classifiers in EEG-Based BCIs. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 814–825. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Y.; Sun, K.; Lu, E. Declaration on the ethics of brain–computer interfaces and augment intelligence. AI Ethics 2021, 1, 209–211. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.-P.; Nie, C.; Jiang, C.-T.; Cao, S.-H.; Tian, K.-X.; Yu, S.; Gu, J.-W. Modulating Brain Activity with Invasive Brain–Computer Interface: A Narrative Review. Brain Sci. 2023, 13, 134. https://doi.org/10.3390/brainsci13010134
Zhao Z-P, Nie C, Jiang C-T, Cao S-H, Tian K-X, Yu S, Gu J-W. Modulating Brain Activity with Invasive Brain–Computer Interface: A Narrative Review. Brain Sciences. 2023; 13(1):134. https://doi.org/10.3390/brainsci13010134
Chicago/Turabian StyleZhao, Zhi-Ping, Chuang Nie, Cheng-Teng Jiang, Sheng-Hao Cao, Kai-Xi Tian, Shan Yu, and Jian-Wen Gu. 2023. "Modulating Brain Activity with Invasive Brain–Computer Interface: A Narrative Review" Brain Sciences 13, no. 1: 134. https://doi.org/10.3390/brainsci13010134